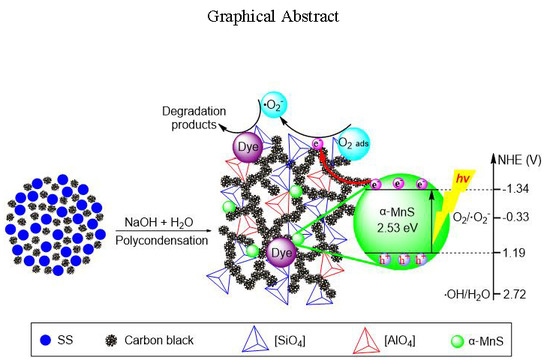
Green Transforming Metallurgical Residue into Alkali-Activated Silicomanganese Slag-Based Cementitious Material as Photocatalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
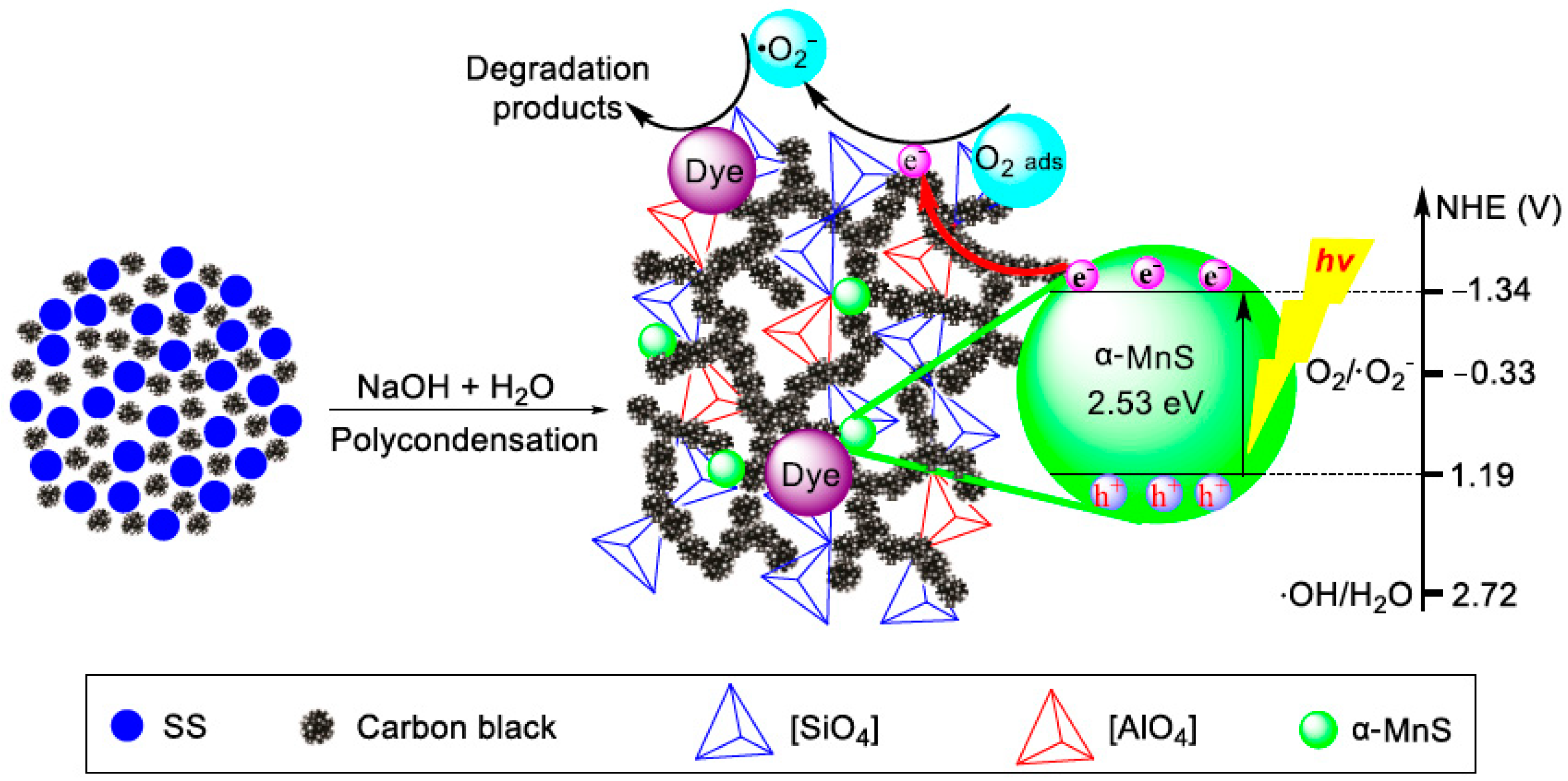
2.2. Preparation of EASSC
