Improving Electrochromic Cycle Life of Prussian Blue by Acid Addition to the Electrolyte
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Sample Preparation
2.3. Characterization and Electrochemical Measurements
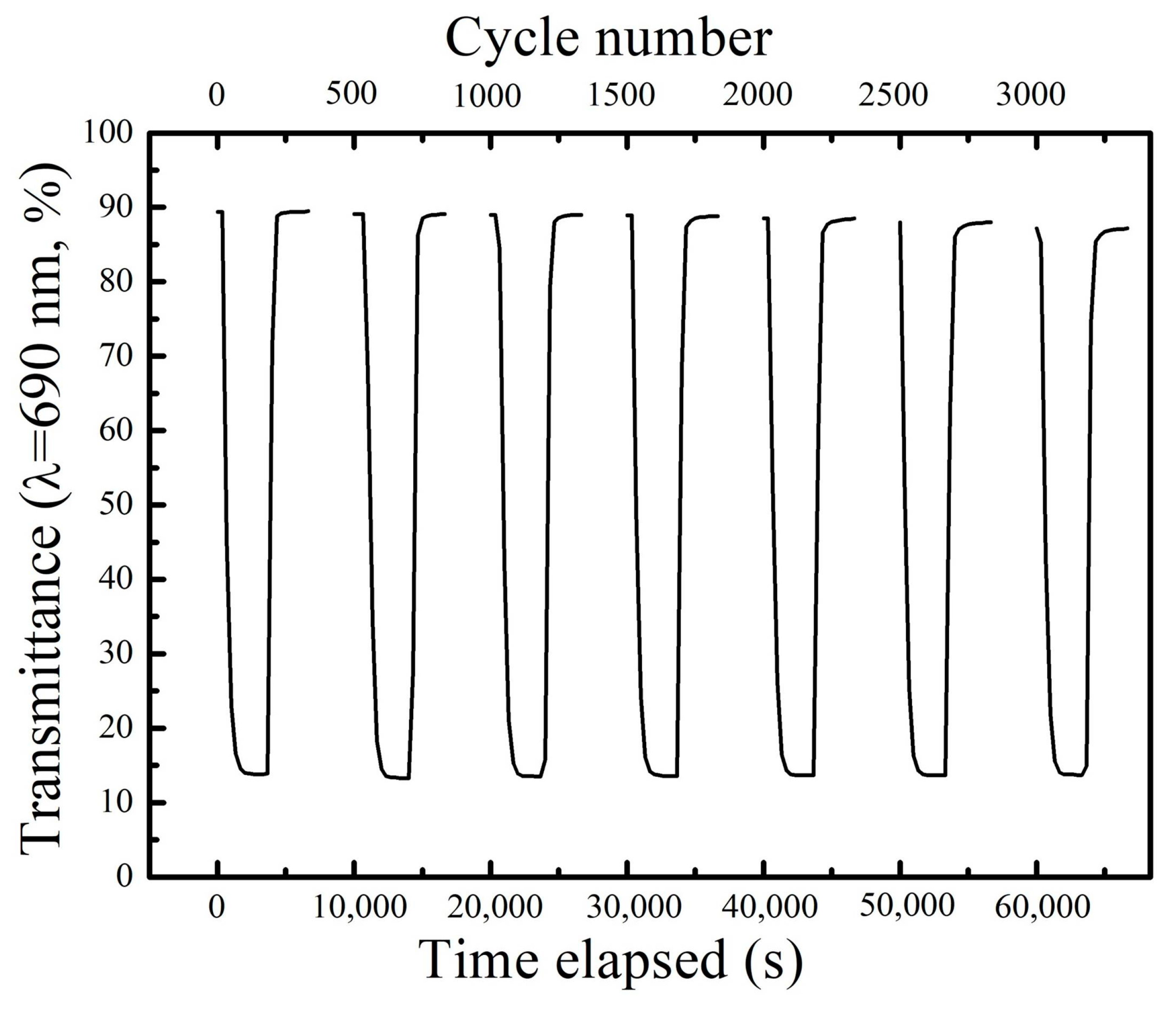
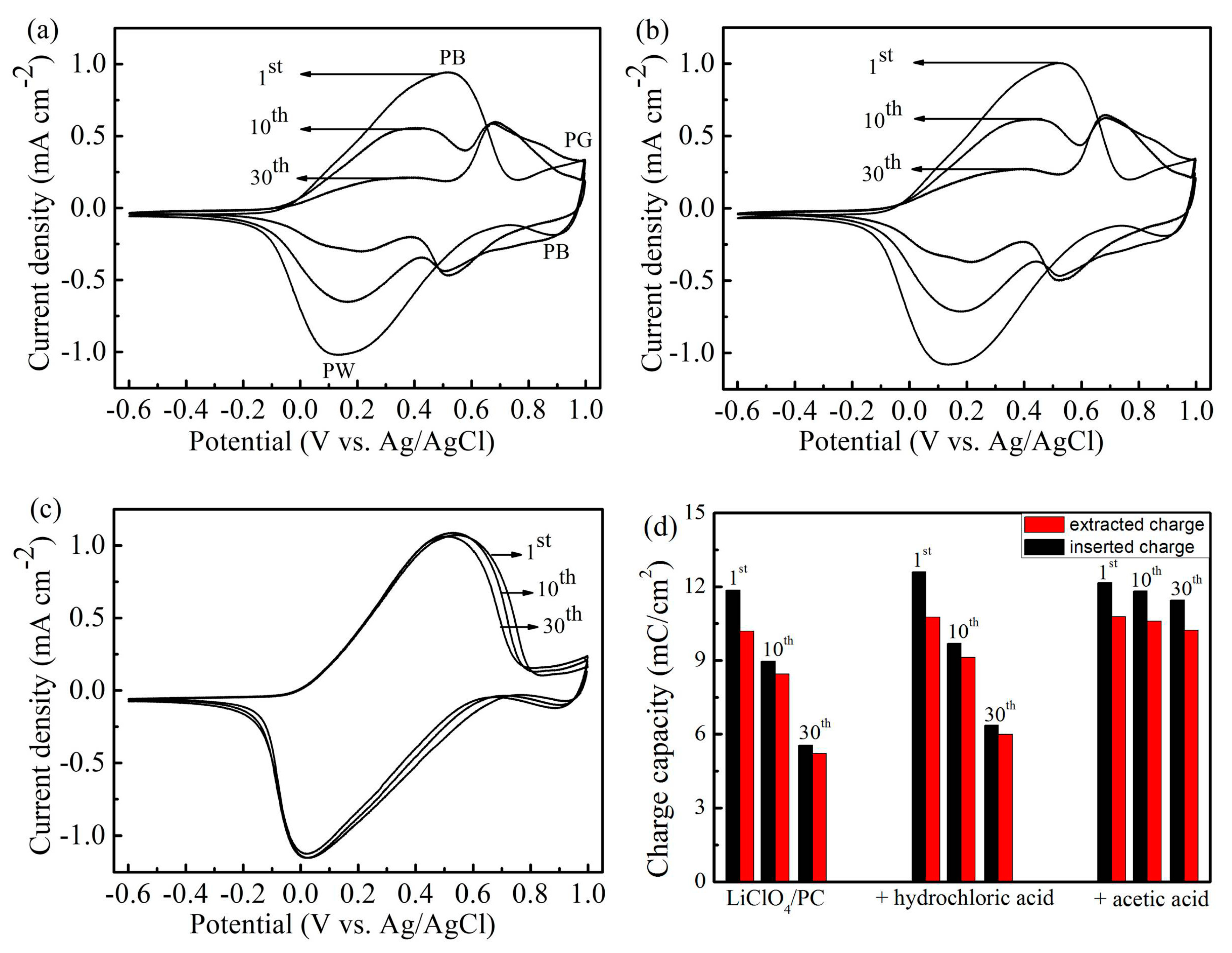
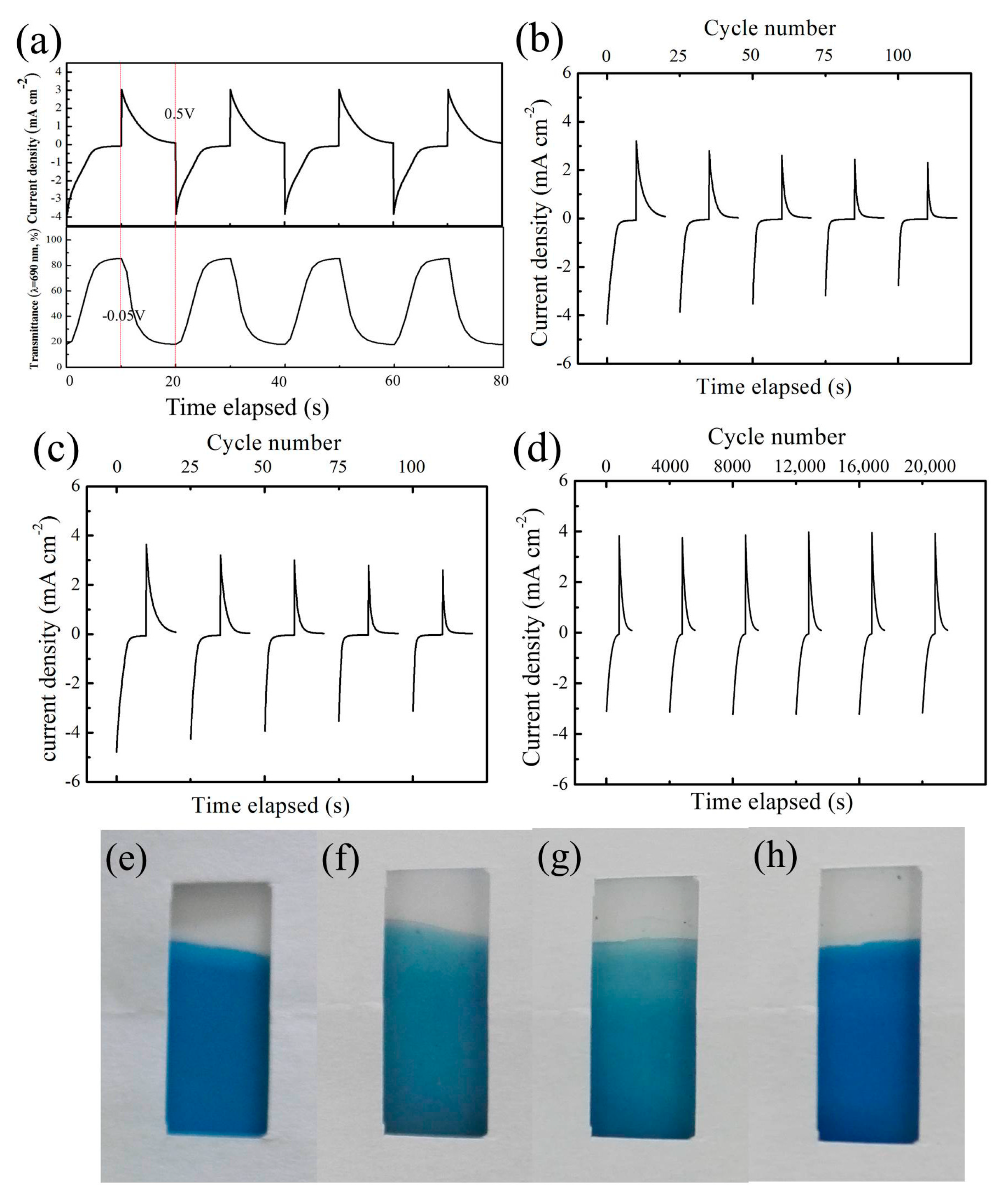
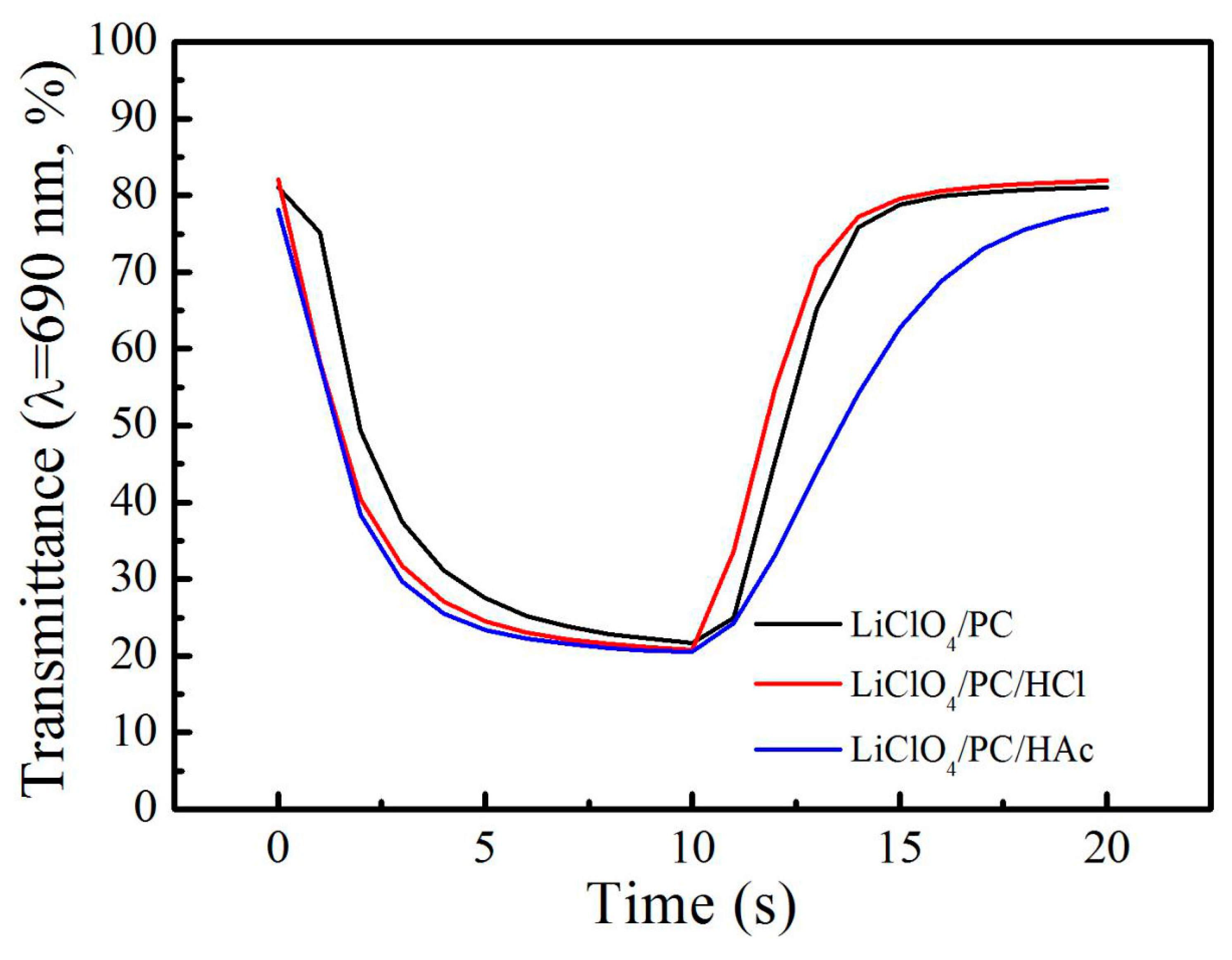
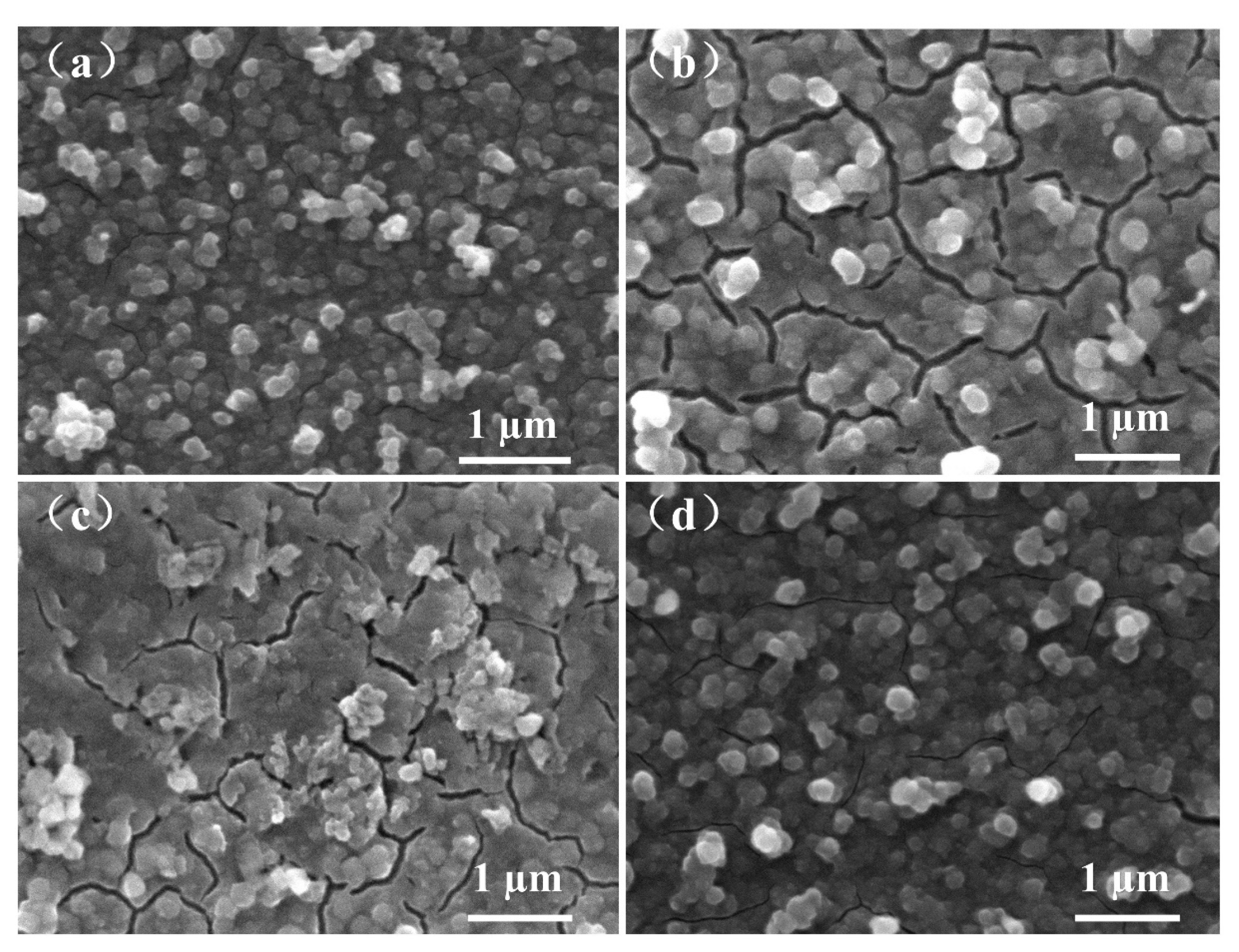
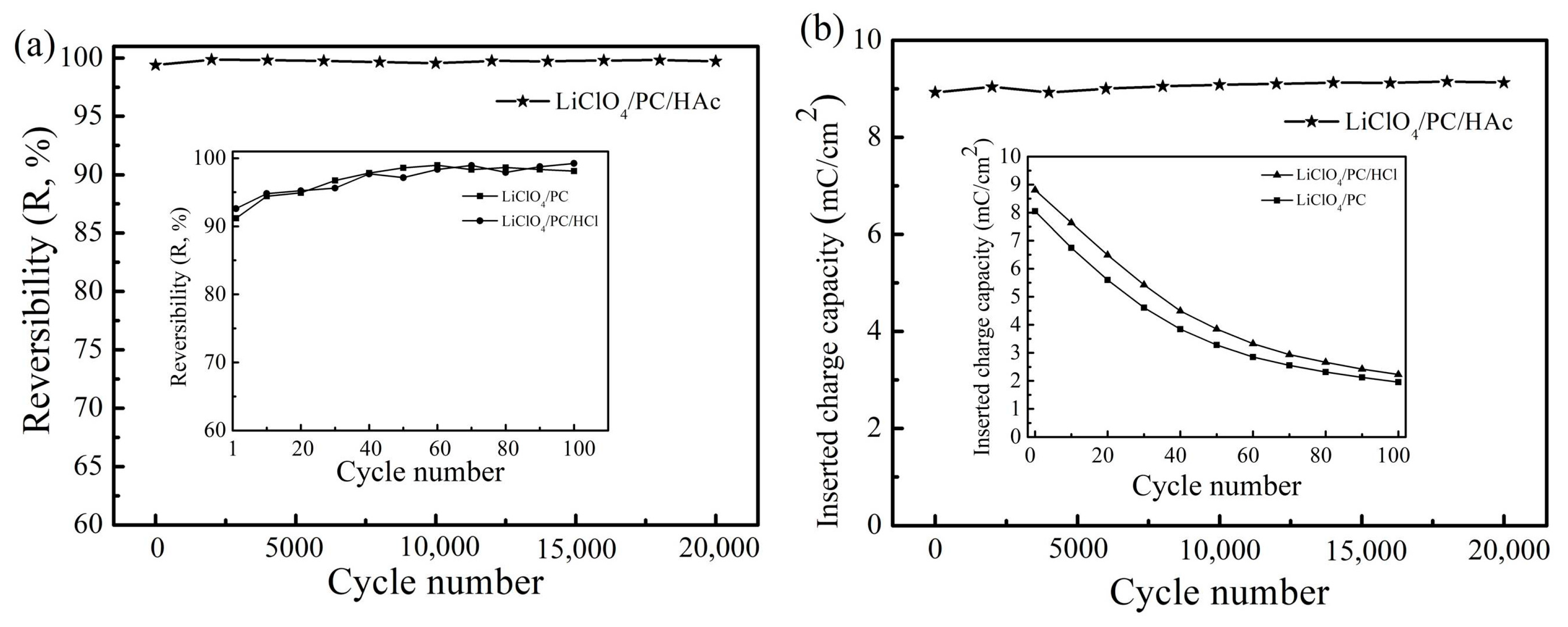
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jensen, J.; Krebs, F.C. Form the bottom up-flexible solid state electrochromic devices. Adv. Mater. 2014, 26, 7231–7234. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.C.; Chen, F.R.; Kai, J.J. WO3-x nanowires based electrochromic devices. Sol. Energy Mater. Sol. Cells 2006, 90, 1147–1155. [Google Scholar] [CrossRef]
- Wang, J.; Khoo, E.; Lee, P.S.; Ma, J. Synthesis, Assembly, and Electrochromic Properties of Uniform Crystalline WO3 Nanorods. J. Phys. Chem. C 2008, 112, 14306–14312. [Google Scholar] [CrossRef]
