Influence of Alloy Substrate Treatment on Microstructure and Surface Performances of Arc-Ion Plated Gold-Like Film
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Instruments
2.2. Characterization
2.3. The Preparation of Gold-Like Film
2.4. The Surface Treatment of Gold-Like Film
3. Results and Discussion
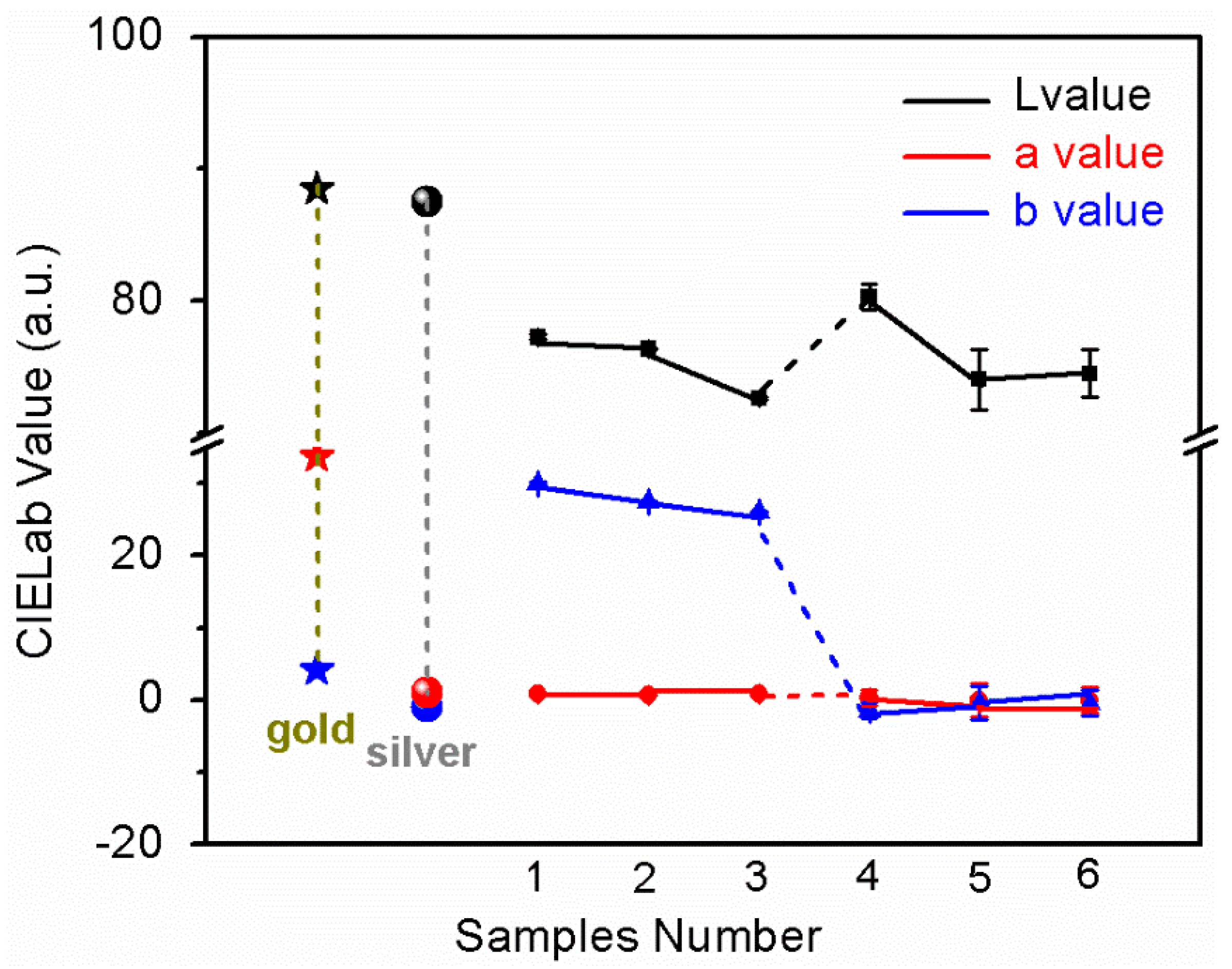
3.1. CIELab Color Coordinate Value Test
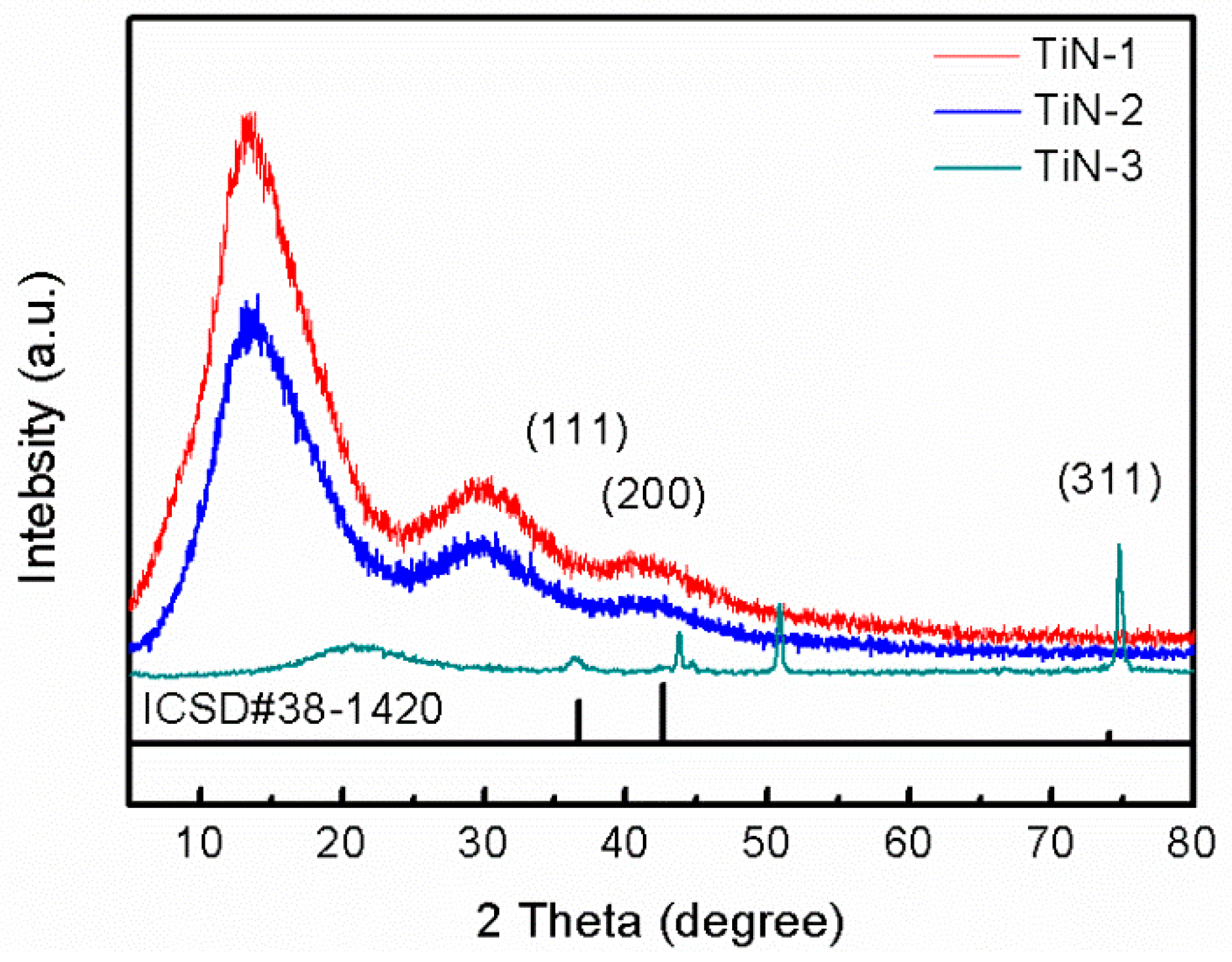
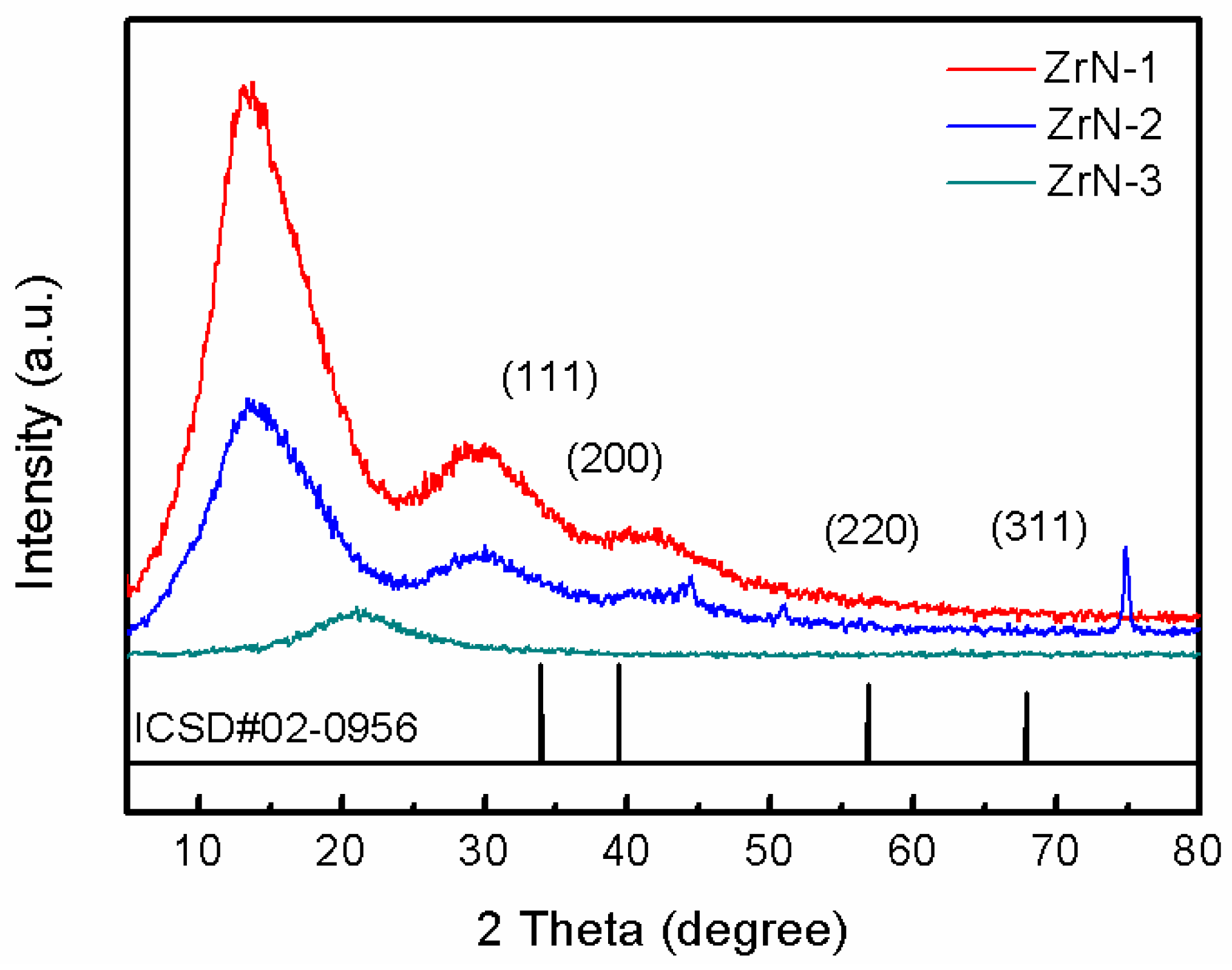