2.3. Characterization of EASSC
2.4. Evaluation of Photocatalytic Activity
3. Results and Discussions
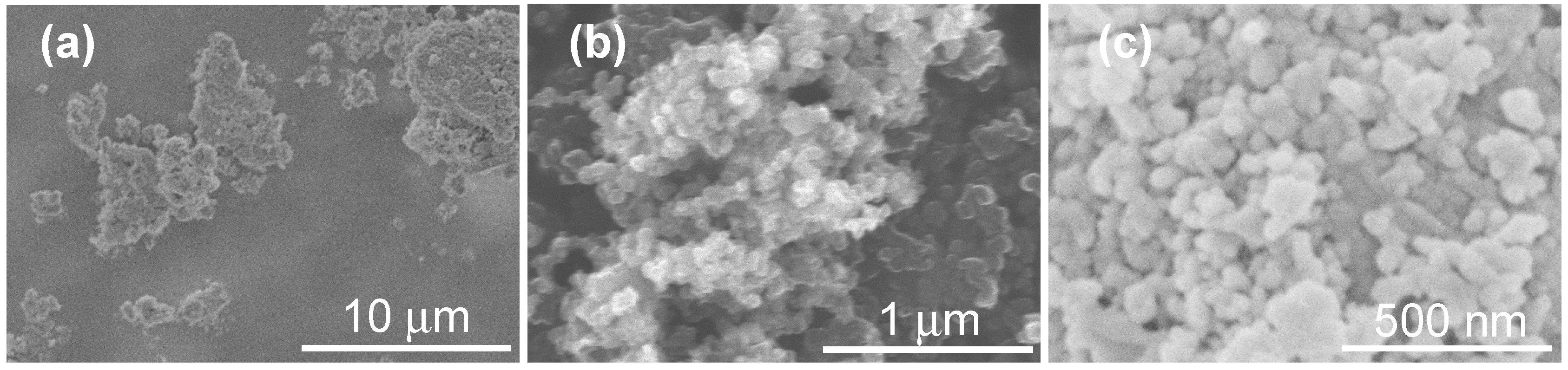
3.1. Interrelationship of Electrical Conductivity, Mechanical Strength and Microstructure
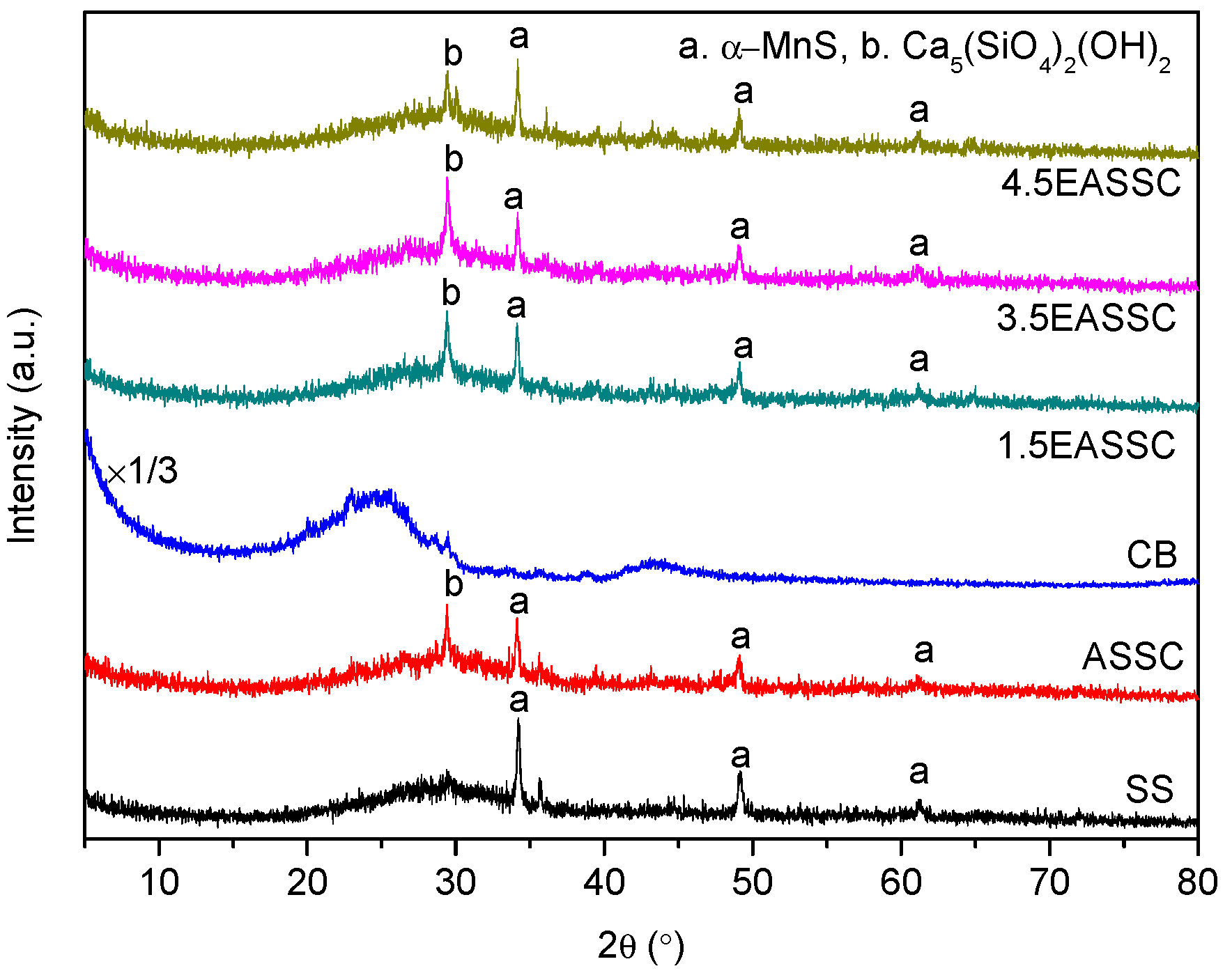
