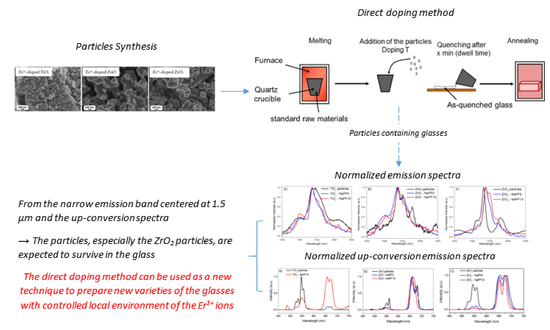
Spectroscopic Properties of Er3+-Doped Particles-Containing Phosphate Glasses Fabricated Using the Direct Doping Method
Abstract
1. Introduction
2. Materials and Methods
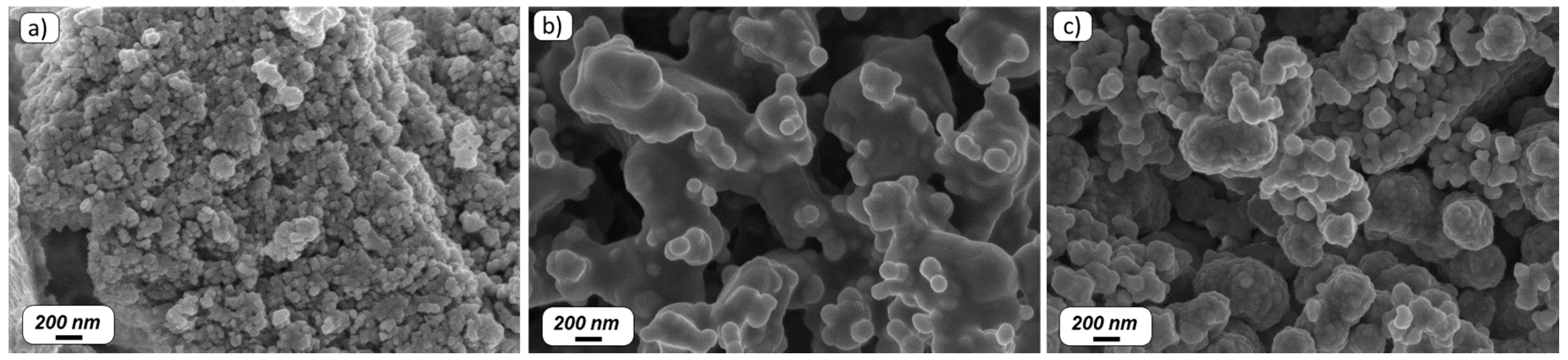
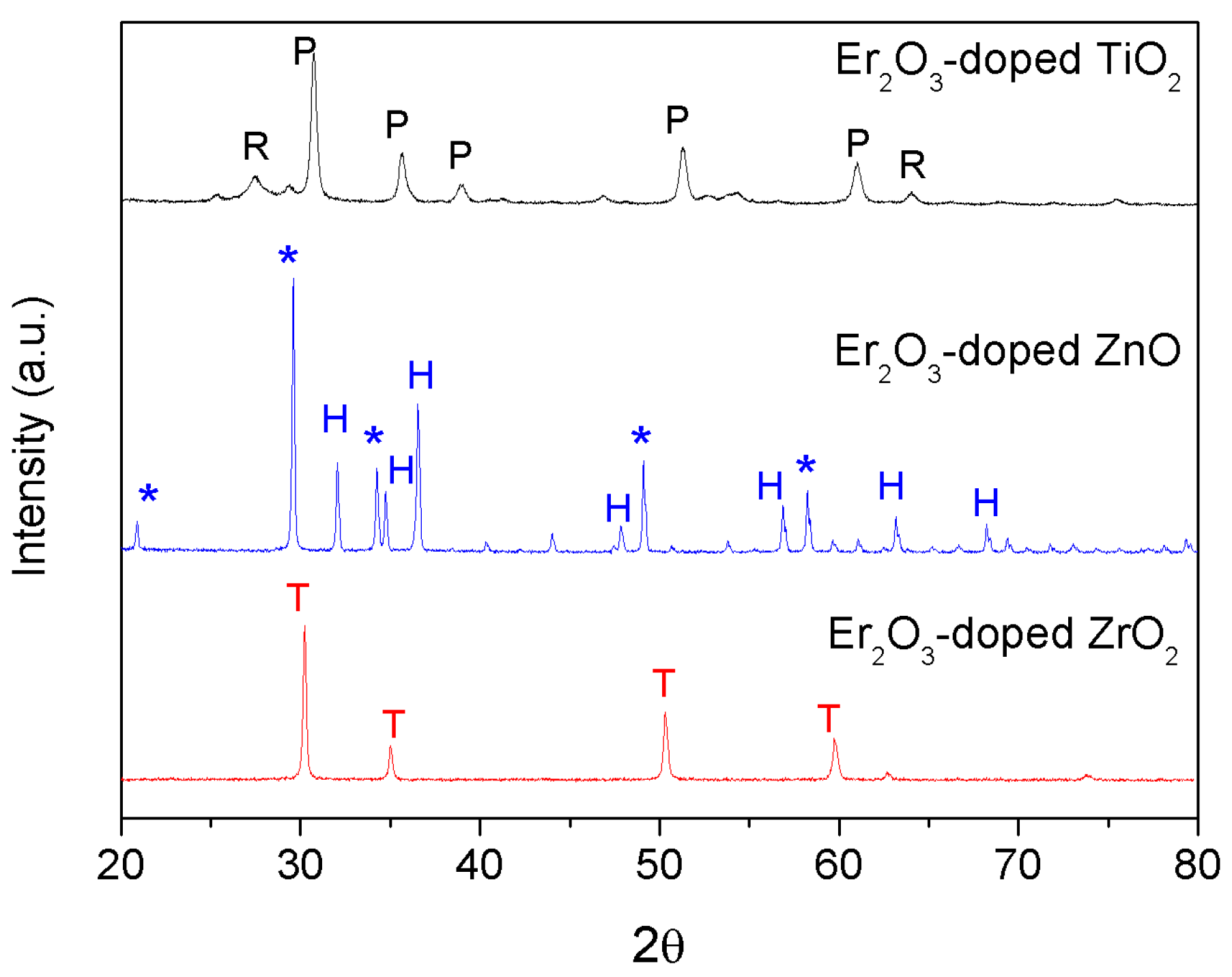
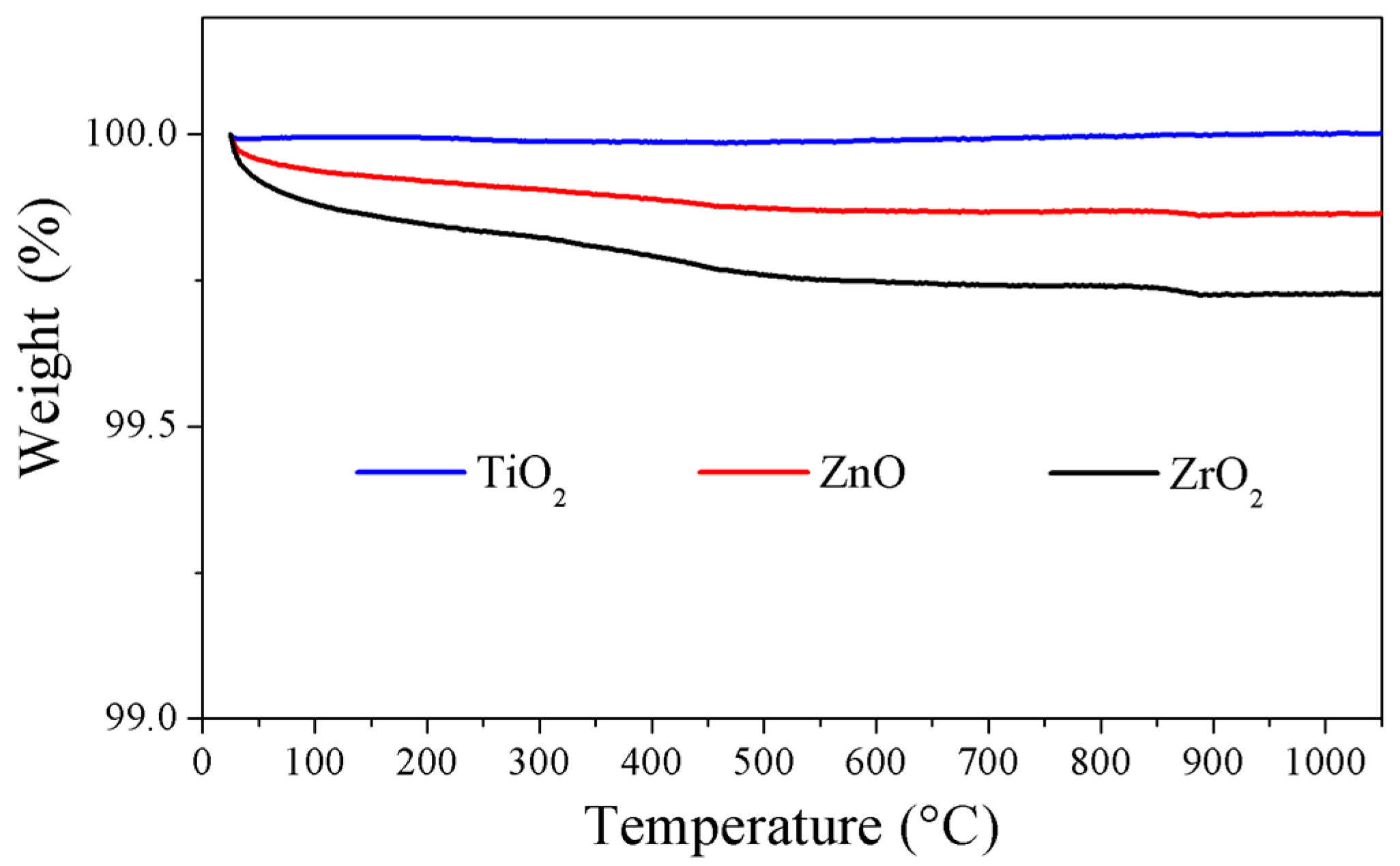
2.1. Particles Synthesis
2.2. Particles-Containing Glasses Preparation
2.3. Characterization Techniques
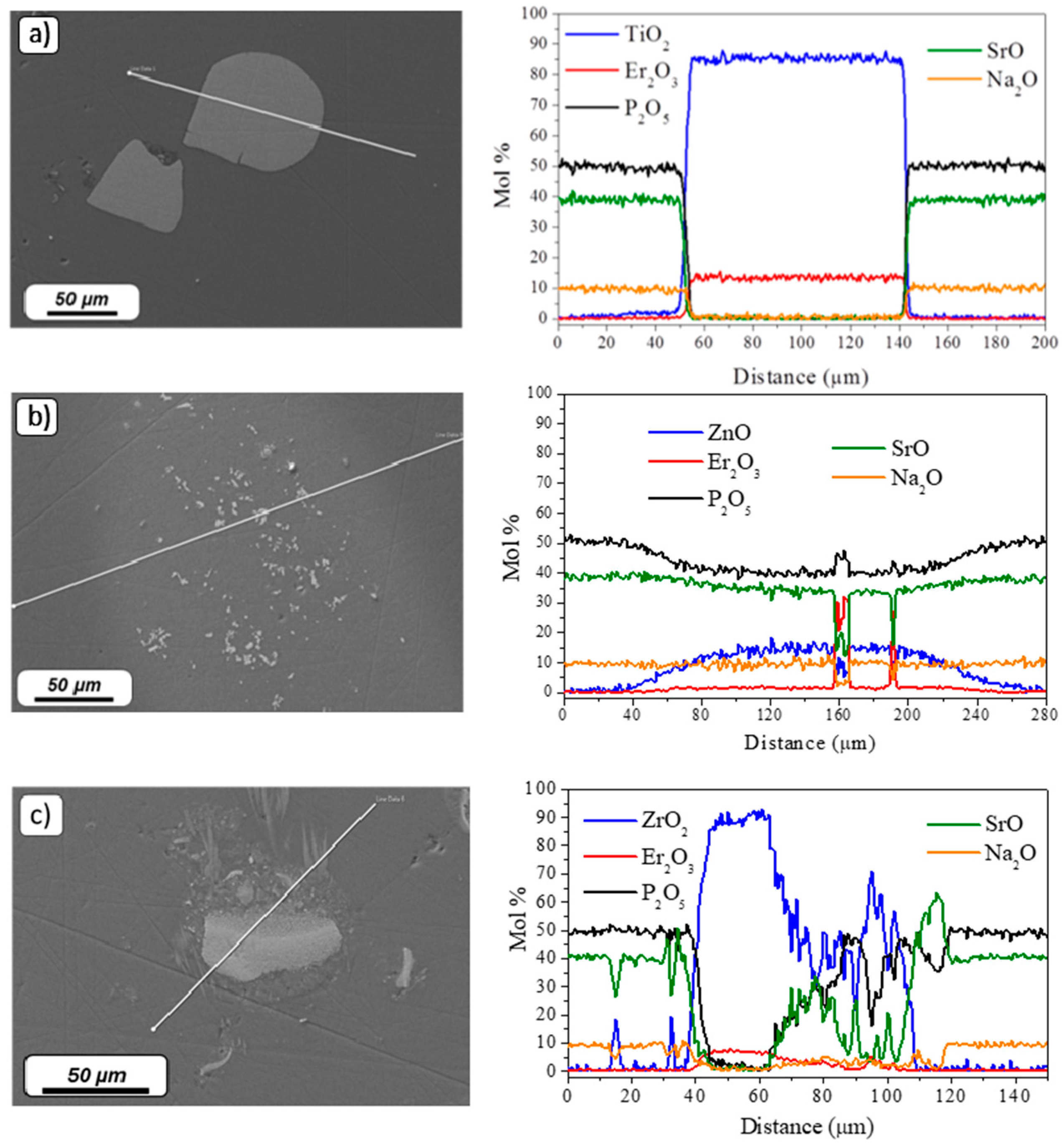
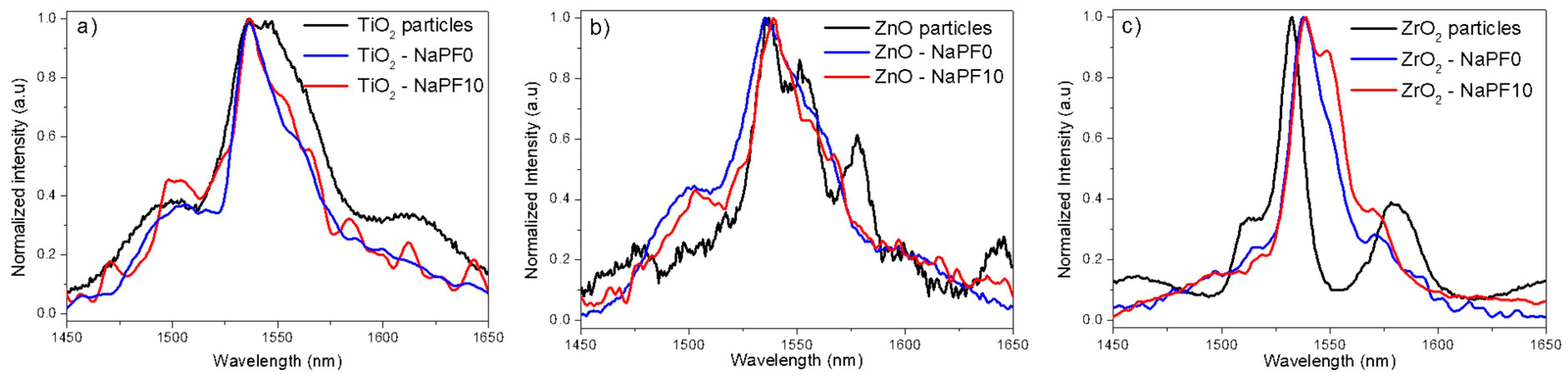
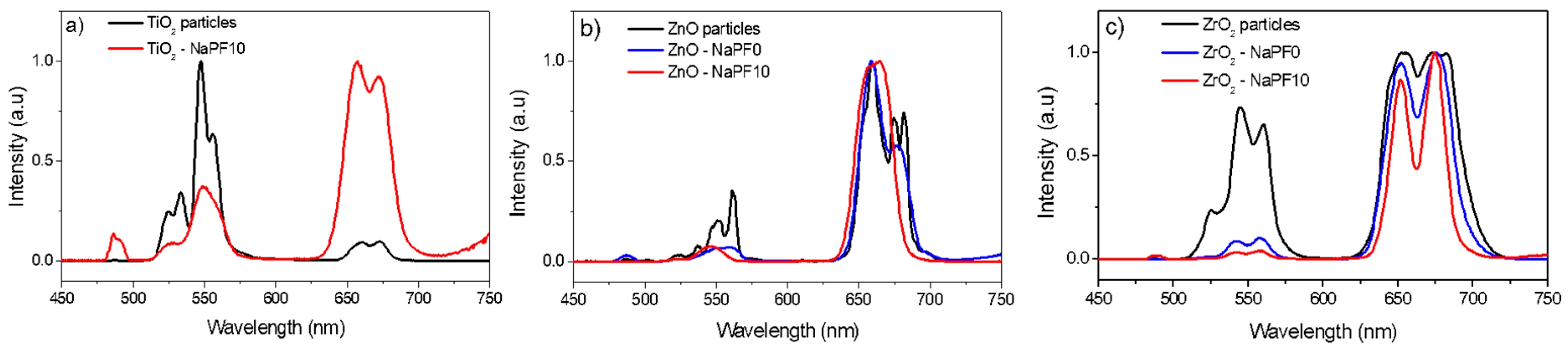
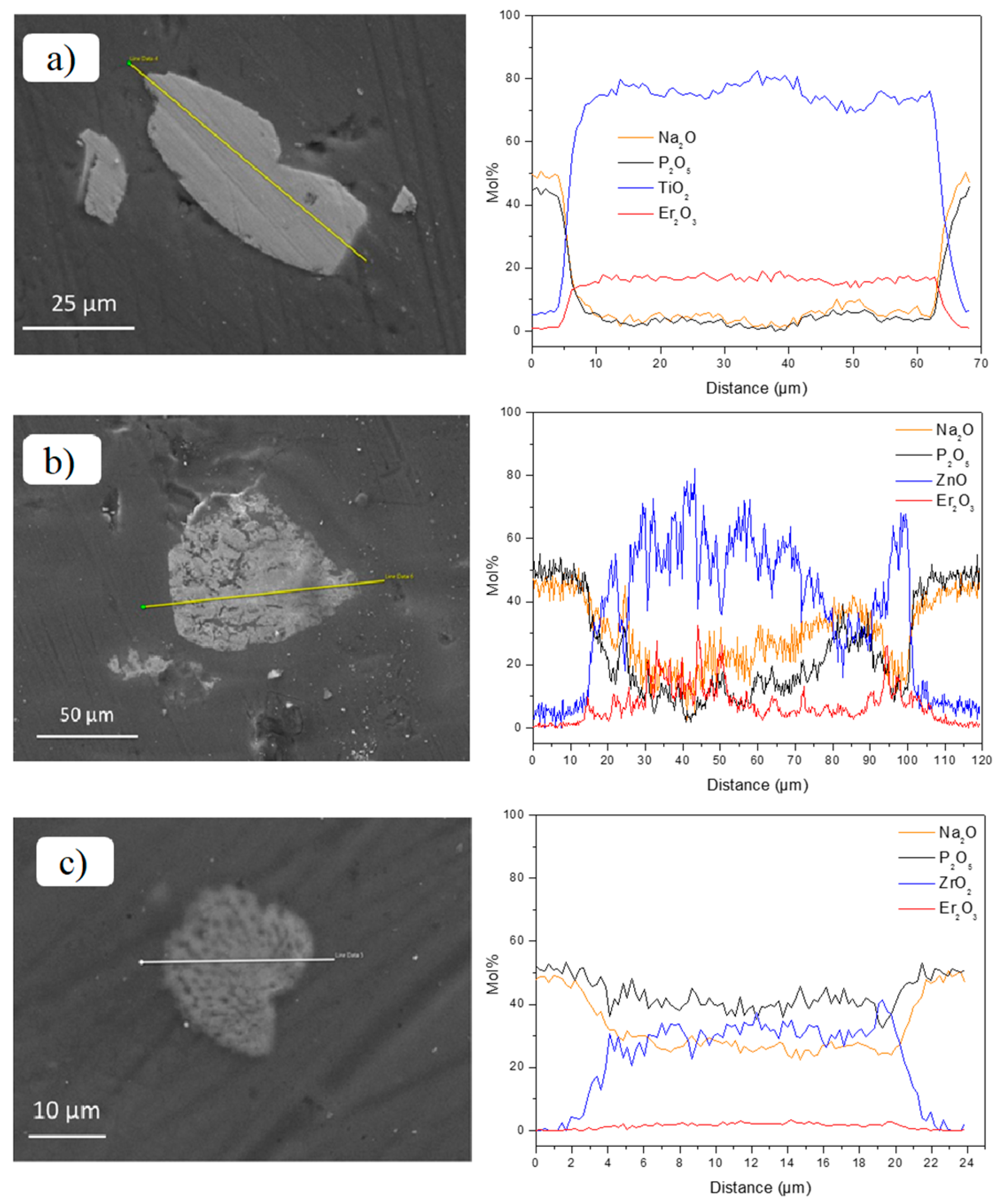
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miniscalco, W.J. Erbium-doped glasses for fiber amplifiers at 1500 nm. J. Lightw. Technol. 1991, 9, 234–250. [Google Scholar] [CrossRef]
- De Wild, J.; Meijerink, A.; Rath, J.K.; van Sark, W.G.J.H.M.; Schropp, R.E.I. Upconverter solar cells: Materials and applications. Energy Environ. Sci. 2011, 4, 4835–4848. [Google Scholar] [CrossRef]
- Rapaport, A.; Milliez, J.; Bass, M.; Cassanho, A.; Jenssen, H. Review of the properties of up-conversion phosphors for new emissive displays. J. Disp. Technol. 2006, 2, 68–78. [Google Scholar] [CrossRef]
