Role of Magnesium Perrhenate in an Oil/Solid Mixed System for Tribological Application at Various Temperatures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
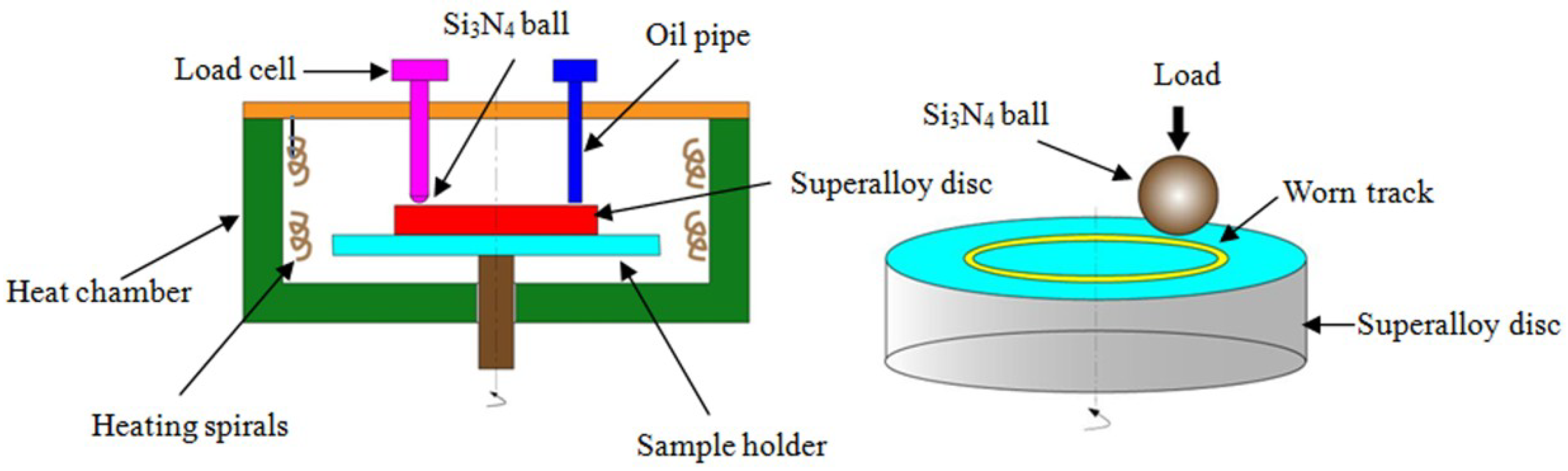
2.2. Four-Ball Test
2.3. Tribological Tests over a Wide Temperature Range
3. Results and Discussion
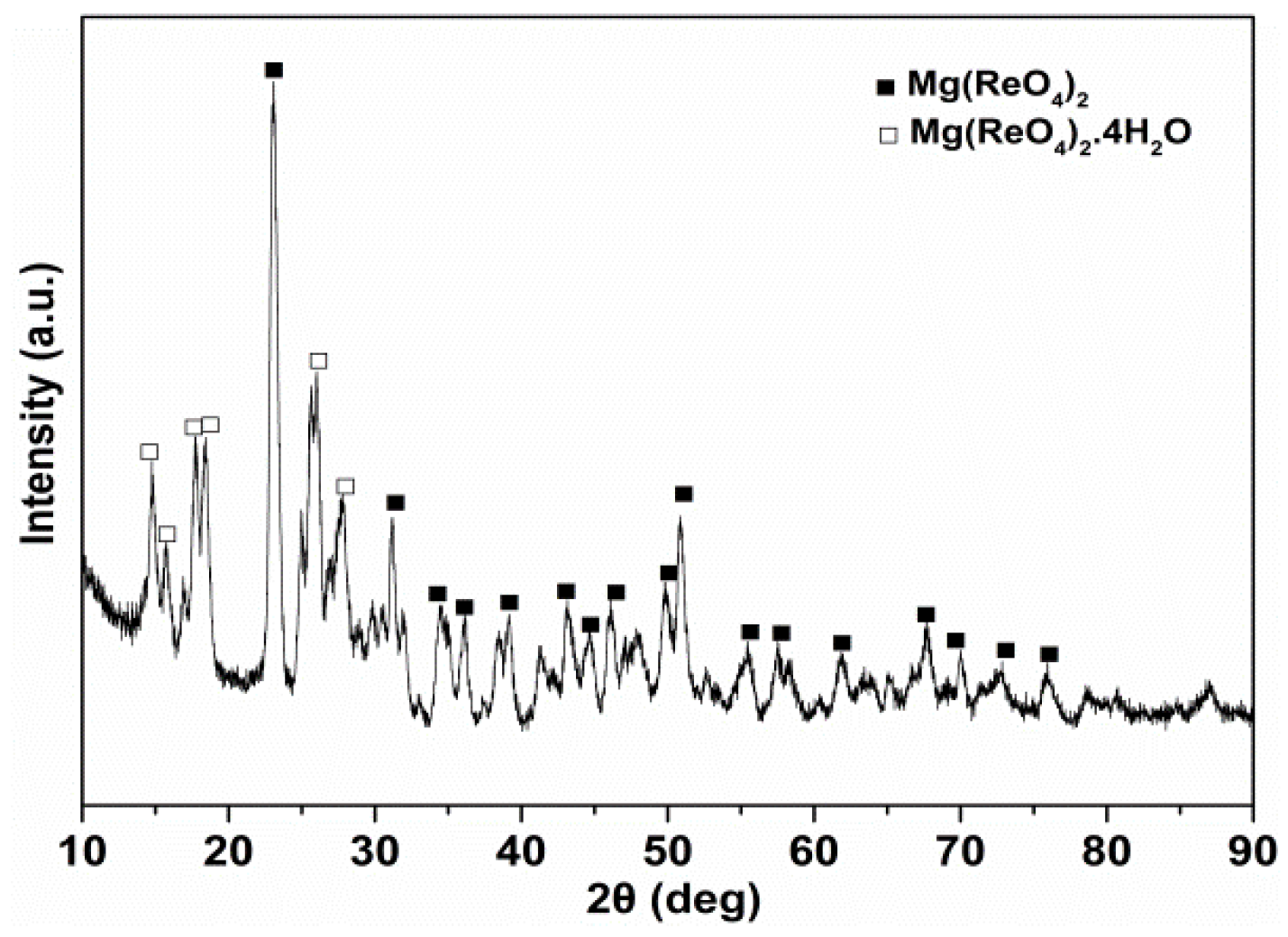
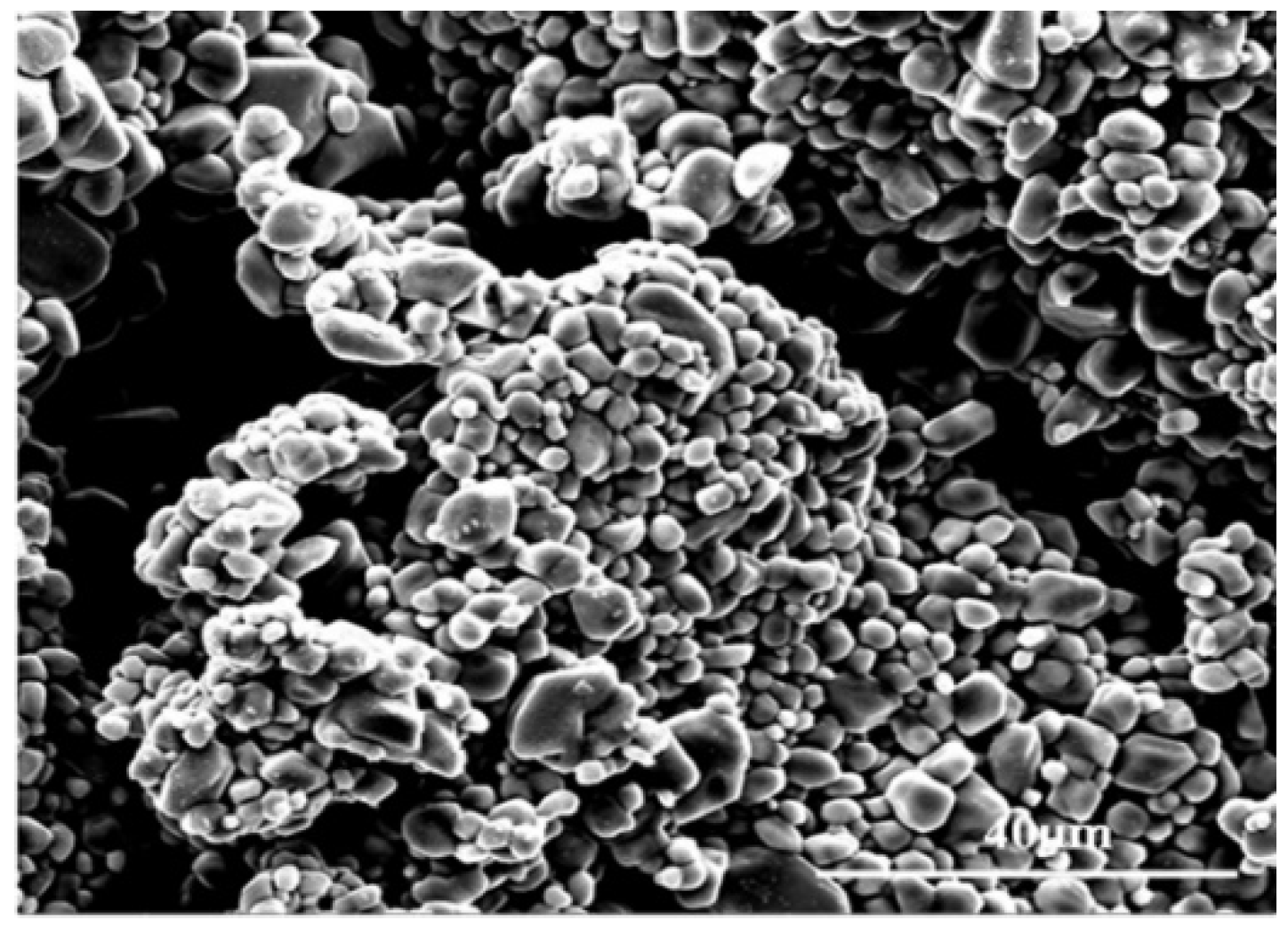
3.1. Characterization of Mg(ReO4)2
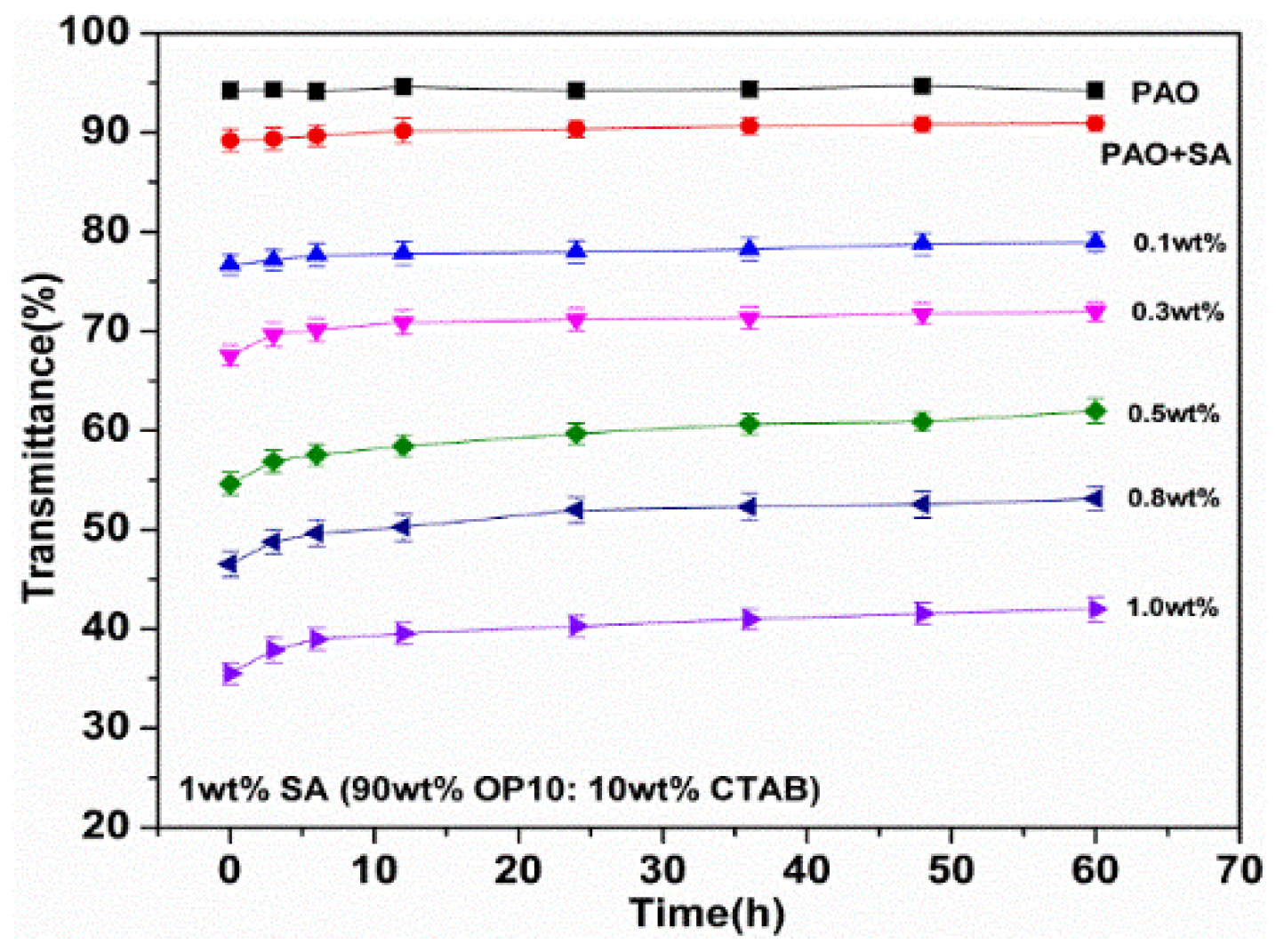
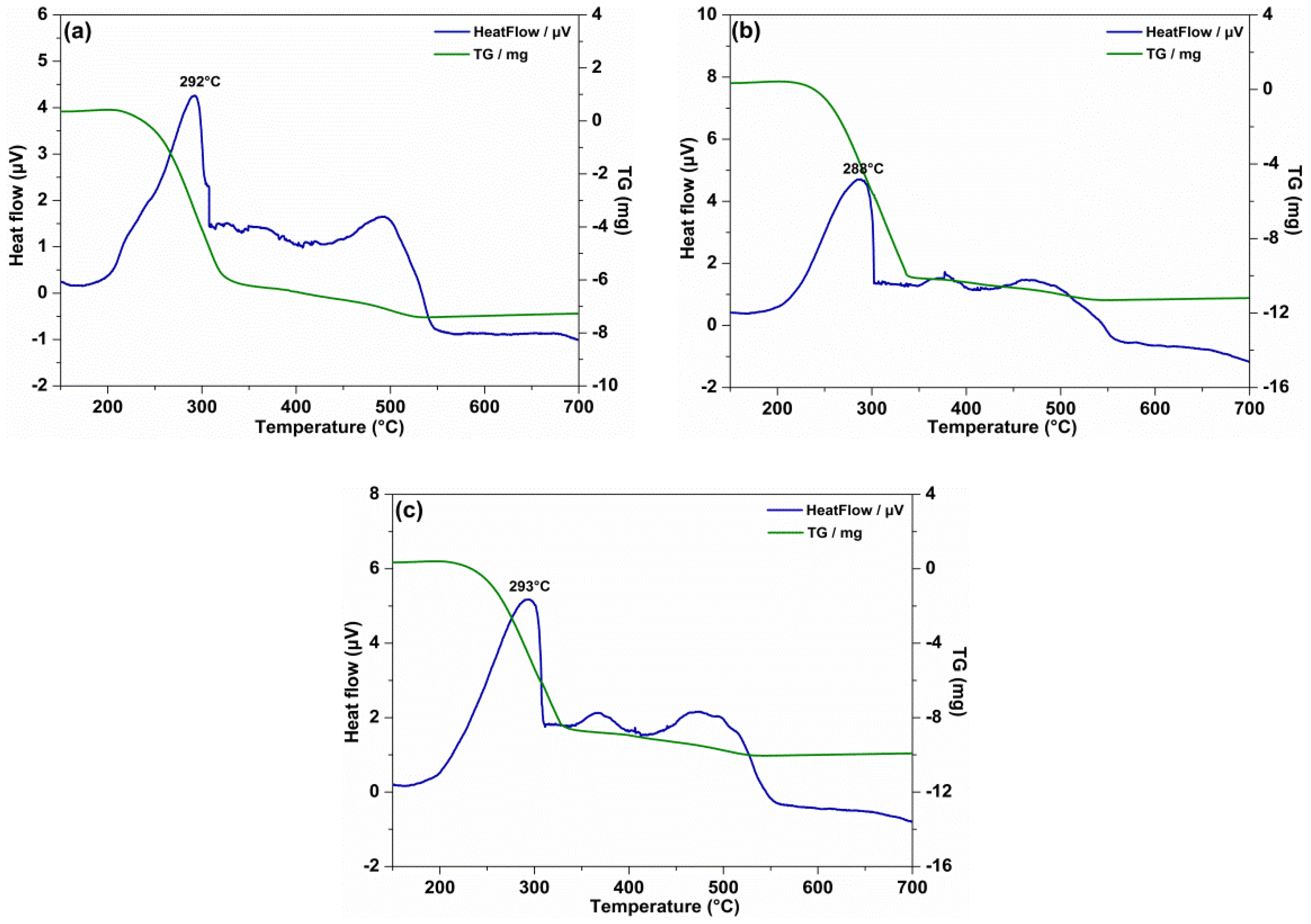
3.2. Dispersion Stability and Thermal Stability
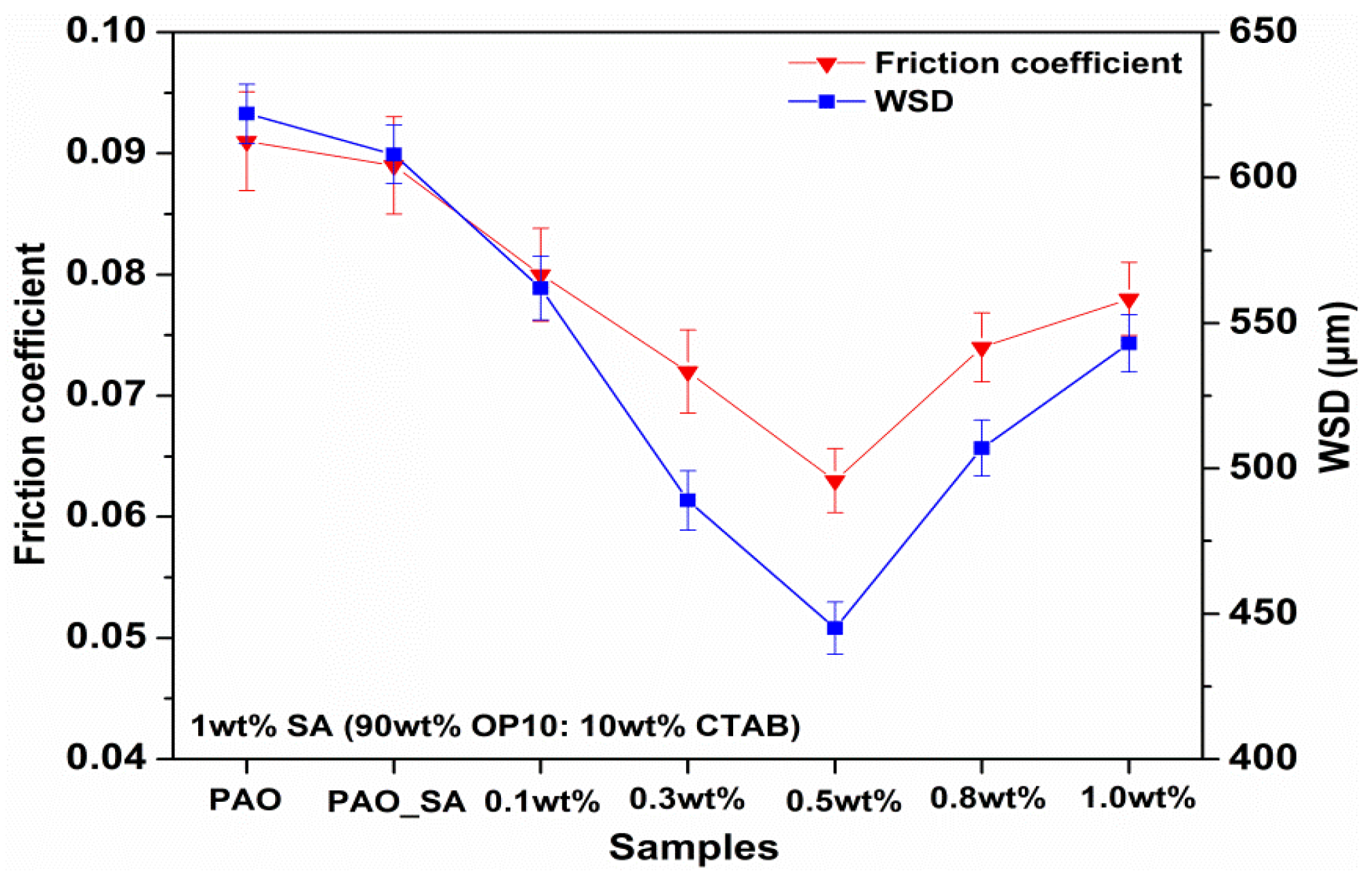
3.3. Four-Ball Test Analysis
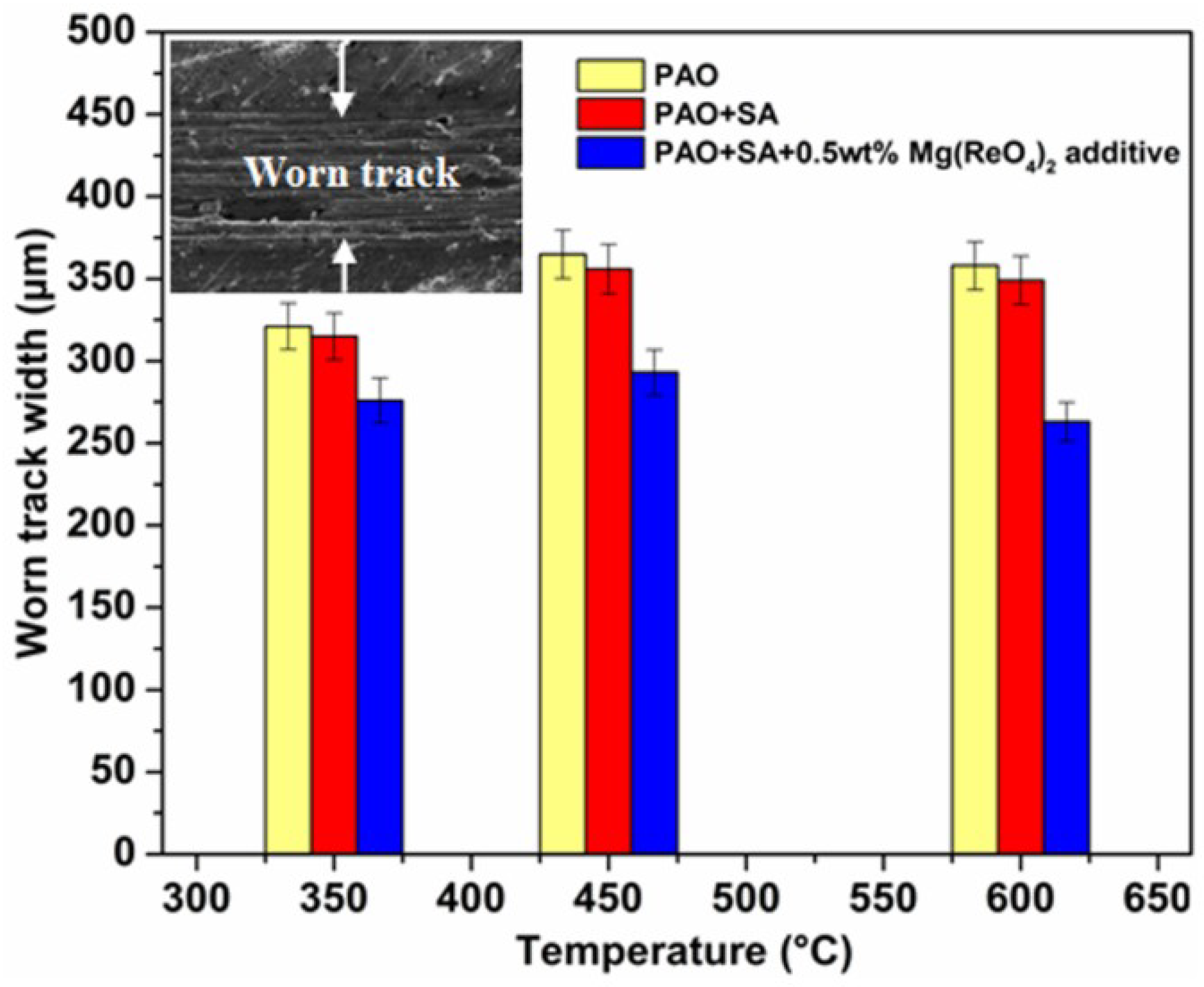
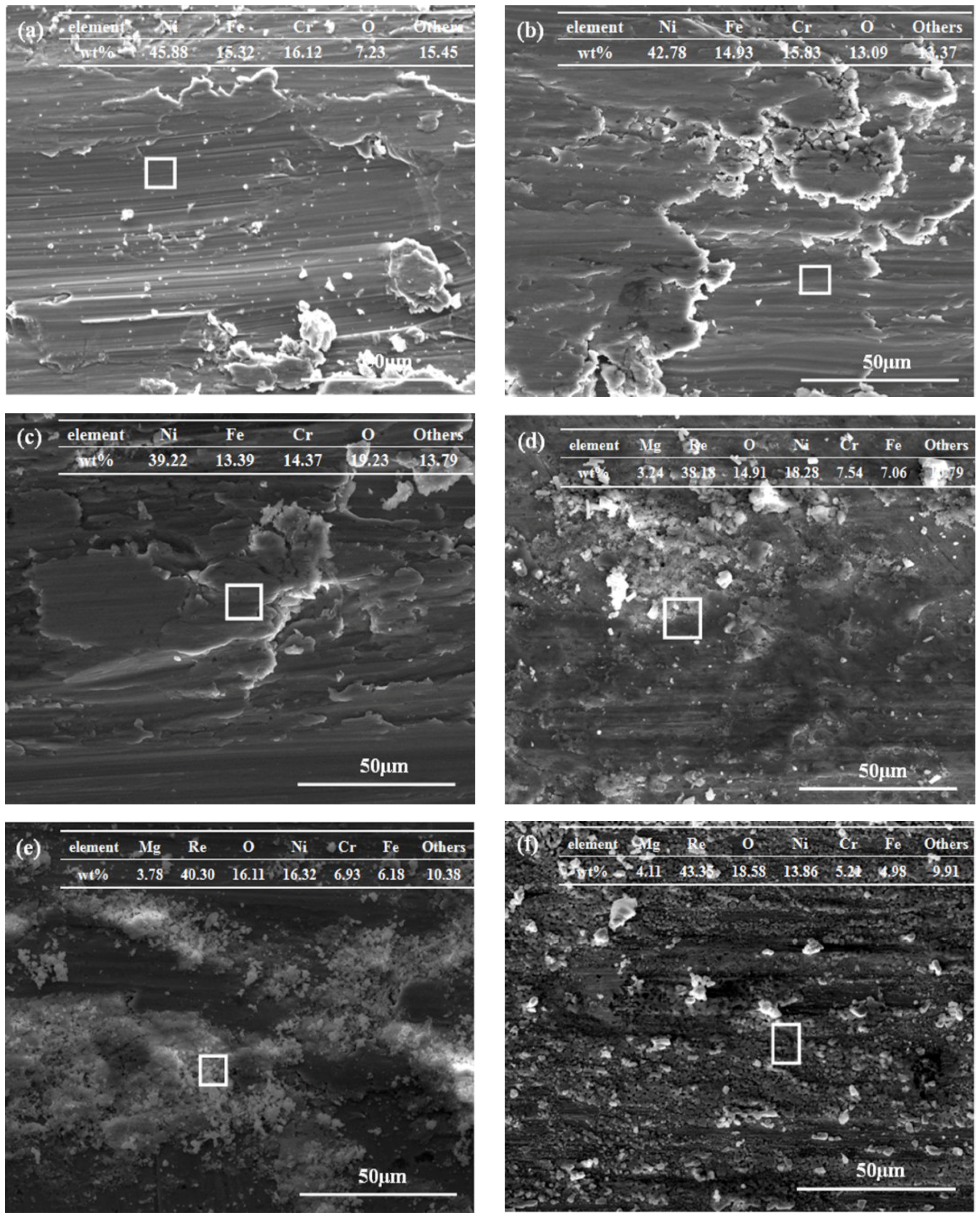
3.4. Tribological Performances at Various Temperatures
4. Conclusions
- (1)
- The Mg(ReO4)2 compound used as lubricating additive significantly improved the lubricating base oil in the condition of the four-ball test. The coefficient of friction and WSD were reduced by 30.8% and 28.5%, respectively, when the optimum concentration of 0.5 wt% Mg(ReO4)2 additive was dispersed.
- (2)
- The Mg(ReO4)2 compound could promote the antifriction property of the base oil at a relatively low temperature and possessed a superb lubrication function at high temperature conditions. The added Mg(ReO4)2 was deposited on the worn surface and generated a protective layer that