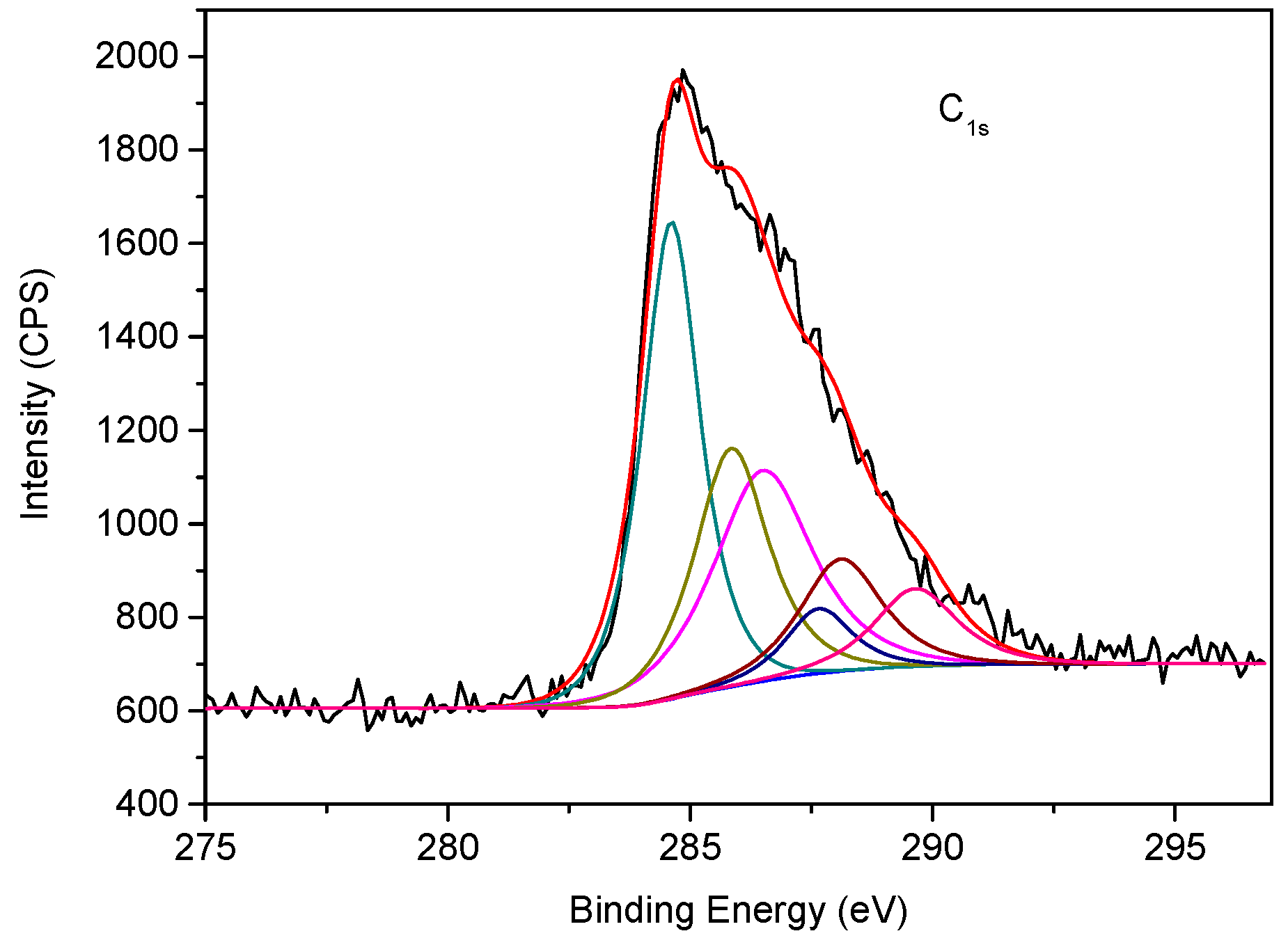
3.2. Spectral Features of EASSC
3.3. Applied EASSC for Removal of Dye Pollutant
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ferro-Alloys.com. Available online: http://www.ferro-alloys.cn/ (accessed on 15 March 2018).