- Wang, M.; Mi, C.-C.; Wang, W.-X.; Liu, C.-H.; Wu, Y.-F.; Xu, Z.-R.; Mao, C.B.; Xu, S.K. Immunolabeling and NIR-excited fluorescent imaging of HeLa cells by using NaYF4:Yb,Er upconversion nanoparticles. ACS Nano 2009, 3, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.H.; Suratwala, T.I. Nd-doped phosphate glasses for high-energy/high-peak-power lasers. J. Non-Cryst. Solids 2000, 263–264, 318–341. [Google Scholar] [CrossRef]
- Jiang, S.; Myers, M.J.; Rhonehouse, D.L.; Hamlin, S.J.; Myers, J.D.; Griebner, U.; Koch, R.; Schonnagel, H. Ytterbium-doped phosphate laser glasses. In Proceedings of the 1997 SPIE International Conference on Photonics West, San Jose, CA, USA, 8–14 February 1997. [Google Scholar]
- Gapontsev, V.P.; Matitsin, S.M.; Isineev, A.A.; Kravchenko, V.B. Erbium glass lasers and their applications. Opt. Laser Technol. 1982, 14, 189–196. [Google Scholar] [CrossRef]
- Jiang, S.; Myers, M.; Peyghambarian, N. Er3+ doped phosphate glasses and lasers. J. Non-Cryst. Solids 1998, 239, 143–148. [Google Scholar] [CrossRef]
- Pugliese, D.; Boetti, N.G.; Lousteau, J.; Ceci-Ginistrelli, E.; Bertone, E.; Geobaldo, F.; Milanese, D. Concentration quenching in an Er-doped phosphate glass for compact optical lasers and amplifiers. J. Alloys Compd. 2016, 657, 678–683. [Google Scholar] [CrossRef]
- Seneschal, K.; Smektala, F.; Bureau, B.; Le Floch, M.; Jiang, S.; Luo, T.; Lucas, J.; Peyghambarian, N. Properties and structure of high erbium doped phosphate glass for short optical fibers amplifiers. Mater. Res. Bull. 2005, 40, 1433–1442. [Google Scholar] [CrossRef]