improved the contact state of the rubbing interface. This layer formed at the elevated temperature range was mainly made up of Mg(ReO4)2 compound and some natural oxides from the metal matrix, while the appearance of the complex layer could effectively prevent direct contact between rubbing pairs. The Mg(ReO4)2 additive had a leading role in friction-reduction as a result of its higher thermal stability and maintaining a shear-susceptible structure with increasing temperature.
- (3)
- The established original characterizations and tribological testing results suggested a tremendous potential for blending proper magnesium perrhenate additive and PAO base oil to obtain a continuous and effective lubrication function over a broad temperature range.
Author Contributions
Funding
Conflicts of Interest
References
- Donnet, C.; Erdenmir, A. Historical developments and new trends in tribological and solid lubricant coatings. Surf. Coat. Technol. 2004, 180, 76–84. [Google Scholar] [CrossRef]
- Spikes, H. Tribology research in the twenty-first century. Tribol. Int. 2001, 34, 789–799. [Google Scholar] [CrossRef]
- Qin, Y.Q.; Xiong, D.S.; Li, J.L.; Jin, Q.T.; He, Y.; Zhang, R.C.; Zou, Y.R. Adaptive-lubricating PEO/Ag/MoS2 multilayered coatings for Ti6Al4V alloy at elevated temperature. Mater. Des. 2016, 107, 311–321. [Google Scholar] [CrossRef]