- Zhang, X.; Ni, W.; Wu, J.; Zhu, L. Hydration mechanism of a cementitious material prepared with Si–Mn slag. Int. J. Miner. Metall. Mater. 2011, 18, 234–239. [Google Scholar] [CrossRef]
- Frias, M.; Sanchez de Rojas, M.I.; Rodriguez, C. The influence of SiMn slag on chemical resistance of blended cement pastes. Constr. Build. Mater. 2009, 23, 1472–1475. [Google Scholar] [CrossRef]
- Patil, A.V.; Pande, A.M. Behaviour of silico manganese slag manufactured aggregate as material for road and rail track construction. Adv. Mater. Res. 2011, 255–260, 3258–3262. [Google Scholar] [CrossRef]
- Kumar, S.; Garcia-Trinanes, P.; Teixeira-Pinto, A.; Bao, M. Development of alkali activated cement from mechanically activated silico-manganese (SiMn) slag. Cem. Concr. Compos. 2013, 40, 7–13. [Google Scholar] [CrossRef]
- Michel, B.; Henning, T.; Jager, C.; Kreibig, U. Optical extinction by spherical carbonaceous particles. Carbon 1999, 37, 391–400. [Google Scholar] [CrossRef]
- Llamas-Jansa, I.; Jager, C.; Mutschke, H.; Henning, T. Far-ultraviolet to near-infrared optical properties of carbon nanoparticles produced by pulsed-laser pyrolysis of hydrocarbons and their relation with structural variations. Carbon 2007, 45, 1542–1557. [Google Scholar] [CrossRef]
- Chhowalla, M.; Wang, H.; Sano, N.; Teo, K.B.K.; Lee, S.B.; Amaratunga, G.A.J. Carbon onions: Carriers of the 217.5 nm interstellar absorption feature. Phys. Rev. Lett. 2003, 90, 155504–155507. [Google Scholar] [CrossRef] [PubMed]