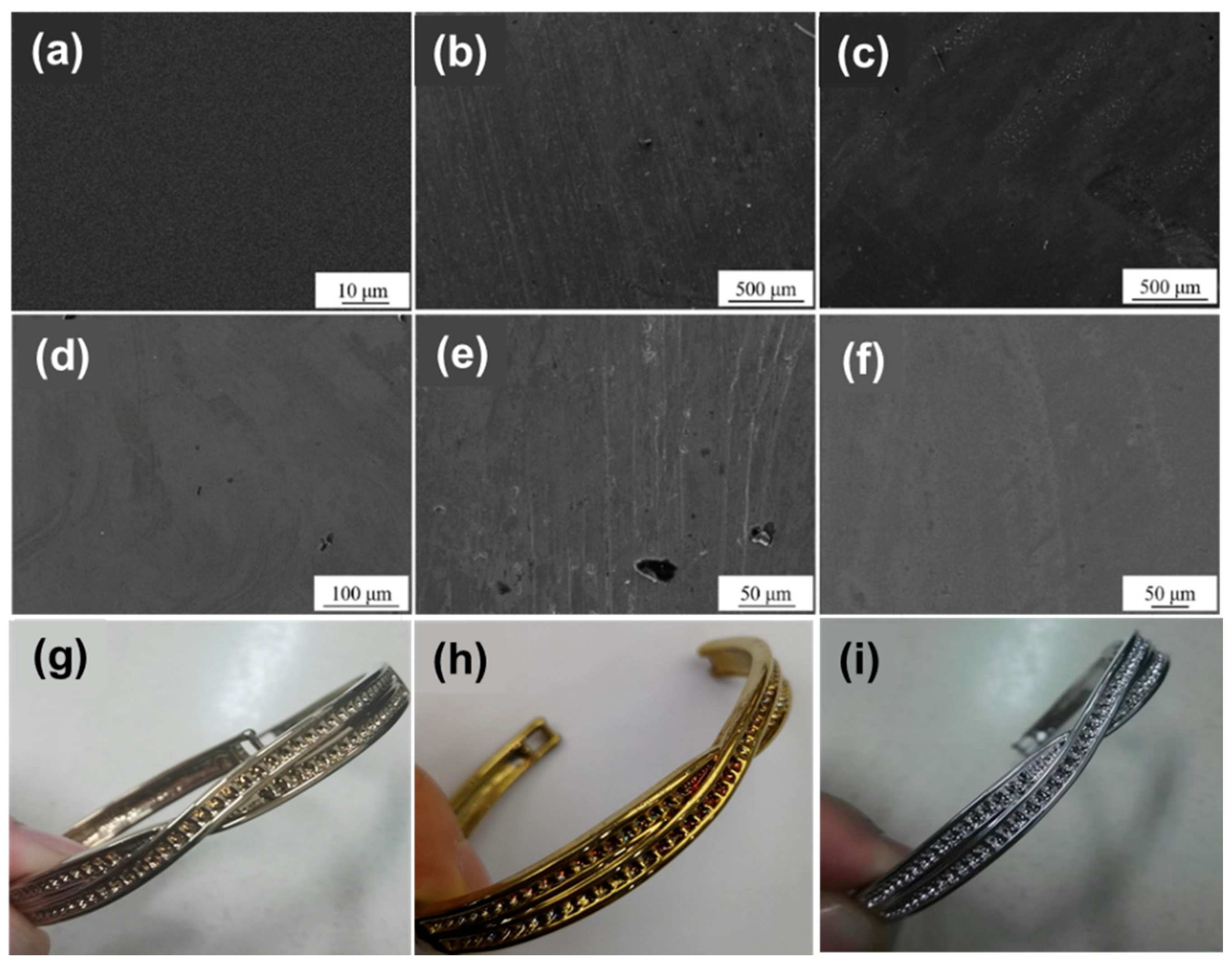
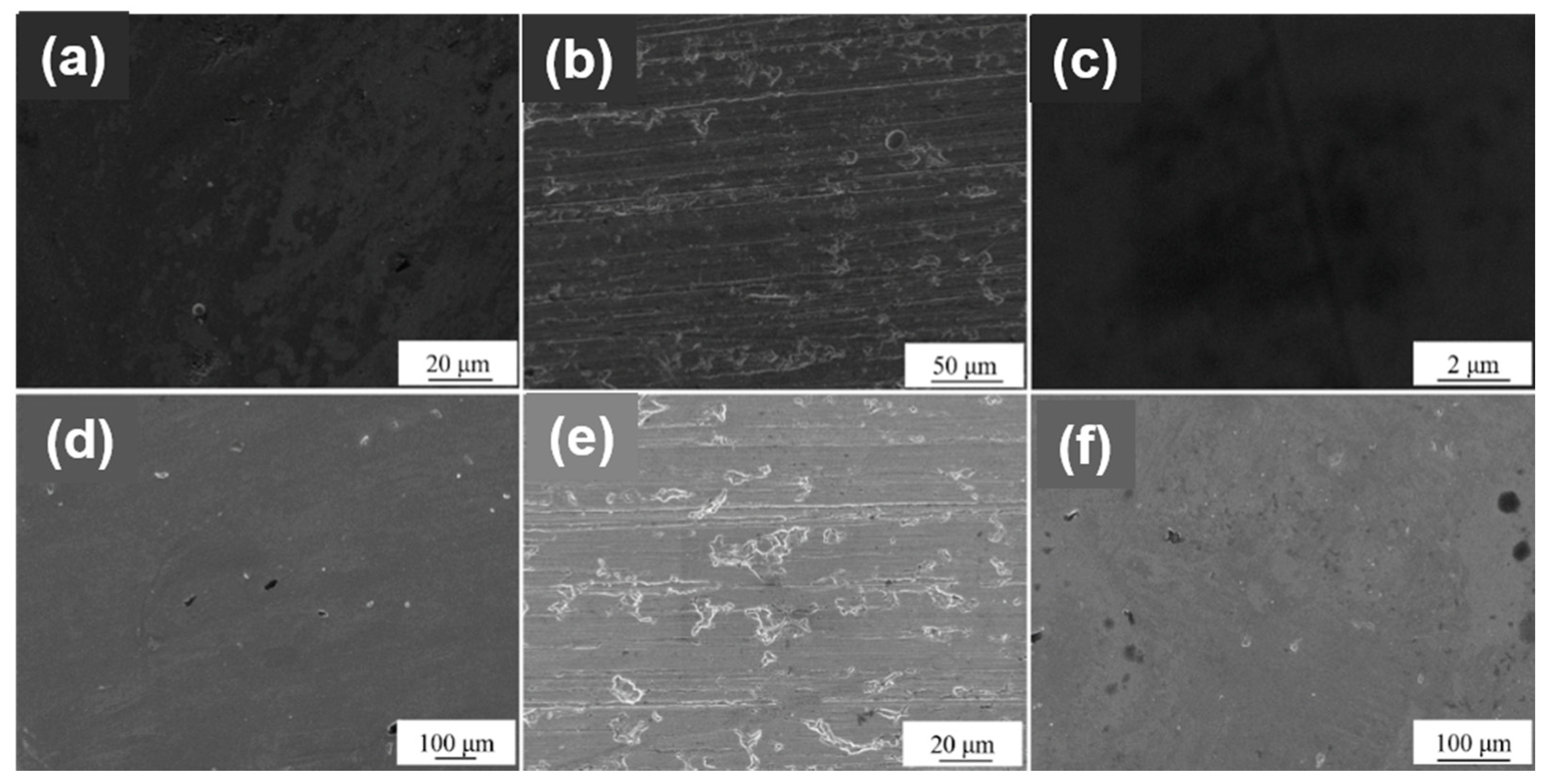
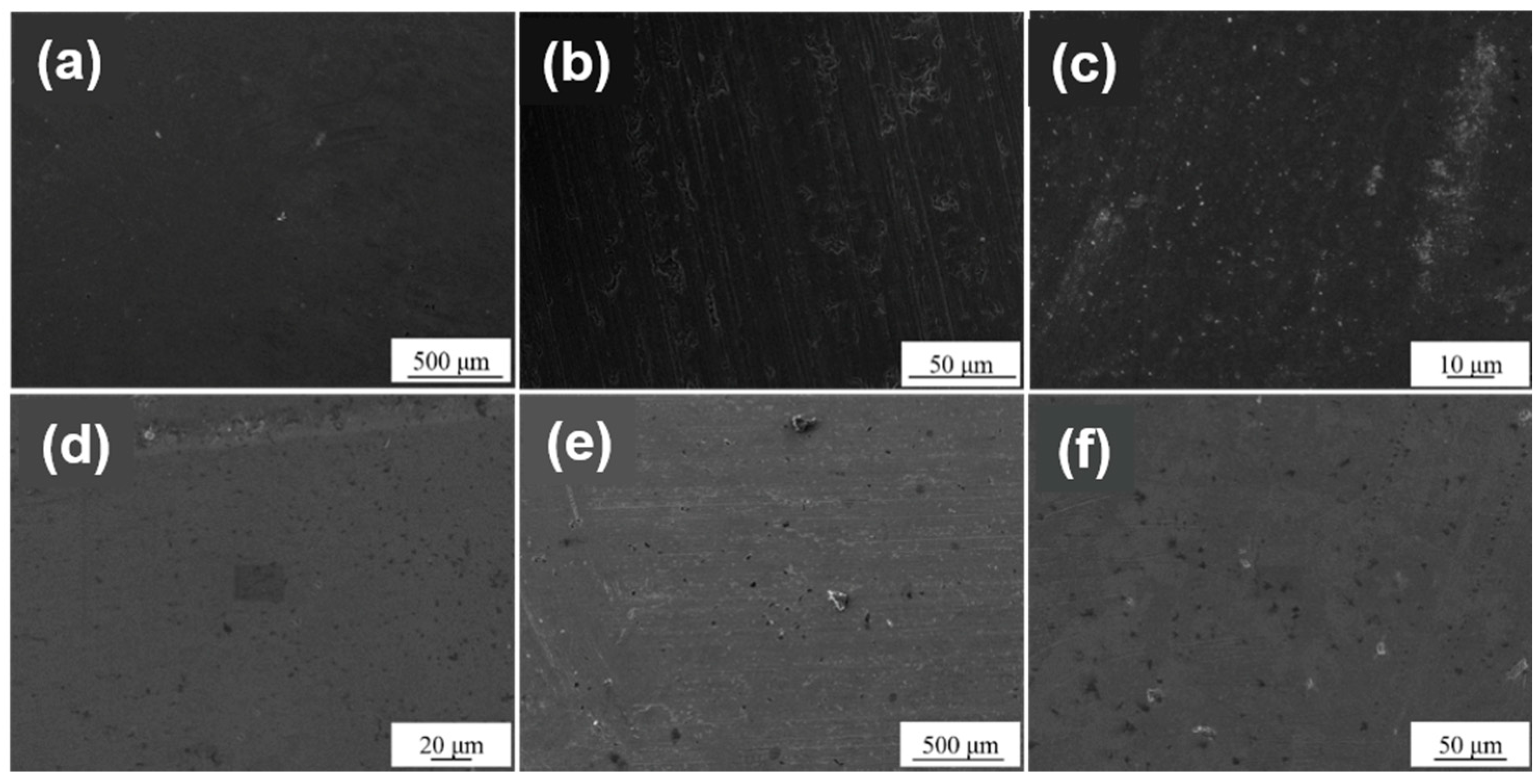
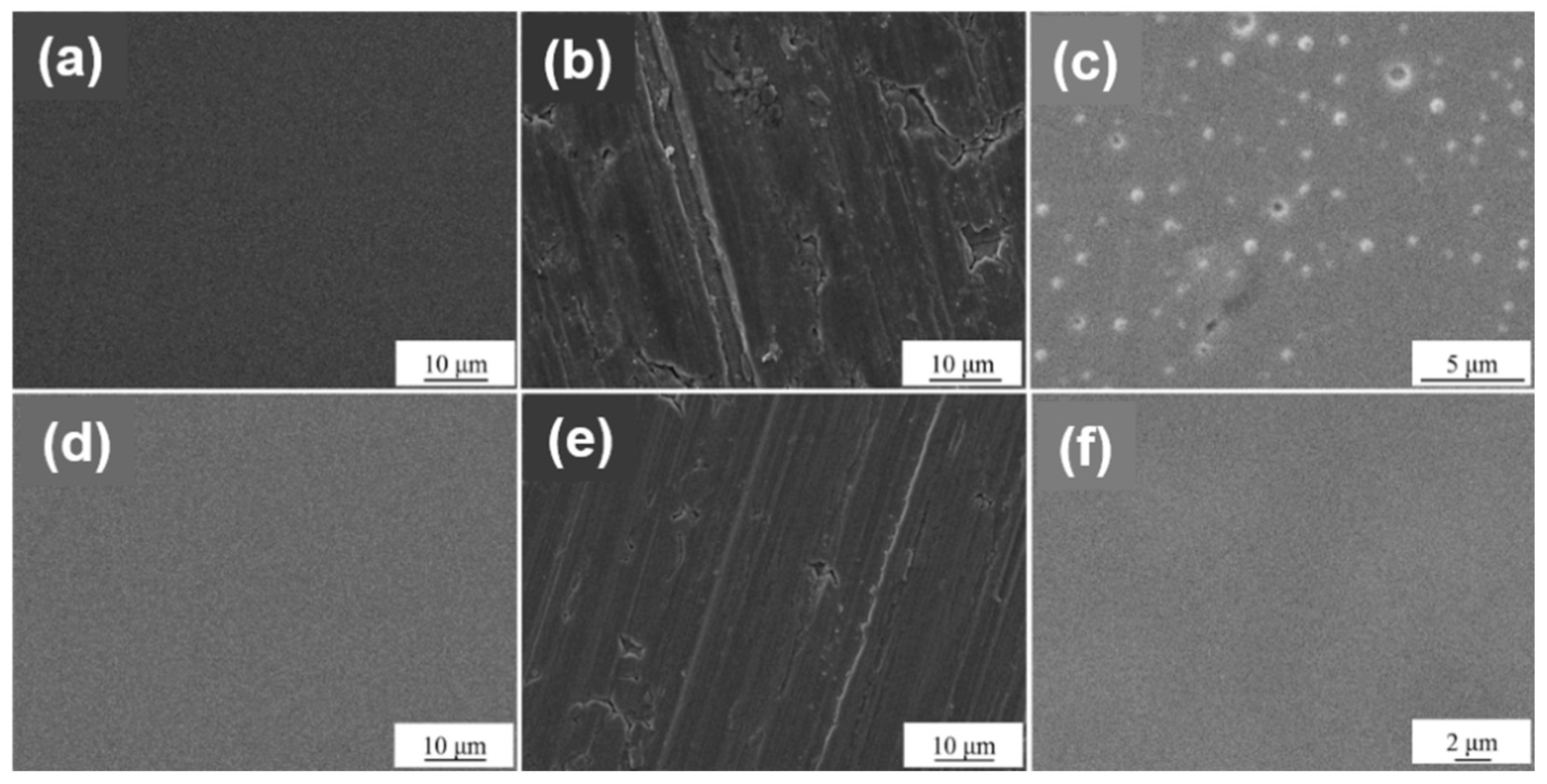
3.2. Crystal Structure and Surface Morphology
3.3. Surface Performances
4. Conclusions
- (1)
- The films with grinding pretreatment are more like gold color; the “L” values are 77.27 cd/m2 and 80.30 cd/m2. The “b” value of TiN film is 29.96, which is the same as that of pure gold. The “a” value of ZrN film is 0.31, which is the same as pure silver. The density of TiN and ZrN films are the best, and both TiN and ZrN films were crystalline. They have the best anti-alkali and anti-oxidation performance. The films with polishing pretreatment have the lowest brightness (72.66 cd/m2) and gold-like color. In terms of the ornamental performance of gold and silver, the rank list for the luminosity of the three surface treatments is: ordinary smoothing treatment > drawing treatment > polishing treatment; TiN film color is closer to pure gold, and ZrN film is closer to pure silver.
- (2)
- The surface treatment method affects the compactness of the film layer and the growth state of the crystal grains. The film obtained by drawing has poor compactness and obvious furrows and cavities; the film obtained by grinding and polishing has good compactness, which is beneficial to improve the corrosion resistance of the film. From grind treatment to drawing and polishing treatment process, the TiN film translates from crystalline to amorphous, and the preferred orientation changes from (311) crystal plane to (200) crystal plane; ZrN film has a good crystallinity, and the preferred orientation along (220) crystal plane appears after disappearing first.
- (3)
- The corrosion resistance and oxidation resistance of the gold-like film are affected by the surface treatment method and film type. The film obtained by the grinding treatment has the best alkali resistance and oxidation resistance. The film obtained by the polishing treatment has the best salt corrosion resistance. The gold-like film obtained by the drawing process has the worst corrosion resistance and oxidation resistance because of a large number of furrows and cavities. TiN film has better corrosion resistance and worse oxidation than ZrN film.