- Campbell, J.H.; Hayden, J.S.; Marker, A. High-power solid-state lasers: A laser glass perspective. Int. J. Appl. Glass Sci. 2011, 2, 3–29. [Google Scholar] [CrossRef]
- Weber, M.J. Science and technology of laser glass. J. Non-Cryst. Solids 1990, 123, 208–222. [Google Scholar] [CrossRef]
- Boetti, N.G.; Scarpignato, G.C.; Lousteau, J.; Pugliese, D.; Bastard, L.; Broquin, J.-E.; Milanese, D. High concentration Yb-Er co-doped phosphate glass for optical fiber amplification. J. Opt. 2015, 17, 065705. [Google Scholar] [CrossRef]
- Jiang, S.; Luo, T.; Hwang, B.-C.; Smekatala, F.; Seneschal, K.; Lucas, J.; Peyghambarian, N. Er3+-doped phosphate glasses for fiber amplifiers with high gain per unit length. J. Non-Cryst. Solids 2000, 263–264, 364–368. [Google Scholar] [CrossRef]
- Knowles, J.C. Phosphate based glasses for biomedical applications. J. Mater. Chem. 2003, 13, 2395–2401. [Google Scholar] [CrossRef]
- Hofmann, P.; Voigtlander, C.; Nolte, S.; Peyghambarian, N.; Schulzgen, A. 550-mW output power from a narrow linewidth all-phosphate fiber laser. J. Lightw. Technol. 2013, 31, 756–760. [Google Scholar] [CrossRef]
- Hwang, B.-C.; Jiang, S.; Luo, T.; Seneschal, K.; Sorbello, G.; Morrell, M.; Smektala, F.; Honkanen, S.; Lucas, J.; Peyghambarian, N. Performance of high-concentration Er3+-doped phosphate fiber amplifiers. IEEE Photonics Technol. Lett. 2001, 13, 197–199. [Google Scholar] [CrossRef]
