Enrichment and Purification of Aucubin from Eucommia ulmoides Ionic Liquid Extract Using Macroporous Resins
Abstract
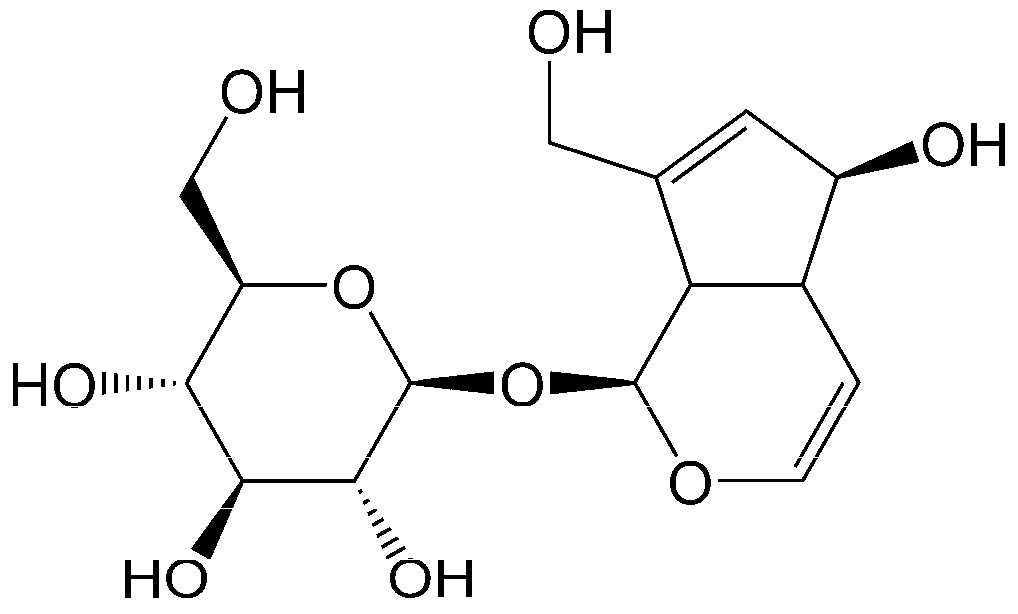
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Pretreatment of Macroporous Resin
2.3. Preparation of Crude Ionic Liquid Extracts
2.4. HPLC Analysis and Quantification
2.5. Static Adsorption and Desorption Tests
2.5.1. Adsorption Capacity and Desorption Ratio
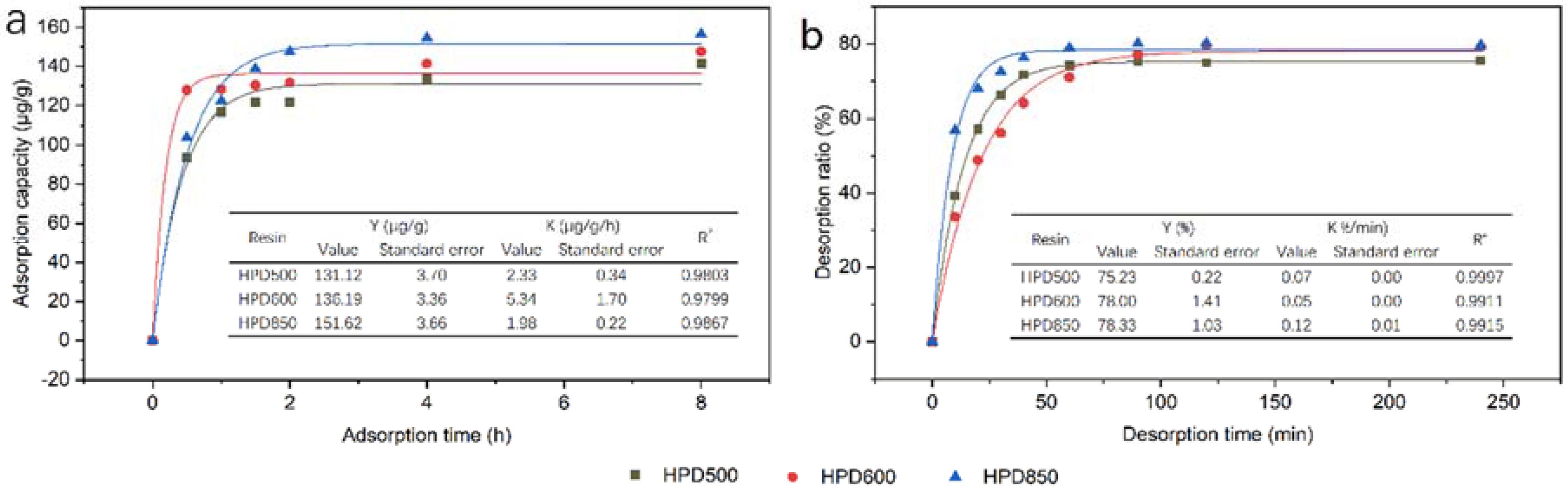
2.5.2. Adsorption and Desorption Kinetics
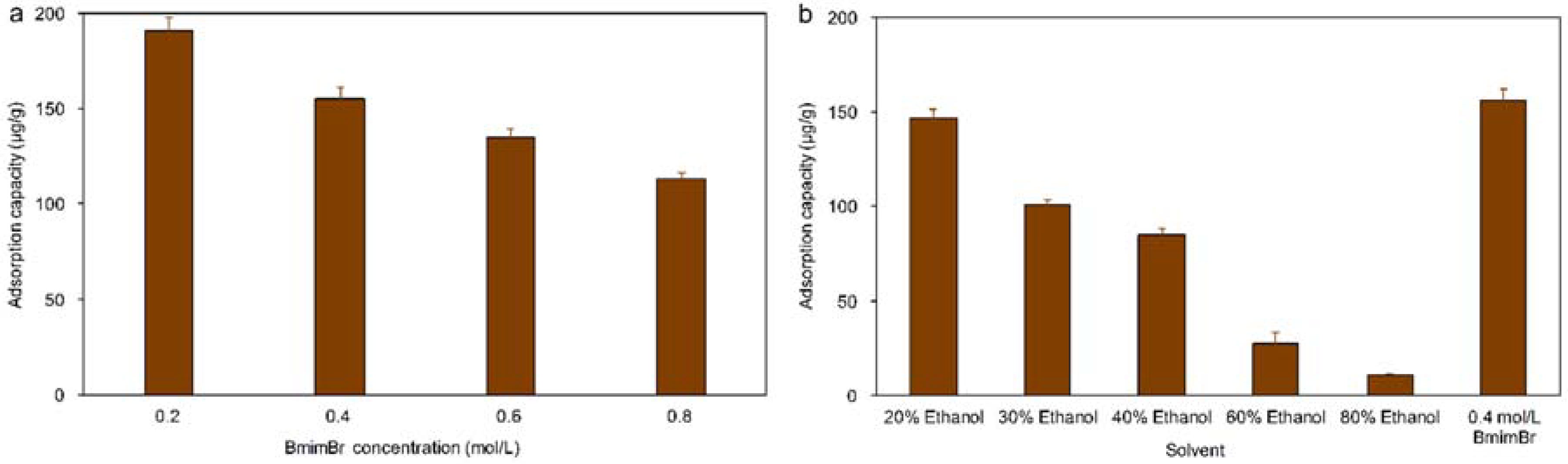
2.5.3. Effect of Extraction Solvent
2.5.4. Adsorption Isotherms
2.6. Dynamic Adsorption and Desorption Tests
2.7. Scale-Up Gradient Elution Tests
3. Results
3.1. Static Adsorption and Desorption Tests
3.1.1. Adsorption Capacity and Desorption Ratio
3.1.2. Adsorption and Desorption Kinetics Curves
3.2. Influence of the Extraction Solvent
3.3. Adsorption Isotherms
3.4. Dynamic Adsorption and Desorption Test
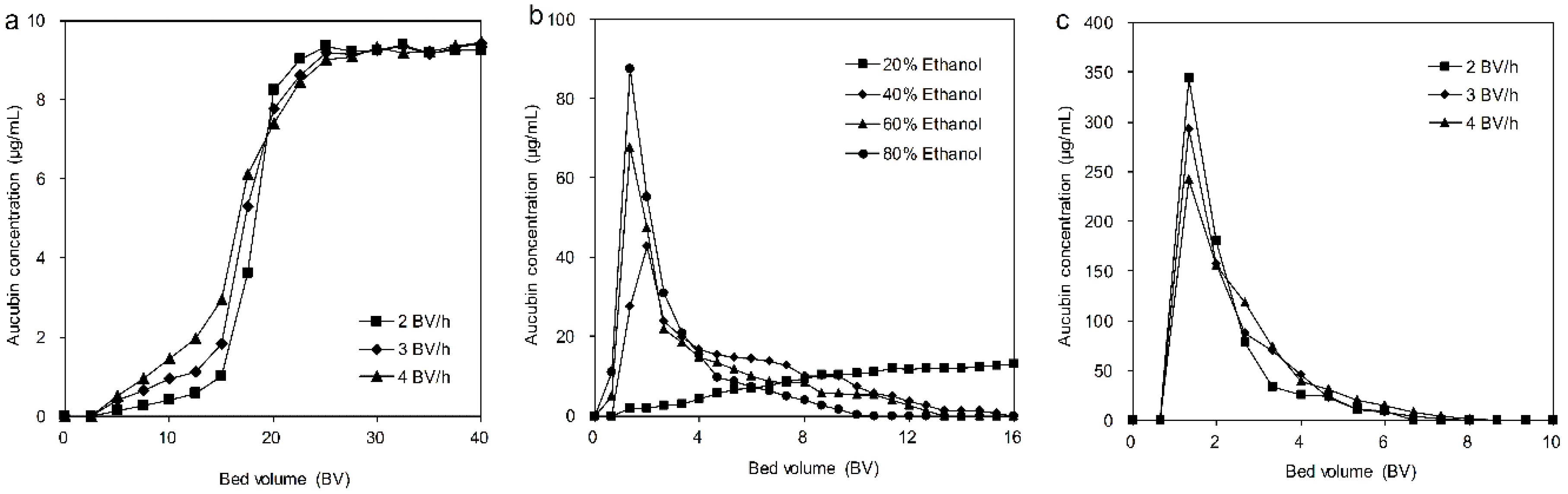
3.4.1. Dynamic Leakage Curves on the HPD850 Resin
3.4.2. Dynamic Water Washing Process for the Ionic Liquid
3.4.3. Dynamic Desorption on the HPD850 Resin
3.5. Scale-Up Gradient Elution Tests for Verification
3.6. Separation Process for Aucubin
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Du, X.L.; Yuan, Q.P.; Li, Y. Equilibrium, thermodynamics and breakthrough studies for adsorption of solanesol onto macroporous resins. Chem. Eng. Process. 2008, 47, 1420–1427. [Google Scholar] [CrossRef]
- Kim, J.; Kim, D.; Gwon, Y.J.; Lee, K.-W.; Lee, T.S. Removal of sodium dodecylbenzenesulfonate by macroporous adsorbent resins. Materials 2018, 11, 1324. [Google Scholar] [CrossRef] [PubMed]