- Schnaiter, M.; Mutschke, H.; Dorschner, J.; Henning, T.; Salama, F. Matrix-isolated nano-sized carbon grains as an analog for the 217.5 nanometer feature carrier. Astrophys. J. 1998, 498, 486–496. [Google Scholar] [CrossRef]
- Jager, C.; Henning, T.; Schlogl, R.; Spillecke, O. Spectral properties of carbon black. J. Non-Cryst. Solids 1999, 258, 161–179. [Google Scholar] [CrossRef]
- Chung, D.D.L. Review: Graphite. J. Mater. Sci. 2002, 37, 1475–1489. [Google Scholar] [CrossRef]
- Wagner, P.; O’Rourke, J.A.; Armstrong, P.E. Porosity effects in polycrystalline graphite. J. Am. Ceram. Soc. 1972, 55, 214–219. [Google Scholar] [CrossRef]
- Sun, T.; Dong, L.; Wang, C.; Guo, W.; Wang, L.; Liang, T. Effect of porosity on the electrical resistivity of carbon materials. New Carbon Mater. 2013, 28, 349–354. [Google Scholar] [CrossRef]
- Song, S.; Jennings, H.M. Pore solution chemistry of alkali-activated ground granulated blast-furnace slag. Cem. Concr. Res. 1999, 29, 159–170. [Google Scholar] [CrossRef]
- Wang, Q.; Su, F.; Tang, Z.; Ling, G.; Yang, Q. Synergetic effect of conductive additives on the performance of high power lithium ion batteries. New Carbon Mater. 2012, 27, 427–432. [Google Scholar] [CrossRef]