- Wang, J.M.; Sun, X.W.; Jiao, Z. Application of nanostructures in electrochromic materials and devices: Recent progress. Materials 2010, 3, 5029–5053. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.H.; Tu, J.P.; Zhang, J.; Wang, X.L.; Zhang, W.K.; Huang, H. Morphology effect on the electrochromic and electrochemical performances of NiO thin film. Electrochim. Acta 2008, 53, 5721–5724. [Google Scholar] [CrossRef]
- Oliveira, S.C.; Torresi, R.M. Uma visão das tendências e perspectivas em eletrocromismo: A busca de novos materiais e desenhos mais simples. Quim. Nova 2000, 23, 79–87. [Google Scholar] [CrossRef]
- Delongchamp, D.M.; Hammond, P.T. Multiple-color electrochromism from layer-by-layer-assembled Polyaniline/Prussian blue nanocomposite thin films. Chem. Mater. 2004, 16, 4799–4805. [Google Scholar] [CrossRef]
- Niklasson, G.A.; Granqvist, C.G. Electrochromics for smart windows: thin films of tungsten oxide and nickel oxide, and devices based on these. J. Mater. Chem. 2007, 17, 127–156. [Google Scholar] [CrossRef]
- Bonhote, P.; Gogniat, E.; Campus, F.; Walder, L.; Gratzel, M. Nanocrystalline electrochromic displays. Displays 1999, 20, 137–144. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Yu, L. A bi-functional device for self-powered electrochromic window and self-rechargeable transparent battery applications. Nat. Commun. 2014, 5, 4921. [Google Scholar] [CrossRef]