- Pi, G.; Ren, P.; Yu, J.; Shi, R.; Yuan, Z.; Wang, C. Separation of sanguinarine and chelerythrine in Macleaya cordata (Willd) R. Br. based on methyl acrylate-co-divinylbenzene macroporous adsorbents. J. Chromatogr. A 2008, 1192, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Ma, C.; Sui, X.; Yang, L.; Zu, Y. Preparation of shikonin by hydrolyzing ester derivatives using basic anion ion exchange resin as solid catalyst. Ind. Crop. Prod. 2012, 36, 47–53. [Google Scholar] [CrossRef]
- Han, F.; Guo, Y.; Gu, H.; Li, F.; Hu, B.; Yang, L. Application of alkyl polyglycoside surfactant in ultrasonic-assisted extraction followed by macroporous resin enrichment for the separation of vitexin-2″-O-rhamnoside and vitexin from Crataegus pinnatifida leaves. J. Chromatogr. B 2016, 1012–1013, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.-J.; Ma, C.-H.; Yang, L.; Zhao, C.-J.; Zhang, Y.; Zu, Y.-G. Enrichment and purification of deoxyschizandrin and γ-schizandrin from the extract of Schisandra chinensis fruit by macroporous resins. Molecules 2012, 17, 3510–3523. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yang, L.; Wang, W.; Liu, Y.; Zhao, C.; Zu, Y. Enrichment and purification of syringin, eleutheroside E and isofraxidin from Acanthopanax senticosus by macroporous resin. Int. J. Mol. Sci. 2012, 13, 8970–8986. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Liu, Z.; Wei, M.; Yang, L. Turpentine as an alternative solvent for the extraction of gutta-percha from Eucommia ulmoides barks. Ind. Crop. Prod. 2018, 121, 142–150. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Huang, W.-H.; Zeng, X.-C.; Li, X.-H.; Li, J.; Zhou, J.; Xiao, J.; Xiao, B.; Ouyang, D.-S.; et al. The protective effect of aucubin from Eucommia ulmoides against status epilepticus by inducing autophagy and inhibiting necroptosis. Am. J. Chin. Med. 2017, 45, 557–573. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S. Aucubin, a naturally occurring iridoid glycoside inhibits TNF-α-induced inflammatory responses through suppression of NF-κB activation in 3T3-L1 adipocytes. Cytokine 2013, 62, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.