Author Contributions
Funding
Conflicts of Interest
References
- Jiang, K.M.; Zhao, D.Q.; Jiang, X.; Huang, Q.; Miao, L.J.; Lu, H.M.; Li, Y. Electronic-structure, corrosion and mechanical properties of nc-CrC/a-C:H films deposited by multi-arc ion plating. J. Alloys Compd. 2018, 750, 560–569. [Google Scholar] [CrossRef]
- Lei, Z.F.; Zhang, Q.Q.; Zhu, X.D.; Ma, D.Y.; Ma, F.; Song, Z.X.; Fu, Y.Q. Corrosion performance of ZrN/ZrO2 multilayer coatings deposited on 304 stainless steel using multi-arc ion plating. Appl. Surf. Sci. 2018, 431, 170–176. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, S.H.; Chen, Z.; Li, J.L.; Li, M.X. Influence of deposition parameters on hard Cr–Al–N coatings deposited by multi-arc ion plating. Appl. Surf. Sci. 2012, 258, 3629–3636. [Google Scholar] [CrossRef]
- Deng, B.; Ye, T.; Guo, D.L. Effects of vanadium ion implantation on microstructure, mechanical and tribological properties of TiN coatings. Appl. Surf. Sci. 2012, 258, 9080–9086. [Google Scholar] [CrossRef]
- Zhang, S.H.; Wang, L.; Wang, Q.M.; Li, M.X. A superhard CrAlSiN superlattice coating deposited by multi-arc ion plating: I. Microstructure and mechanical properties. Surf. Coat. Technol. 2013, 214, 160–167. [Google Scholar] [CrossRef]
- Garcia, E.; Flores, M.; Rodriguez, E.; Rivera, L.P.; Camps, E.; Muhl, S. Tribological, Tribocorrosion and Wear Mechanism Studies of TaZrN Coatings Deposited by Magnetron Sputtering on TiAlV Alloy. Coatings 2018, 8, 295. [Google Scholar] [CrossRef]
- Liu, T.Y.; Yang, X.; Wang, Z.L.; Yan, X.X. Enhanced chitosan beads-supported Fe0-nanoparticles for removal of heavy metals from electroplating wastewater in permeable reactive barriers. Water Res. 2013, 47, 6691–6700. [Google Scholar] [CrossRef]
- Wang, C.T.; Gao, N.; Gee, M.G.; Wood, R.J.K.; Langdon, T.G. Processing of an ultrafine-grained titanium by high-pressure torsion: An evaluation of the wear properties with and without a TiN coatin. J. Mech. Behav. Biomed. Mater. 2013, 17, 166–175. [Google Scholar] [CrossRef]
- Aelion, C.M.; Davis, H.T.; Mcdermott, S.; Lawson, A.B. Metal concentrations in rural topsoil in South Carolina: Potential for human health impact. Sci. Total Environ. 2008, 402, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.Y.; Teng, Y.G.; Lu, S.J.; Wang, Y.Y.; Wang, J.S. Contamination features and health risk of soil heavy metals in China. Sci. Total Environ. 2015, 512–513, 143–153. [Google Scholar] [CrossRef]
- Sobol, O.V.; Meilekhov, A.A. Conditions of Attaining a Superhard State at a Critical Thickness of Nanolayers in Multiperiodic Vacuum-Arc Plasma Deposited Nitride Coatings. Tech. Phys. Lett. 2018, 44, 63–66. [Google Scholar] [CrossRef]
- Mei, H.J.; Zhao, S.S.; Chen, W.; Wang, Q.M.; Liang, H.F. Microstructure and residual stress of TiN films deposited at low temperature by arc ion plating. Trans. Nonferr. Met. Soc. China 2018, 28, 1368–1376. [Google Scholar] [CrossRef]
- Kravchenko, Y.O.; Coy, L.E.; Peplinska, B.; Iatsunskyi, I.; Załeski, K.; Kempinski, M.; Beresnev, V.M.; Konarski, P.; Jurga, S.; Pogrebnjak, A.D. Nano-multilayered coatings of (TiAlSiY)N/MeN (Me=Mo, Cr and Zr): Influence of composition of the alternating layer on their structural and mechanical properties. J. Alloys Compd. 2018, 767, 483–495. [Google Scholar] [CrossRef]
- Xu, Y.X.; Chen, L.; Pei, F.; Chang, K.K.; Du, Y. Effect of the modulation ratio on the interface structure of TiAlN/TiN and TiAlN/ZrN multilayers: First-principles and experimental investigation. Acta Mater. 2017, 130, 281–288. [Google Scholar] [CrossRef]
- Valerini, D.; Signore, M.A.; Tapfer, L.; Piscopiello, E.; Galietti, U.; Rizzo, A. Adhesion and wear of ZrN films sputtered on tungsten carbide substrates. Thin Solid Films 2013, 538, 42–47. [Google Scholar] [CrossRef]
- Fayomi, O.S.I.; Monyai1, T.; Popoola, A.P.I. Wear stress mitigation and structural characteristics of highly performing zinc-based induced ZrO2/ZrN composite alloy coating on mild steel. Int. J. Adv. Manuf. Tech. 2018, 96, 1813–1821. [Google Scholar] [CrossRef]