- Xu, Z.S.; Shi, X.L.; Wang, M.; Zhai, W.Z.; Yao, J.; Song, S.Y.; Zhang, Q.X. Effect of Ag and Ti3SiC2 on tribological properties of TiAl Matrix self-lubricating composites at room and increased temperatures. Tribol. Lett. 2014, 53, 617–629. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, Y.; Zhang, X.Y.; Cao, W.F.; Jia, J.H. Tribological properties of NiCr-ZrO2(Y2O3)-SrSO4 composites at elevated temperatures. Ceram. Int. 2016, 42, 12981–12987. [Google Scholar] [CrossRef]
- Tatarko, P.; Kasiarova, M.; Chlup, Z.; Dusza, J.; Sajgalik, P.; Vávra, I. Influence of rare-earth oxide additives and SiC nanoparticles on the wear behavior of Si3N4-based composites at temperatures up to 900 °C. Wear 2013, 300, 155–162. [Google Scholar] [CrossRef]
- Zhang, J.X.; Hu, L.T.; Chen, J.M.; Liu, W.M. Lubrication behaviour of Y-TZP/Al2O3/Mo nanocomposites at high temperature. Wear 2010, 268, 1091–1094. [Google Scholar] [CrossRef]
- Zhen, J.M.; Zhu, S.Y.; Cheng, J.; Qiao, Z.H.; Liu, W.M.; Yang, J. Effects of sliding speed and testing temperature on the tribological behaviour of a nickel-alloy based solid-lubricating composite. Wear 2016, 368–369, 45–52. [Google Scholar] [CrossRef]
- Sliney, H.E. Solid lubricant materials for high temperature. Tribol. Int. 1982, 15, 305–313. [Google Scholar] [CrossRef]
- Allam, L.M. Solid lubricants for applications at elevated temperatures. J. Mater. Sci. 1991, 26, 3977–3984. [Google Scholar] [CrossRef]