- Ho, K.C. Cycling and at-rest satbilities of a complementary electrochromic device based on tungsten oxide and Prussian blue. Electrochim. Acta 1999, 44, 3227–3235. [Google Scholar] [CrossRef]
- Takada, K.; Sakamoto, R.; Yi, S.T.; Katagiri, S.; Kambe, T.; Nishihara, H. Electrochromic bis (terpyridine) metal complex nanosheets. J. Am. Chem. Soc. 2015, 137, 4681–4689. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.; Moos, M.; Schreck, M.H.; Lambert, C. Green-to-Red Electrochromic Fe (II) Metallo-Supramolecular Polyelectrolytes Self-Assembled from Fluorescent 2, 6-Bis (2-pyridyl) pyrimidine Bithiophene. Inorg. Chem. 2017, 56, 1418–1432. [Google Scholar] [CrossRef] [PubMed]
- Wałęsa-Chorab, M.; Banasz, R.; Marcinkowski, D.; Kubicki, M.; Patroniak, V. Electrochromism and electrochemical properties of complexes of transition metal ions with benzimidazole-based ligand. RSC Adv. 2017, 7, 50858–50867. [Google Scholar] [CrossRef]
- Burdukov, A.B.; Vershinin, M.A.; Pervukhina, N.V.; Boguslvaskii, E.G.; Eltsov, V.; Shundrin, L.A.; Selector, S.L. Towards the clathrochelate-based electrochromic materials: The case study of the first iron (II) cage complex with an annelated quinoxaline fragment. Inorg. Chem. Commun. 2014, 44, 183–187. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, G.; Xia, J.; Xia, Y.; Zhang, F.; Xia, L.; Song, D.; Liu, J. Facile preparation of a Pt/Prussian blue/graphene composite and its application as an enhanced catalyst for methanol oxidation. Electrochim. Acta 2014, 121, 245–252. [Google Scholar] [CrossRef]
- Soloyev, A.A.; Zakharov, A.N.; Robatkin, S.V.; Kovsharov, N.F. Electrochromic device with polymer electrolyte. J. Electron. Mater. 2016, 45, 3866–3871. [Google Scholar] [CrossRef]
- Wang, J.Y.; Wang, M.C.; Jan, D.J. Synthesis of poly(methyl methacrylate)-succinonitrile composite polymer electrolyte and its application for flexible elcetrochromic devices. Sol. Energy Mater. Sol. Cells 2017, 160, 476–483. [Google Scholar] [CrossRef]
- Fan, M.S.; Kao, S.Y.; Chang, T.H.; Vittal, R.; Ho, K.C. A high contrast solid-state electrochromic device based on nano-structural Prussian blue and poly(butyl viologen) thin films. Sol. Energy Mater. Sol. Cells 2016, 145, 35–41. [Google Scholar] [CrossRef]