- Adam, J.-L.; Matecki, M.; L’Helgoualch, H.; Jacquier, B. Non-radiative emissions in Er3+-doped chloro-fluoride glasses. Eur. J. Solid State Inorg. Chem. 1994, 31, 337–349. [Google Scholar]
- Tikhomirov, V.K.; Naftaly, M.; Jha, A. Effects of the site symmetry and host polarizability on the hypersensitive transition 3P0 → 3F2 of Pr3+ in fluoride glasses. J. Appl. Phys. 1999, 86, 351–354. [Google Scholar] [CrossRef]
- Lopez-Iscoa, P.; Petit, L.; Massera, J.; Janner, D.; Boetti, N.G.; Pugliese, D.; Fiorilli, S.; Novara, C.; Giorgis, F.; Milanese, D. Effect of the addition of Al2O3, TiO2 and ZnO on the thermal, structural and luminescence properties of Er3+-doped phosphate glasses. J. Non-Cryst. Solids 2017, 460, 161–168. [Google Scholar] [CrossRef]
- Lopez-Iscoa, P.; Salminen, T.; Hakkarainen, T.; Petit, L.; Janner, D.; Boetti, N.G.; Lastusaari, M.; Pugliese, D.; Paturi, P.; Milanese, D. Effect of partial crystallization on the structural and luminescence properties of Er3+-doped phosphate glasses. Materials 2017, 10, 473. [Google Scholar] [CrossRef] [PubMed]
- Dantelle, G.; Mortier, M.; Vivien, D.; Patriarche, G. Influence of Ce3+ doping on the structure and luminescence of Er3+-doped transparent glass-ceramics. Opt. Mater. 2006, 28, 638–642. [Google Scholar] [CrossRef]