-Q.; Xiao, Y.; Duan, M.-X.; Yuan, Y.; Jiang, X.-H.; Yang, Z.; Liao, H.-H.; Deng, W.; Tang, Q.-Z. Aucubin protects against pressure overload-induced cardiac remodelling via the β3-adrenoceptor–neuronal NOS cascades. Br. J. Pharmacol. 2018, 175, 1548–1566. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Han, M.; Kwon, Y.K. Effect of aucubin on neural precursor cell survival during neuronal differentiation. Int. J. Neurosci. 2018. [CrossRef] [PubMed]
- Lee, G.-H.; Lee, M.-R.; Lee, H.-Y.; Kim, S.H.; Kim, H.-K.; Kim, H.-R.; Chae, H.-J. Eucommia ulmoides cortex, geniposide and aucubin regulate lipotoxicity through the inhibition of lysosomal BAX. PLoS ONE 2014, 9, e88017. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, Y.; Zhang, L.; Li, C.; Coffie, J.W.; Geng, X.; Qiu, L.; You, X.; Fang, Z.; Song, M.; et al. Aucubin promotes angiogenesis via estrogen receptor beta in a mouse model of hindlimb ischemia. J. Steroid Biochem. 2017, 172, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Kartini; Islamie, R.; Handojo, C.S. Wound healing activity of aucubin on hyperglycemic rat. J. Young Pharm. 2018, 10, s136–s139. [Google Scholar] [CrossRef]
- Li, Y.; Hu, W.; Han, G.; Lu, W.; Jia, D.; Hu, M.; Wang, D. Involvement of bone morphogenetic protein-related pathways in the effect of aucubin on the promotion of osteoblast differentiation in MG63 cells. Chem-Biol. Interact. 2018, 283, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Peng, M.; Bu, X.; Wu, G.; Hu, W. Extraction and separation of aucubin from the leave of Eucommia ulmoides in aqueous two-phase system. Nat. Prod. Res. Dev. 2010, 22, 264–267. [Google Scholar] [CrossRef]
- Li, H.; Wang, L.; Peng, Y.; Zhang, Y. Purification of aucubin from the crude extract of Eucommia ulmoides seeds by silica gel chromatography. Food Sci. 2010, 31, 58–61. [Google Scholar] [CrossRef]