- Chen, Y.G.; Zhang, M.; Fan, D.M.; Fan, K.; Wang, X.C. Linear Regression between CIE-Lab Color Parameters and Organic Matter in Soils of Tea Plantations. Eurasian Soil Sci. 2018, 51, 199–203. [Google Scholar] [CrossRef]
- Meints, T.; Teischinger, A.; Stingl, R.; Hansmann, C. Wood colour of central European wood species: CIELAB characterisation and colour intensification. Eur. J. Wood Wood Prod. 2016, 75, 499–509. [Google Scholar] [CrossRef]
- Han, K.C.; Lin, G.Q.; Dong, C.; Tai, K.P.; Jiang, X. Influence of nitrogen vacancy concentration on mechanical and electrical properties of rocksalt zirconium nitride films. Acta Metall. Sin. 2017, 30, 1100–1108. [Google Scholar] [CrossRef]
- Pogrebnjak, A.; Ivashchenko, V.; Bondar, O.; Beresnevc, V.; Sobold, O.; Załęskie, K.; Jurgae, S.; Coye, E.; Konarskif, P.; Postolnyia, B. Multilayered vacuum-arc nanocomposite TiN/ZrN coatings before and after annealing: Structure, properties, first-principles calculations. Mater. Charact. 2017, 134, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Achour, A.; Porto, R.L.; Soussou, M.A.; Islam, M.; Boujtita, M.; Aissa, K.A.; Le Brizoual, L.; Djouadi, A.; Brousse, T. Titanium nitride films for micro-supercapacitors: Effect of surface chemistry and film morphology on the capacitance. J. Power Sources 2015, 300, 525–532. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Z.; Yang, X.F.; Fan, X.W.; Chen, B.; Zhang, J.Y.; Li, D. First-principles study of the TiN(111)/ZrN(111) interface. Surf. Interface Anal. 2018, 50, 321–327. [Google Scholar] [CrossRef]
- Chen, Q.; Xie, Z.W.; Chen, T.; Gong, F. Tribocorrosion failure mechanism of TiN/SiOx duplex coating deposited on AISI304 stainless steel. Materials 2016, 9, 936. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.M.; Liu, J.H.; Zhang, R.J. Corrosion behaviour of electrodeposited Ni/Al2O3 composite coating covered with a NaCl salt film at 800 °C. Mater. Corros. 2015, 62, 920–925. [Google Scholar] [CrossRef]
- You, Y.; Li, H.Q.; Huang, Y.Q.; Tang, Q.; Zhang, J.; Xu, J. (SiC/AlN)2 multilayer film as an effective protective coating for sintered NdFeB by magnetron sputtering. Mater. Res. Express 2017, 4, 086407. [Google Scholar] [CrossRef]
- Ju, H.B.; Jia, P.; Xu, J.H.; Geng, Y.X.; Chen, Y.H.; Liu, M.H.; Wei, T.Y. The effects of adding aluminum on crystal structure, mechanical, oxidation resistance, friction and wear properties of nanocomposite vanadium nitride hard films by reactive magnetron sputtering. Mater. Chem. Phys. 2018, 215, 368–375. [Google Scholar] [CrossRef]
| Numbers | Samples | Surface Treatments | Films |
|---|---|---|---|
| 1 | TiN-1 | Grinding surface | TiN film |
| 2 | TiN-2 | Drawing surface | TiN film |
| 3 | TiN-3 | Polished surface | TiN film |
| 4 | ZrN-1 | Grinding surface | ZrN film |
| 5 | ZrN-2 | Drawing surface | ZrN film |
| 6 | ZrN-3 | Polished surface | ZrN film |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Q.; Pei, J.; Huang, J.; Zhang, Y.; Ma, D. Influence of Alloy Substrate Treatment on Microstructure and Surface Performances of Arc-Ion Plated Gold-Like Film. Materials 2019, 12, 180. https://doi.org/10.3390/ma12010180
Yu Q, Pei J, Huang J, Zhang Y, Ma D. Influence of Alloy Substrate Treatment on Microstructure and Surface Performances of Arc-Ion Plated Gold-Like Film. Materials. 2019; 12(1):180. https://doi.org/10.3390/ma12010180
Chicago/Turabian StyleYu, Qinghai, Jingxuan Pei, Jiankang Huang, Yuejuan Zhang, and Di Ma. 2019. "Influence of Alloy Substrate Treatment on Microstructure and Surface Performances of Arc-Ion Plated Gold-Like Film" Materials 12, no. 1: 180. https://doi.org/10.3390/ma12010180
APA StyleYu, Q., Pei, J., Huang, J., Zhang, Y., & Ma, D. (2019). Influence of Alloy Substrate Treatment on Microstructure and Surface Performances of Arc-Ion Plated Gold-Like Film. Materials, 12(1), 180. https://doi.org/10.3390/ma12010180