- Stone, D.; Liu, J.; Singh, D.P.; Muratore, C.; Voevodin, A.A.; Mishra, S.; Rebholz, C.; Ge, Q.; Aouadi, S.M. Layered atomic structures of double oxides for low shear strength at high temperatures. Scr. Mater. 2010, 62, 735–738. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Demydov, D.; Jahan, M.P.; Mistry, K.; Erdermir, A. Fundamental understanding of the tribological and thermal behavior of Ag-MoS2 nanoparticle-based multi-component lubricating system. Wear 2012, 288, 9–16. [Google Scholar] [CrossRef]
- Stone, D.S.; Migas, J.; Martini, A.; Smith, T.; Muratore, C.; Voevodin, A.A.; Aouadi, S.M. Adaptive NbN/Ag coatings for high temperature tribological applications. Surf. Coat. Technol. 2012, 206, 4316–4321. [Google Scholar] [CrossRef]
- Zhu, S.Y.; Bi, Q.L.; Kong, L.Q.; Yang, J.; Liu, W.M. Barium chromate as a solid lubricant for nickel aluminum. Tribol. Trans. 2012, 55, 218–223. [Google Scholar] [CrossRef]
- Niu, M.Y.; Bi, Q.L.; Zhu, S.Y.; Yang, J.; Liu, W.M. Microstructure, phase transition and tribological performances of Ni3Si-based self-lubricating composite coatings. J. Alloys Compd. 2013, 555, 367–374. [Google Scholar] [CrossRef]
- Yu, L.H.; Zhao, H.J.; Xu, J.H. Influence of silver content on structure, mechanical and tribological properties of WCN-Ag films. Mater. Charact. 2016, 114, 136–145. [Google Scholar] [CrossRef]