- Liu, W.; Wu, X.; Huang, H.; Lai, X.; Huang, G.; Xu, J.; Cao, L.; Zhou, Z. Separation of aucubin by reversed-phase preparative high performance liquid chromatography. J. Jiangxi Coll. Tradit. Chin. Med. 2003, 15, 43–44. [Google Scholar]
- Li, H.; Wen, C.; Li, Y.; Peng, Y.; Wang, L.; Zhang, Y. Separation and purification of aucubin from Eucommia ulmoides seed meal. Food Sci. 2011, 32, 32–35. [Google Scholar]
- Ma, L.; Zhou, X.; Xu, H. Study on separation and purification process of aucubin from Eucommia seeds by macroporous resins. Sci. Technol. Food Ind. 2012, 33, 249–252. [Google Scholar] [CrossRef]
- Chen, X.; He, Q.; Cao, H.; Liu, N.; Cao, Z. Extraction and purification of aucubin from Eucommia ulmoide Oliver. J. Cent. South Univ. (Sci. Technol.) 2005, 36, 60–64. [Google Scholar]
- Li, H.; Hu, J.; Ouyang, H.; Li, Y.; Shi, H.; Ma, C.; Zhang, Y. Extraction of aucubin from seeds of Eucommia ulmoides Oliv. using supercritical carbon dioxide. J. AOAC Int. 2009, 92, 103–110. [Google Scholar] [PubMed]
- Silva, E.M.; Pompeu, D.R.; Larondelle, Y.; Rogez, H. Optimisation of the adsorption of polyphenols from Inga edulis leaves on macroporous resins using an experimental design methodology. Sep. Purif. Tech. 2007, 53, 274–280. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, X.; Zhang, Q.; Du, X.; Yang, L.; Zu, Y.; Yang, F. Simultaneous synergistic microwave-ultrasonic extraction and hydrolysis for preparation of trans-resveratrol in tree peony seed oil-extracted residues using imidazolium-based ionic liquid. Ind. Crop. Prod. 2016, 94, 266–280. [Google Scholar] [CrossRef]