- Zhao, J.; Zheng, X.; Schartner, E.P.; Ionescu, P.; Zhang, R.; Nguyen, T.-L.; Jin, D.; Ebendorff-Heidepriem, H. Upconversion nanocrystal-doped glass: A new paradigm for photonic materials. Adv. Opt. Mater. 2016, 4, 1507–1517. [Google Scholar] [CrossRef]
- Boivin, D.; Föhn, T.; Burov, E.; Pastouret, A.; Gonnet, C.; Cavani, O.; Collet, C.; Lempereur, S. Quenching investigation on new erbium doped fibers using MCVD nanoparticle doping process. In Proceedings of the SPIE 7580, Fiber Lasers VII: Technology, Systems, and Application (SPIE LASE 2010), San Francisco, CA, USA, 23–28 January 2010. [Google Scholar]
- Ojha, N.; Tuomisto, M.; Lastusaari, M.; Petit, L. Upconversion from fluorophosphate glasses prepared with NaYF4:Er3+,Yb3+ nanocrystals. RSC Adv. 2018, 8, 19226–19236. [Google Scholar] [CrossRef]
- Nguyen, H.; Tuomisto, M.; Oksa, J.; Salminen, T.; Lastusaari, M.; Petit, L. Upconversion in low rare-earth concentrated phosphate glasses using direct NaYF4:Er3+, Yb3+ nanoparticles doping. Scr. Mater. 2017, 139, 130–133. [Google Scholar] [CrossRef]
- Johannsen, S.R.; Roesgaard, S.; Julsgaard, B.; Ferreira, R.A.S.; Chevallier, J.; Balling, P.; Ram, S.K.; Nylandsted Larsen, A. Influence of TiO2 host crystallinity on Er3+ light emission. Opt. Mater. Express 2016, 6, 1664–1678. [Google Scholar] [CrossRef]