- Tilllmann, W.; Kokalj, D.; Stangier, D.; Paulus, M.; Sternemann, C.; Tolan, M. Investigation on the oxidation behavior of AlCrVxN thin films by means of synchrotron radiation and influence on the high temperature friction. Appl. Surf. Sci. 2018, 427, 511–521. [Google Scholar] [CrossRef]
- Liu, E.Y.; Wang, W.Z.; Gao, Y.M.; Jia, J.H. Tribological properties of adaptive Ni-based composites with addition of lubricious Ag2MoO4 at elevated temperatures. Tribol. Lett. 2012, 47, 21–30. [Google Scholar] [CrossRef]
- Bondarev, A.V.; Kiryukhantsev-Korneev, P.V.; Sidorenko, D.A.; Shtansky, D.V. A new insight into hard low friction MoCN-Ag coatings intended for applications in wide temperature range. Mater. Des. 2016, 93, 63–72. [Google Scholar] [CrossRef]
- Liu, C.C.; Chen, L.; Zhou, J.S.; Zhou, H.D.; Chen, J.M. Tribological properties of adaptive phosphate composite coatings with addition of silver and molybdenum disulfide. Appl. Surf. Sci. 2014, 300, 111–116. [Google Scholar] [CrossRef]
- Fang, Y.; Fan, H.Z.; Song, J.J.; Zhang, Y.S.; Hu, L.T. Surface engineering design of Al2O3/Mo self-lubricating structural ceramics—Part II: Continuous lubrication effects of a three-dimensional lubricating layer at temperature from 25 to 800 °C. Wear 2016, 360–361, 97–103. [Google Scholar] [CrossRef]