- Liu, S.; Yi, L.; Liang, Y. Traditional Chinese medicine and separation science. J. Sep. Sci. 2008, 31, 2113–2137. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhou, R.; Sui, J.; Liu, Y. Simultaneous preparation of salidroside and p-tyrosol from Rhodiola crenulata by DIAION HP-20 macroporous resin chromatography combined with silica gel chromatography. Molecules 2018, 23, 1602. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Mo, K.; Liu, Z.; Yang, F.; Hou, K.; Li, S.; Zu, Y.; Yang, L. Ionic liquid-based vacuum microwave-assisted extraction followed by macroporous resin enrichment for the separation of the three glycosides salicin, hyperin and rutin from populus bark. Molecules 2014, 19, 9689–9711. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Chen, F.; Zhang, Q.; Zang, J. Application of ionic liquids in vacuum microwave-assisted extraction followed by macroporous resin isolation of three flavonoids rutin, hyperoside and hesperidin from Sorbus tianschanica leaves. J. Chromatogr. B 2016, 1014, 45–55. [Google Scholar] [CrossRef] [PubMed]


| Resin | Surface Area (m2/g) | Average Pore Diameter (nm) | Particle Diameter (mm) | Polarity | Moisture Content (%) | Adsorption Capacity (μg/g) | Desorption Ratio (%) |
|---|---|---|---|---|---|---|---|
| HPDD | 650–750 | 9.0–11.0 | 0.30–1.25 | Non-polar | 44.45 | 144.89 ± 4.24 | 51.06 ± 1.52 |
| HPD100A | 650–700 | 9.5–10.0 | 0.30–1.20 | Non-polar | 45.65 | 68.64 ± 1.63 | 66.05 ± 2.14 |
| HPD700 | 650–700 | 8.5–9.0 | 0.30–1.20 | Non-polar | 52.31 | 123.56 ± 4.72 | 49.94 ± 1.34 |
| DM130 | 500–550 | 9.0–10.0 | 0.30–1.25 | Middle-polar | 46.56 | 117.40 ± 2.37 | 68.30 ± 2.42 |
| HPD750 | 650–700 | 8.5–9.0 | 0.30–1.20 | Middle-polar | 51.28 | 122.14 ± 4.05 | 67.22 ± 2.79 |
| HPD850 | 1100–1300 | 8.5–9.5 | 0.30–1.20 | Middle-polar | 47.82 | 154.96 ± 4.38 | 79.09 ± 2.33 |
| HPD400 | 500–550 | 7.5–8.0 | 0.30–1.20 | Polar | 48.91 | 125.71 ± 4.22 | 67.88 ± 2.28 |
| HPD500 | 500–550 | 5.5–7.5 | 0.30–1.20 | Polar | 50.26 | 147.00 ± 4.53 | 75.08 ± 4.01 |
| HPD600 | 550–600 | 8.0 | 0.30–1.20 | Polar | 49.55 | 149.70 ± 4.08 | 59.81 ± 1.41 |
| Temperature (°C) | Langmuir Equation | Freundlich Equation | ||||