- Bahtat, A.; Bouazaoui, M.; Bahtat, M.; Mugnier, J. Fluorescence of Er3+ ions in TiO2 planar waveguides prepared by a sol-gel process. Opt. Commun. 1994, 111, 55–60. [Google Scholar] [CrossRef]
- John, R.; Rajakumari, R. Synthesis and characterization of rare earth ion doped nano ZnO. Nano-Micro Lett. 2012, 4, 65–72. [Google Scholar] [CrossRef]
- Choi, S.R.; Bansal, N.P. Mechanical behavior of zirconia/alumina composites. Ceram. Int. 2005, 31, 39–46. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Hu, L.; Liu, W. Pressureless-sintering behavior of nanocrystalline ZrO2–Y2O3–Al2O3 system. Mater. Lett. 2006, 60, 2302–2305. [Google Scholar] [CrossRef]
- Maeda, N.; Wada, N.; Onoda, H.; Maegawa, A.; Kojima, K. Spectroscopic properties of Er3+ in sol–gel derived ZrO2 films. Thin Solid Films 2003, 445, 382–386. [Google Scholar] [CrossRef]
- Lopez-Iscoa, P.; Pugliese, D.; Boetti, N.G.; Janner, D.; Baldi, G.; Petit, L.; Milanese, D. Design, synthesis, and structure-property relationships of Er3+-doped TiO2 luminescent particles synthesized by sol-gel. Nanomaterials 2018, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Jayachandraiah, C.; Krishnaiah, G. Erbium induced raman studies and dielectric properties of Er-doped ZnO nanoparticles. Adv. Mater. Lett. 2015, 6, 743–748. [Google Scholar] [CrossRef]