- Qi, X.W.; Jia, Z.N.; Yang, Y.L.; Fan, B.L. Characterization and auto- restoration mechanism of nanoscale serpentine powder as lubricating oil additive under high temperature. Tribol. Int. 2011, 7–8, 805–810. [Google Scholar] [CrossRef]
- Qi, X.W.; Lu, L.; Jia, Z.N.; Yang, Y.L.; Liu, H.R. Comparative tribological properties of magnesium hexasilicate and serpentine powder as lubricating oil additives under high temperature. Tribol. Int. 2012, 49, 53–57. [Google Scholar] [CrossRef]
- Wang, X.B.; Bai, Z.M.; Zhao, D.; Zhao, F.Y. Friction behavior of Mg-Al-CO3 layered double hydroxide prepared by magnesite. Appl. Surf. Sci. 2013, 277, 134–138. [Google Scholar] [CrossRef]
- Standard Test Method for Wear Preventive Characteristics of Lubricating Fluid (Four-Ball Method); ASTM D4172-94(2004); ASTM International: West Conshohocken, PA, USA, 2004.
- The Powder Diffraction File Search Website. Available online: http://www.icdd.com/index.php/pdf-2/ (accessed on 4 January 2018).
- Lu, H.S.; Tang, W.W.; Liu, X.; Wang, B.G.; Huang, Z.Y. Oleylamine-modified carbon nanoparticles as a kind of efficient lubricating additive of polyalphaolefin. J. Mater. Sci. 2017, 52, 4483–4492. [Google Scholar] [CrossRef]
- Chen, X.; Han, Z.; Li, X.Y.; Lu, K. Lowering coefficient of friction in Cu alloys with stable gradient nanostructures. Sci. Adv. 2016, 2, e1601943. [Google Scholar] [CrossRef] [PubMed]