- Tung, T.S.; Ho, K.C. Cycling and at-rest stabilities of a complementary electrochromic device containing poly(3,4-ethylenedioxythiophene) and Prussian blue. Sol. Energy Mater. Sol. Cells 2006, 90, 521–537. [Google Scholar] [CrossRef]
- Qian, J.; Ma, D.; Xu, Z.; Li, D.; Wang, J. Electrochromic properties of hydrothermally grown Prussian blue film and device. Sol. Energy Mater. Sol. Cells 2018, 177, 9–14. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.; Gao, B.; Tong, Y.; Zhang, X.; Su, L. Stability improvement of Prussian blue in nonacidic solutions via an electrochemical post-treatment method and the shape evolution of Prussian blue from nanospheres to nanocubes. Analyst 2014, 139, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.C.; Kai, J.J.; Chen, F.R. Improving the durability of Prussian blue based on nano-composite thin film in Li+ based liquid electrolyte. Electrochim. Acta 2007, 52, 6554–6560. [Google Scholar] [CrossRef]
- Seelandt, B.; Wark, M. Electrodeposited Prussian blue in mesoporous TiO2 as electrochromic hybrid material. Microporous Mesoporous Mater. 2012, 164, 67–70. [Google Scholar] [CrossRef]
- Eftekhari, A. Potassium secondary cell based on Prussian blue cathode. J. Power Sources 2004, 126, 221–228. [Google Scholar] [CrossRef]
- Agnihotry, S.A.; Singh, P.; Joshi, A.G.; Singh, D.P.; Sood, K.N.; Shivaprasad, S.M. Electrodeposited Prussian blue film: Annealing effect. Electrochim. Acta 2006, 51, 4291–4301. [Google Scholar] [CrossRef]
- Agrisuelas, J.; García-Jareño, J.J.; Moreno-Guerrero, C.; Vicente, R.F. Identification of electroactive sites in Prussian Yellow films. Electrochim. Acta 2013, 113, 825–833. [Google Scholar] [CrossRef]
- Stilwell, D.E.; Park, K.H.; Miles, M.H. Electrochemical studies of the factors influencing the cyclic stability of Prussian Blue films. J. Appl. Electrochem. 1992, 22, 325–331. [Google Scholar] [CrossRef]
- Shinde, P.S.; Deshmukh, H.P.; Mujawar, S.H.; Inamdar, A.I.; Patil, P.S. Spray deposited titanium oxide thin films as passive counter electrodes. Electrochim. Acta 2007, 52, 3114–3120. [Google Scholar] [CrossRef]