- Freris, I.; Riello, P.; Enrichi, F.; Cristofori, D.; Benedetti, A. Synthesis and optical properties of sub-micron sized rare earth-doped zirconia particles. Opt. Mater. 2011, 33, 1745–1752. [Google Scholar] [CrossRef]
- Ojha, N.; Nguyen, H.; Laihinen, T.; Salminen, T.; Lastusaari, M.; Petit, L. Decomposition of persistent luminescent microparticles in corrosive phosphate glass melt. Corros. Sci. 2018, 135, 207–214. [Google Scholar] [CrossRef]
- Li, J.-G.; Wang, X.-H.; Kamiyama, H.; Ishigaki, T.; Sekiguchi, T. RF plasma processing of Er-doped TiO2 luminescent nanoparticles. Thin Solid Films 2006, 506–507, 292–296. [Google Scholar] [CrossRef]
- Patra, A.; Friend, C.S.; Kapoor, R.; Prasad, P.N. Upconversion in Er3+:ZrO2 nanocrystals. J. Phys. Chem. B 2002, 106, 1909–1912. [Google Scholar] [CrossRef]
- Maschio, S.; Bruckner, S.; Pezzotti, G. Synthesis and sintering of zirconia-erbia tetragonal solid solutions. J. Ceram. Soc. Jpn. 1999, 107, 1111–1114. [Google Scholar] [CrossRef]
- Ojha, N.; Laihinen, T.; Salminen, T.; Lastusaari, M.; Petit, L. Influence of the phosphate glass melt on the corrosion of functional particles occurring during the preparation of glass-ceramics. Ceram. Int. 2018, 44, 11807–11811. [Google Scholar] [CrossRef]

| Sample Code | Er3+:4I13/2 Lifetime ±0.2 ms | ||
|---|---|---|---|
| Particles Alone | Particles-Containing Glasses | ||