- Morina, A.; Neville, A. Tribofilms: Aspects of formation, stability and removal. J. Phys. D Appl. Phys. 2007, 40, 5476–5487. [Google Scholar] [CrossRef]
- Perterson, M.B.; Calabrese, S.J.; Li, S.Z.; Jiang, X.X. Friction of alloys at high temperature. J. Mater. Sci. Technol. 1994, 10, 313–320. [Google Scholar]
- Zheng, D.; Wu, Y.P.; Li, Z.Y.; Cai, Z.B. Tribological properties of WS2/graphene nanocomposites as lubricating oil additives. RSC Adv. 2017, 7, 14060–14068. [Google Scholar] [CrossRef]
- Jin, F.J.; Yang, G.B.; Peng, Y.; Zhang, S.M.; Yu, L.G.; Zhang, P.Y. Preparation of borate ester and evaluation of its tribological properties as an additive in different base oils. Lubr. Sci. 2016, 28, 505–519. [Google Scholar] [CrossRef]
- Erdemir, A. A crystal-chemical approach to lubrication by solid oxides. Tribol. Lett. 2000, 8, 97–102. [Google Scholar] [CrossRef]
- Erdemir, A. A crystal chemical approach to the formulation of self-lubricating nanocomposite coatings. Surf. Coat. Technol. 2005, 200, 1792–1796. [Google Scholar] [CrossRef]



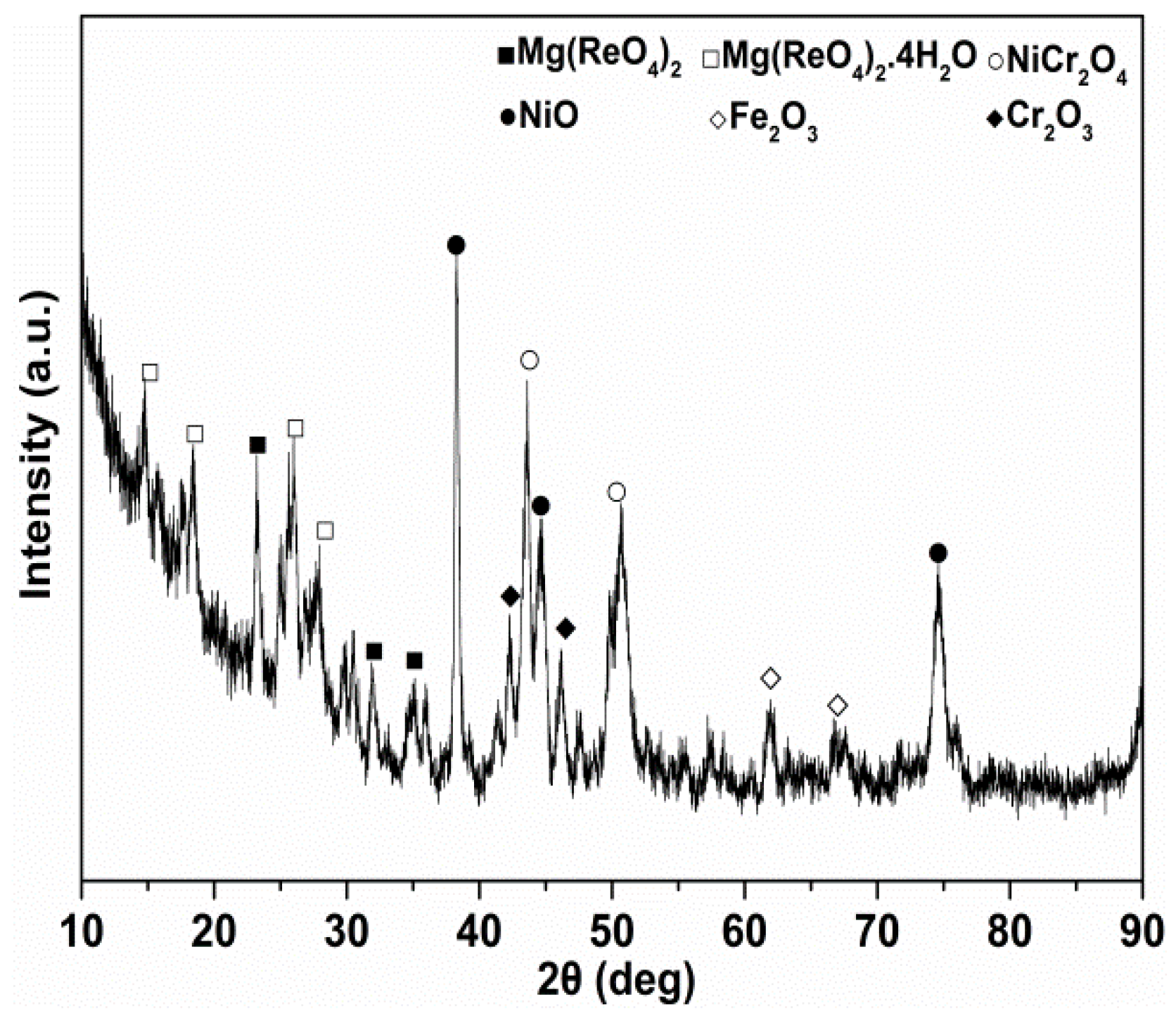
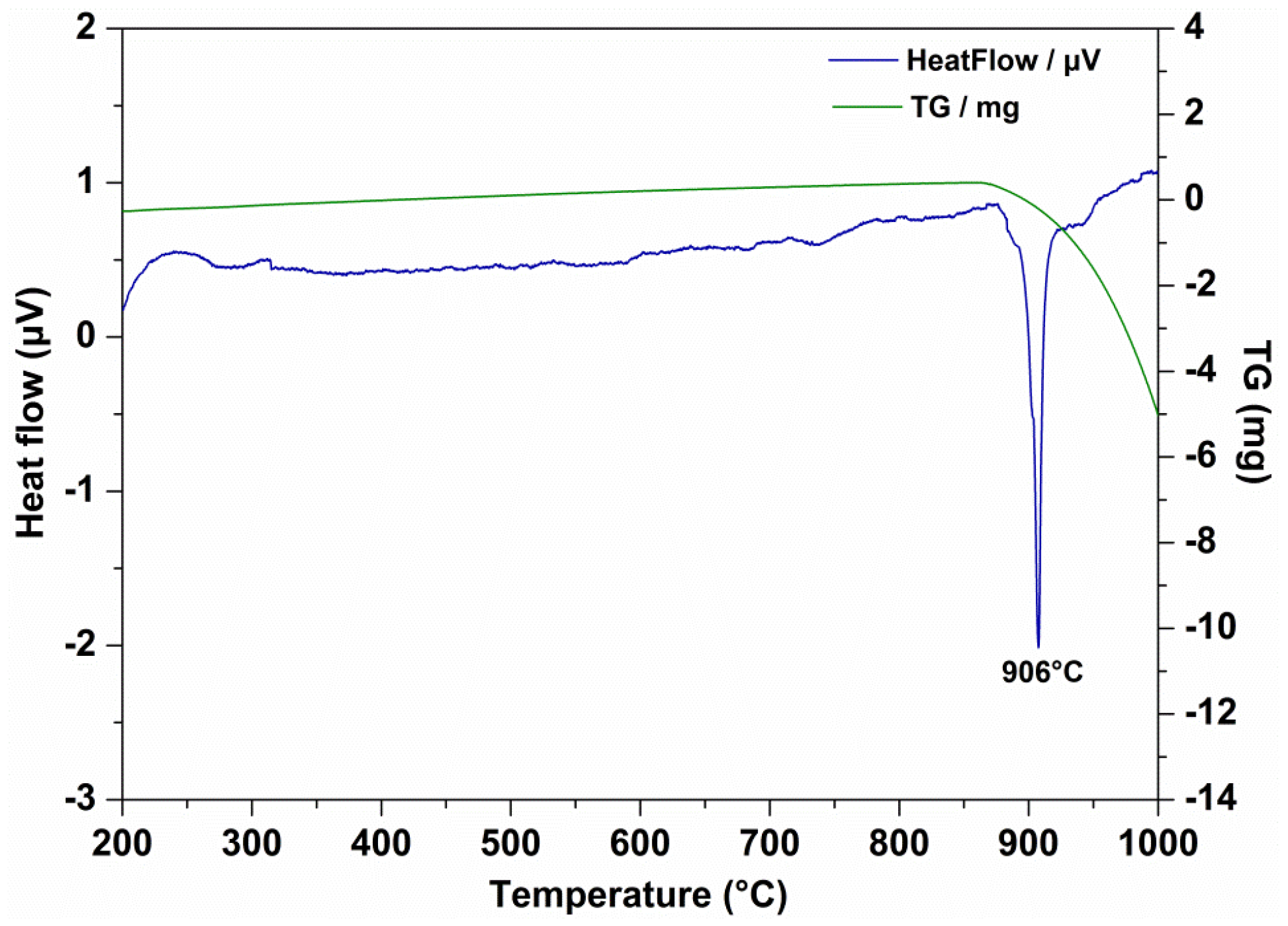
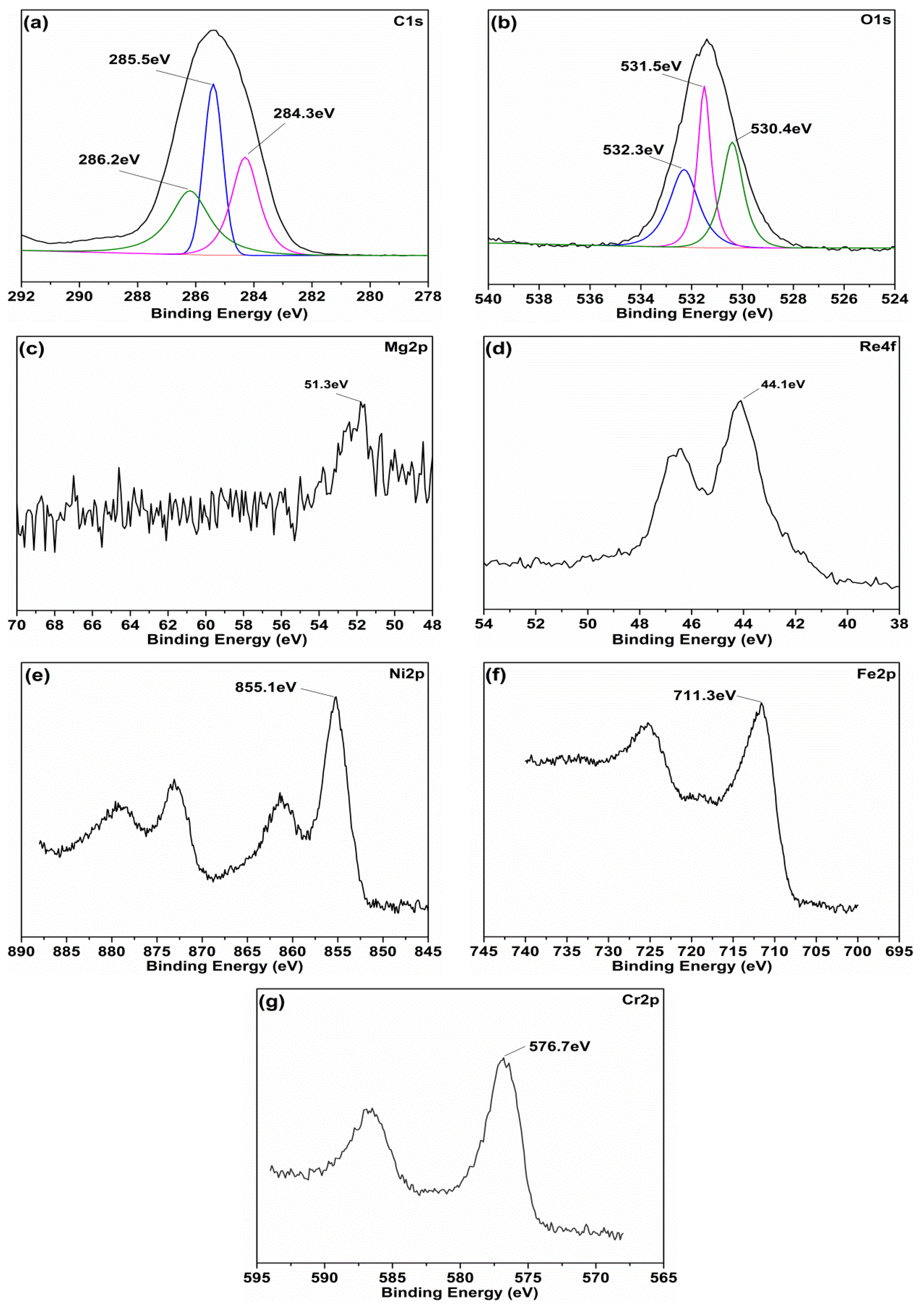
| Parameter | Density (15 °C, g/cm3) | Kinematic Viscosity (mm2/s) | Viscosity Index | Pour Point (°C) | Flash Point (°C) | |
|---|---|---|---|---|---|---|
| 40 °C | 100 °C | |||||
| PAO6 | 0.819 | 31 | 5.8 | 138 | −68 | 246 |