|---|---|---|---|---|---|---|
| Qmax | KL | R2 | KF | 1/n | R2 | |
| 5 | 0.4229 | 0.3187 | 0.9993 | 0.2857 | 0.2998 | 0.9199 |
| 25 | 0.3424 | 0.5991 | 0.9958 | 0.1822 | 0.3687 | 0.9749 |
| 50 | 0.2779 | 1.3265 | 0.9933 | 0.1070 | 0.4674 | 0.9143 |
| Mode | Ethanol Volume Fraction (%) | Mass of Dried Residue (mg) | Mass of Aucubin (mg) | Aucubin Purity (%) | Aucubin Recovery (%) |
|---|---|---|---|---|---|
| Isocratic mode | 60 | 1077.42 | 793.63 | 73.66 | 82.47 |
| Gradient mode | 10 | 46.91 | 0.00 | 0.00 | 0.00 |
| 20 | 24.44 | 11.5 | 47.05 | 1.20 | |
| 30 | 36.44 | 23.01 | 63.14 | 2.39 | |
| 40 | 112.44 | 92.03 | 81.85 | 9.56 | |
| 50 | 188.56 | 161.05 | 85.41 | 16.74 | |
| 60 | 240.56 | 195.56 | 81.29 | 20.32 | |
| 70 | 188.02 | 149.55 | 79.54 | 15.54 | |
| 80 | 81.67 | 46.01 | 56.34 | 4.78 | |
| 90 | 156.27 | 23.01 | 14.72 | 2.39 | |
| Collection of 40–80 | 811.25 | 644.2 | 79.41 | 72.92 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Wei, M.; Tian, H.; Liu, T.; Yang, L. Enrichment and Purification of Aucubin from Eucommia ulmoides Ionic Liquid Extract Using Macroporous Resins. Materials 2018, 11, 1758. https://doi.org/10.3390/ma11091758
Yang X, Wei M, Tian H, Liu T, Yang L. Enrichment and Purification of Aucubin from Eucommia ulmoides Ionic Liquid Extract Using Macroporous Resins. Materials. 2018; 11(9):1758. https://doi.org/10.3390/ma11091758
Chicago/Turabian StyleYang, Xinyu, Mengxia Wei, Hao Tian, Tingting Liu, and Lei Yang. 2018. "Enrichment and Purification of Aucubin from Eucommia ulmoides Ionic Liquid Extract Using Macroporous Resins" Materials 11, no. 9: 1758. https://doi.org/10.3390/ma11091758
APA StyleYang, X., Wei, M., Tian, H., Liu, T., & Yang, L. (2018). Enrichment and Purification of Aucubin from Eucommia ulmoides Ionic Liquid Extract Using Macroporous Resins. Materials, 11(9), 1758. https://doi.org/10.3390/ma11091758