- Darmstadt, H.; Roy, C.; Kaliaguine, S.; Xu, G.; Auger, M.; Tuel, A.; Ramaswamy, V. Solid state 13C-NMR spectroscopy and XRD studies of commercial and pyrolytic carbon blacks. Carbon 2000, 38, 1279–1287. [Google Scholar] [CrossRef]
- Park, S.J.; Cho, K.S.; Ryu, S.K. Filler–elastomer interactions: Influence of oxygen plasma treatment on surface and mechanical properties of carbon black/rubber composites. Carbon 2003, 41, 1437–1442. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, F.Q.; Huang, Z.J.; Chen, H.; Zhuang, Z.F.; Pan, Z.Z.; Long, J.F.; Gu, F.L. Photochemical fabrication of SnO2 dense layers on reduced graphene oxide sheets for application in photocatalytic degradation of p-nitrophenol. Appl. Catal. B Environ. 2017, 215, 8–17. [Google Scholar] [CrossRef]
- Trzcinski, K.; Szkoda, M.; Sawczak, M.; Karczewski, J.; Lisowska-Oleksiak, A. Visible light activity of pulsed layer deposited BiVO4/MnO2 filmsdecorated with gold nanoparticles: The evidence for hydroxyl radicals formation. Appl. Surf. Sci. 2016, 385, 199–208. [Google Scholar] [CrossRef]
- Dan, M.; Zhang, Q.; Yu, S.; Prakash, A.; Lin, Y.; Zhou, Y. Noble-metal-free MnS/In2S3 composite as highly efficient visible light driven photocatalyst for H2 production from H2S. Appl. Catal. B Environ. 2017, 217, 530–539. [Google Scholar] [CrossRef]
- Hong, Y.; Jiang, Y.; Li, C.; Fan, W.; Yan, X.; Yan, M.; Shi, W. In-situ synthesis of direct solid-state Z-scheme V2O5/g-C3N4 heterojunctions with enhanced visible light efficiency in photocatalytic degradation of pollutants. Appl. Catal. B Environ. 2016, 180, 663–673. [Google Scholar] [CrossRef]