- Sagane, F.; Abe, T.; Iriyama, Y. Li+ and Na+ transfer through interfaces between inorganic solid electrolytes and polymer or liquid electrolytes. J. Power Sources 2005, 146, 749–752. [Google Scholar] [CrossRef]
- Wen, R.T.; Granqvist, C.G.; Niklasson, G.A. Eliminating degradation and uncovering ion-trapping dynamics in electrochromic WO3 thin films. Nat. Mater. 2015, 14, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Kondalkar, V.V.; Patil, P.B.; Mane, R.M.; Patil, P.S.; Choudhury, S. Electrochromic performance of nickel oxide thin film: Synthesis via electrodeposition technique. Macromol. Symp. 2016, 361, 47–50. [Google Scholar] [CrossRef]
- Buser, H.J.; Schwarzenbach, D.; Petter, W.; Ludi, A. The crystal structure of Prussian blue: Fe4(CN6)3·xH2O. Inorg. Chem. 1977, 16, 2704–2710. [Google Scholar] [CrossRef]
- Salazar, P.; Martín, M.; O’Neill, R.D.; Gonzalez-Mora, J.L. Improvement and characterization of surfactant-modified Prussian blue screen-printed carbon electrodes for selective H2O2 detection at low applied potentials. J. Electroanal. Chem. 2012, 674, 48–56. [Google Scholar] [CrossRef]