| Samples | PAO | PAO–SA | 0.1 wt% | 0.3 wt% | 0.5 wt% | 0.8 wt% | 1.0 wt% |
|---|---|---|---|---|---|---|---|
| Original temperature (°C) | 25.8 | 26.4 | 26.7 | 26.4 | 26.2 | 26.1 | 26.2 |
| Finishing temperature (°C) | 63.2 | 62.9 | 60.6 | 57.6 | 55.8 | 58.4 | 59.3 |
| Temperature rise (°C) | 37.4 | 36.5 | 33.9 | 31.2 | 29.6 | 32.3 | 33.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Li, T.; Yan, T.; Zhang, L.; Zhang, K.; Qu, X. Role of Magnesium Perrhenate in an Oil/Solid Mixed System for Tribological Application at Various Temperatures. Materials 2018, 11, 1754. https://doi.org/10.3390/ma11091754
Wang J, Li T, Yan T, Zhang L, Zhang K, Qu X. Role of Magnesium Perrhenate in an Oil/Solid Mixed System for Tribological Application at Various Temperatures. Materials. 2018; 11(9):1754. https://doi.org/10.3390/ma11091754
Chicago/Turabian StyleWang, Junhai, Ting Li, Tingting Yan, Lixiu Zhang, Ke Zhang, and Xin Qu. 2018. "Role of Magnesium Perrhenate in an Oil/Solid Mixed System for Tribological Application at Various Temperatures" Materials 11, no. 9: 1754. https://doi.org/10.3390/ma11091754
APA StyleWang, J., Li, T., Yan, T., Zhang, L., Zhang, K., & Qu, X. (2018). Role of Magnesium Perrhenate in an Oil/Solid Mixed System for Tribological Application at Various Temperatures. Materials, 11(9), 1754. https://doi.org/10.3390/ma11091754