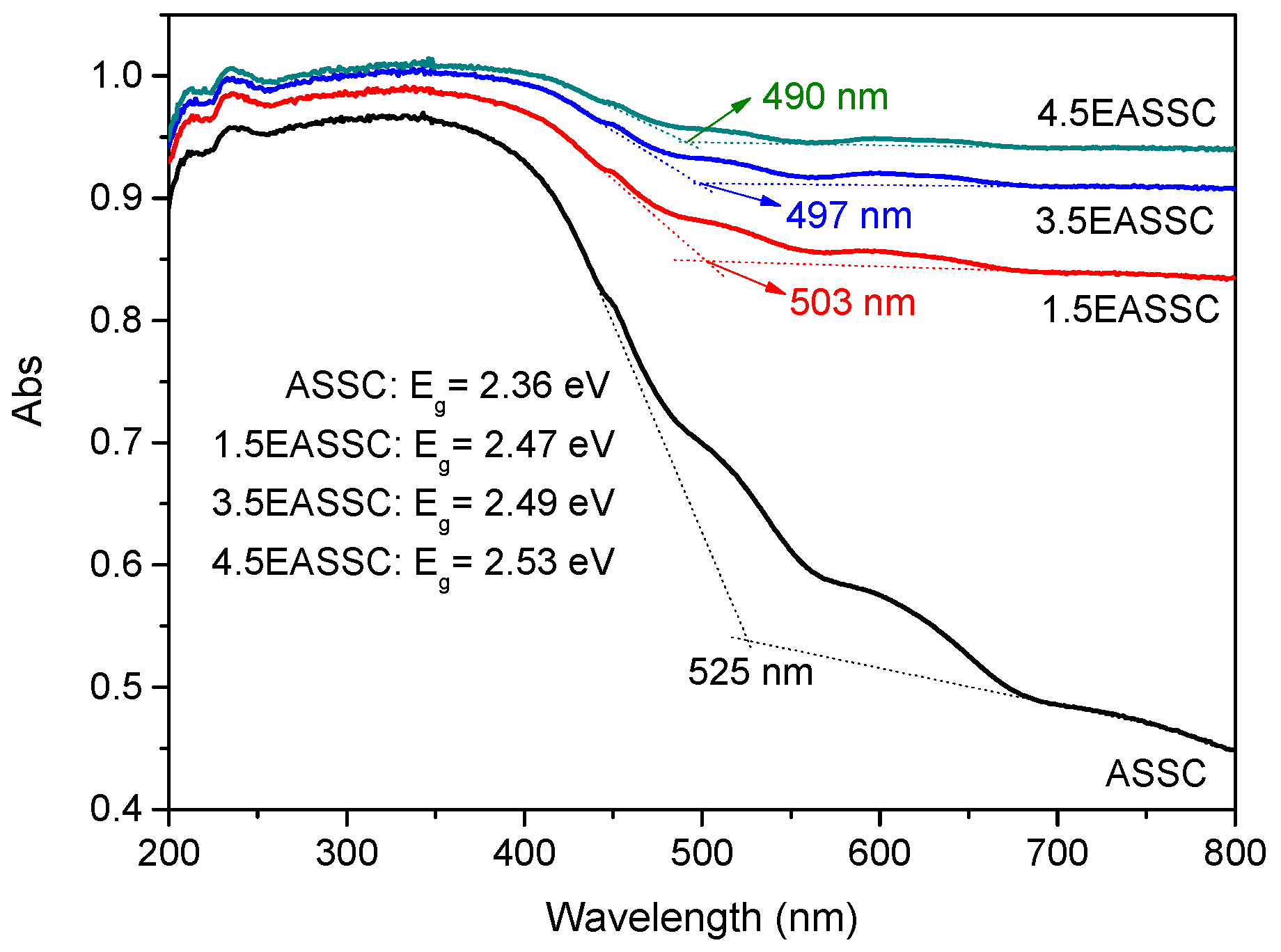
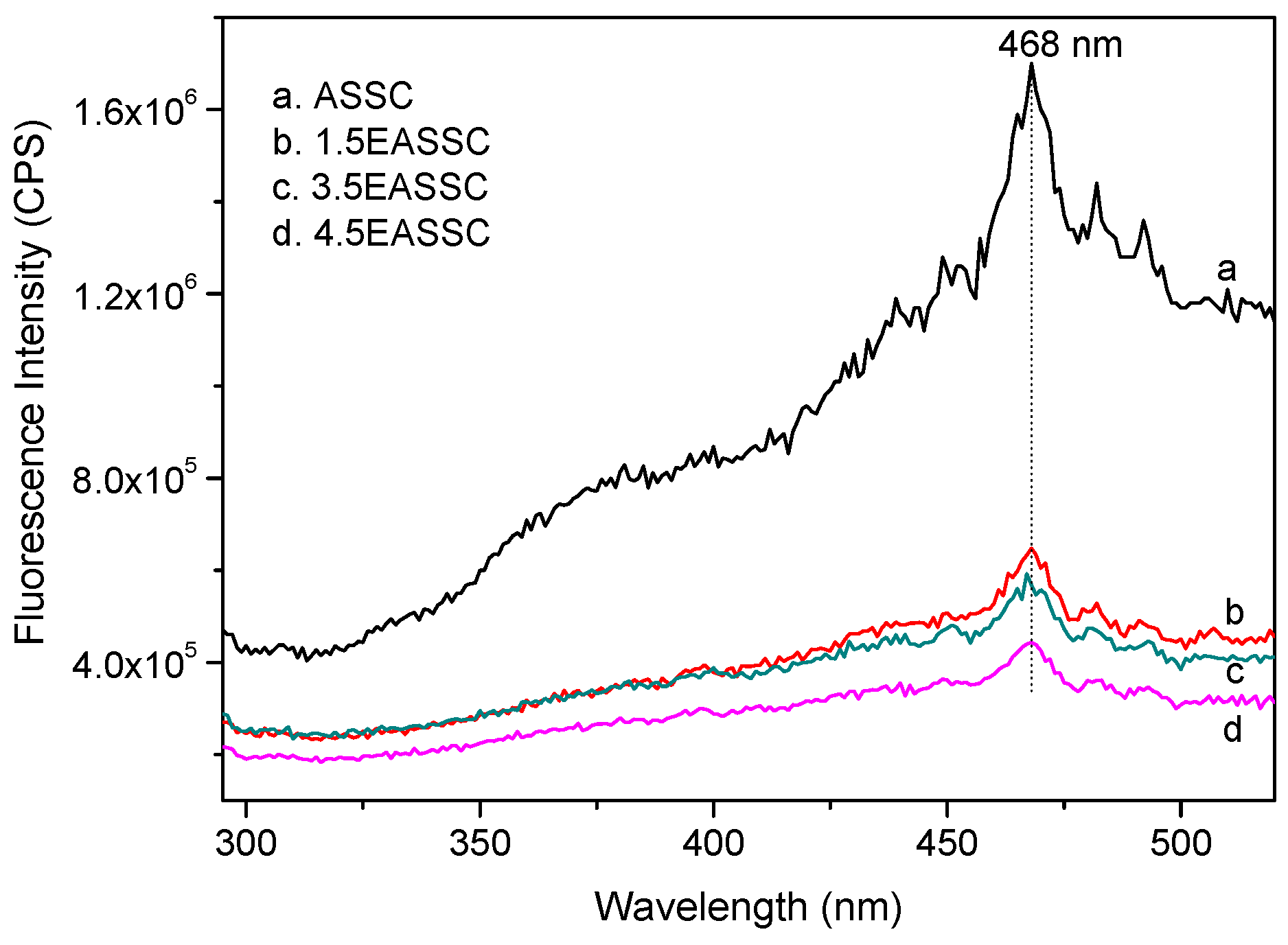
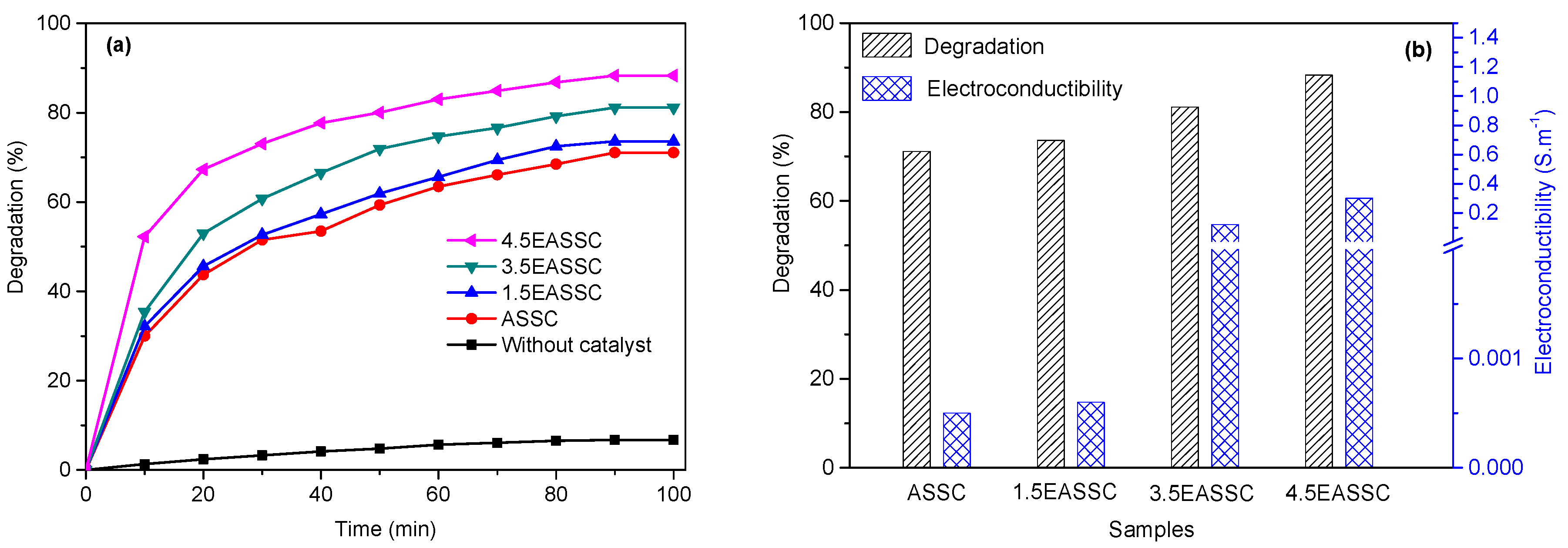
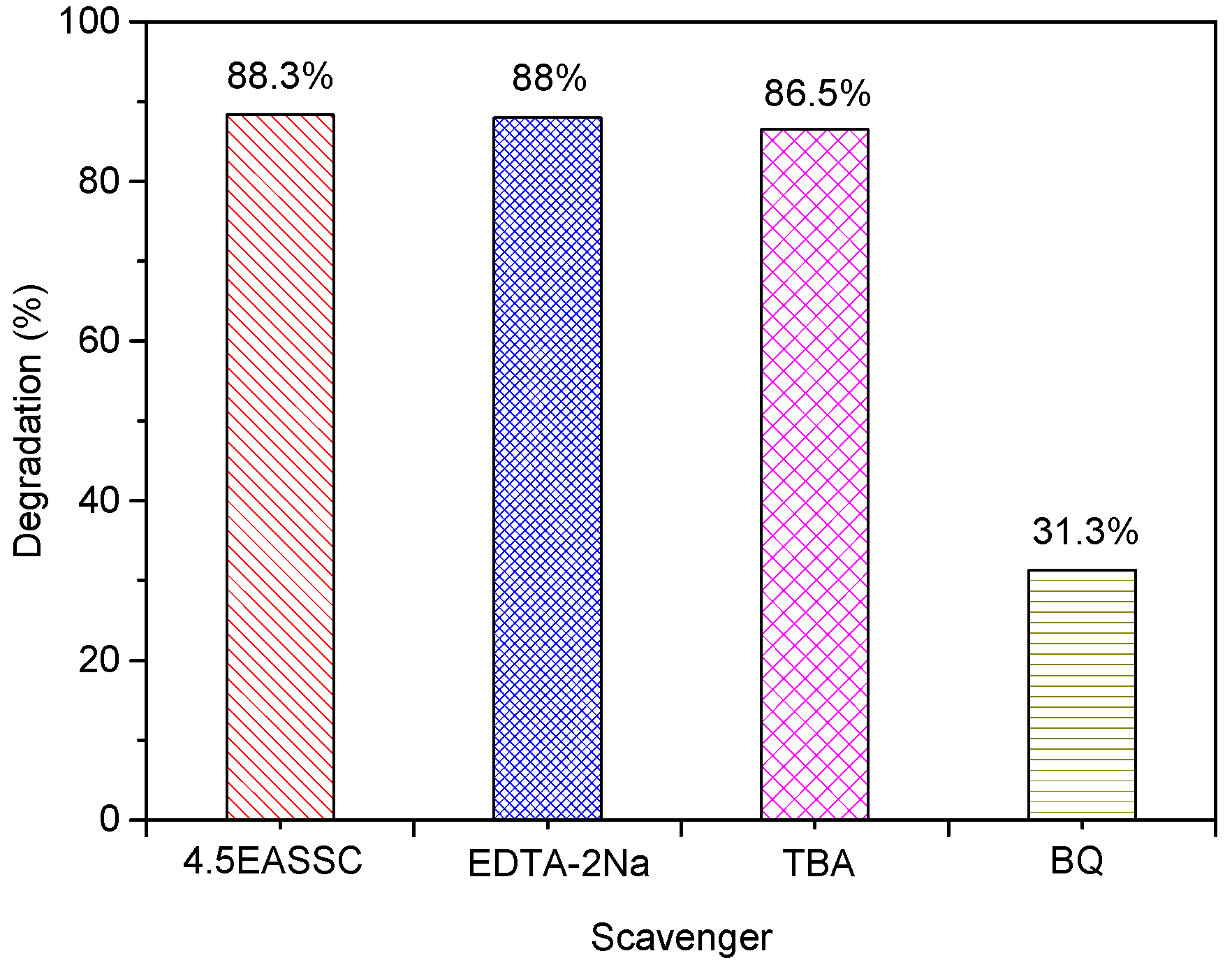
| Sample | Na2O | CaO | SiO2 | Al2O3 | MgO | K2O | SO3 | MnO | Fe2O3 | TiO2 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SS | 0.41 | 21.86 | 28.34 | 18.45 | 4.72 | 1.04 | 2.23 | 11.58 | 0.72 | 0.27 | 10.38 |
| 4.5EASSC | 5.72 | 18.29 | 26.89 | 17.24 | 4.67 | 1.05 | 2.15 | 11.64 | 0.75 | 0.25 | 11.35 |
| Samples | Carbon Black (wt %) | Eectrical Conductivity at Different Curing Ages (S·m−1) | Compressive Strength of 28 Days (MPa) | |||
|---|---|---|---|---|---|---|
| 3 Days | 7 Days | 14 Days | 28 Days | |||
| ASSC | 0 | 0.0008 | 0.0006 | 0.0005 | 0.0005 | 45.6 |
| 1.5EASSC | 1.5 | 0.0011 | 0.0010 | 0.0007 | 0.0006 | 33.0 |
| 3.5EASSC | 3.5 | 0.1249 | 0.1209 | 0.1169 | 0.1155 | 17.6 |
| 4.5EASSC | 4.5 | 0.3109 | 0.3008 | 0.2976 | 0.2976 | 13.0 |
| Samples | Zero Order Reaction Kinetics | First Order Kinetic Equations | Second Order Reaction Kinetics Equations | Third Order Kinetics Equations | ||
|---|---|---|---|---|---|---|
| R02 | R12 | Reaction Kinetics Equation | R22 | t1/2 (min) | R32 | |
| Without catalyst | 0.93534 | 0.93938 | 1/Ct = 0.000184t + 0.25221 | 0.9434 | 1357.316 | 0.94692 |