- Koncki, R.; Wolfbeis, O.S. Composite film of Prussian Blue and N-substituted polypyrroles: Fabrication and application to optical determination of Ph. Anal. Chem. 1998, 70, 2544–2550. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Dong, S. Electrochemistry of vanadium hexacyanoferrate film. Electroanalysis 1997, 9, 838–842. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Tang, Y.; Zhou, K.; Wang, H.; Yan, H. Improving Electrochromic Cycle Life of Prussian Blue by Acid Addition to the Electrolyte. Materials 2019, 12, 28. https://doi.org/10.3390/ma12010028
Li Z, Tang Y, Zhou K, Wang H, Yan H. Improving Electrochromic Cycle Life of Prussian Blue by Acid Addition to the Electrolyte. Materials. 2019; 12(1):28. https://doi.org/10.3390/ma12010028
Chicago/Turabian StyleLi, ZiTong, YunHui Tang, KaiLing Zhou, Hao Wang, and Hui Yan. 2019. "Improving Electrochromic Cycle Life of Prussian Blue by Acid Addition to the Electrolyte" Materials 12, no. 1: 28. https://doi.org/10.3390/ma12010028
APA StyleLi, Z., Tang, Y., Zhou, K., Wang, H., & Yan, H. (2019). Improving Electrochromic Cycle Life of Prussian Blue by Acid Addition to the Electrolyte. Materials, 12(1), 28. https://doi.org/10.3390/ma12010028