| NaPF0 | NaPF10 | ||
| Er2O3-doped TiO2 particles | 0.1 | 2.3 | 0.7 |
| Er2O3-doped ZnO particles | n.a. | 1.4 | 1.3 |
| Er2O3-doped ZrO2 particles | 1.0 | 1.6 | 1.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez-Iscoa, P.; Ojha, N.; Aryal, U.; Pugliese, D.; Boetti, N.G.; Milanese, D.; Petit, L. Spectroscopic Properties of Er3+-Doped Particles-Containing Phosphate Glasses Fabricated Using the Direct Doping Method. Materials 2019, 12, 129. https://doi.org/10.3390/ma12010129
Lopez-Iscoa P, Ojha N, Aryal U, Pugliese D, Boetti NG, Milanese D, Petit L. Spectroscopic Properties of Er3+-Doped Particles-Containing Phosphate Glasses Fabricated Using the Direct Doping Method. Materials. 2019; 12(1):129. https://doi.org/10.3390/ma12010129
Chicago/Turabian StyleLopez-Iscoa, Pablo, Nirajan Ojha, Ujjwal Aryal, Diego Pugliese, Nadia G. Boetti, Daniel Milanese, and Laeticia Petit. 2019. "Spectroscopic Properties of Er3+-Doped Particles-Containing Phosphate Glasses Fabricated Using the Direct Doping Method" Materials 12, no. 1: 129. https://doi.org/10.3390/ma12010129
APA StyleLopez-Iscoa, P., Ojha, N., Aryal, U., Pugliese, D., Boetti, N. G., Milanese, D., & Petit, L. (2019). Spectroscopic Properties of Er3+-Doped Particles-Containing Phosphate Glasses Fabricated Using the Direct Doping Method. Materials, 12(1), 129. https://doi.org/10.3390/ma12010129