| ASSC | 0.76186 | 0.9066 | 1/Ct = 0.00619t + 0.29147 | 0.98656 | 40.78303 | 0.98596 |
| 1.5EASSC | 0.75671 | 0.91077 | 1/Ct = 0.00714t + 0.29672 | 0.98216 | 35.01401 | 0.97857 |
| 3.5EASSC | 0.70929 | 0.90203 | 1/Ct = 0.01112t + 0.29384 | 0.98965 | 22.48201 | 0.97690 |
| 4.5EASSC | 0.57034 | 0.86509 | 1/Ct = 0.01922t + 0.32543 | 0.99147 | 13.00728 | 0.95239 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.J.; He, P.Y.; Chen, H.; Liu, L.C. Green Transforming Metallurgical Residue into Alkali-Activated Silicomanganese Slag-Based Cementitious Material as Photocatalyst. Materials 2018, 11, 1773. https://doi.org/10.3390/ma11091773
Zhang YJ, He PY, Chen H, Liu LC. Green Transforming Metallurgical Residue into Alkali-Activated Silicomanganese Slag-Based Cementitious Material as Photocatalyst. Materials. 2018; 11(9):1773. https://doi.org/10.3390/ma11091773
Chicago/Turabian StyleZhang, Yao Jun, Pan Yang He, Hao Chen, and Li Cai Liu. 2018. "Green Transforming Metallurgical Residue into Alkali-Activated Silicomanganese Slag-Based Cementitious Material as Photocatalyst" Materials 11, no. 9: 1773. https://doi.org/10.3390/ma11091773
APA StyleZhang, Y. J., He, P. Y., Chen, H., & Liu, L. C. (2018). Green Transforming Metallurgical Residue into Alkali-Activated Silicomanganese Slag-Based Cementitious Material as Photocatalyst. Materials, 11(9), 1773. https://doi.org/10.3390/ma11091773