Investigation on the Thermal Behavior, Mechanical Properties and Reaction Characteristics of Al-PTFE Composites Enhanced by Ni Particle
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Specimen Preparation
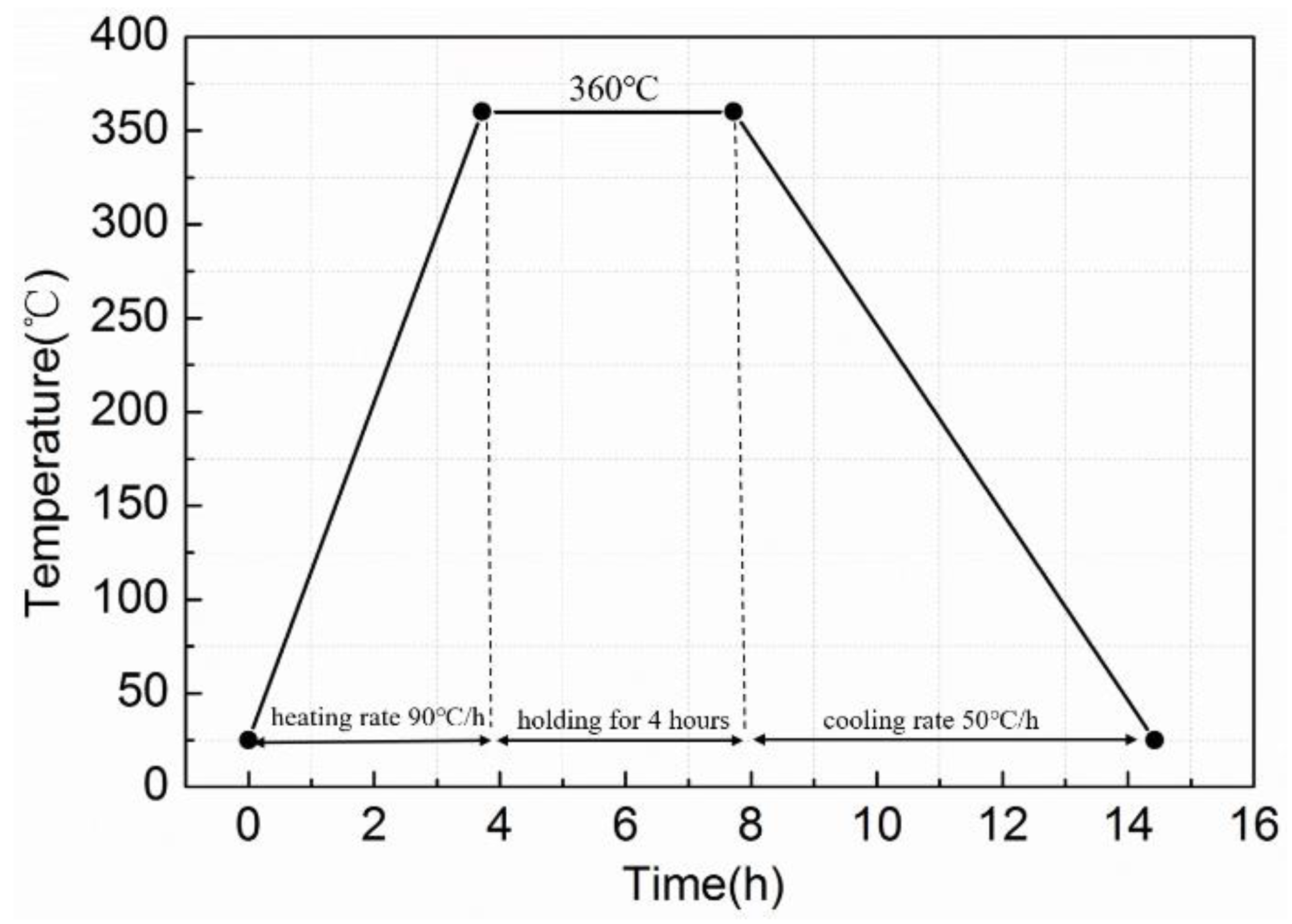
2.3. Experimental Procedures
3. Results and Discussion
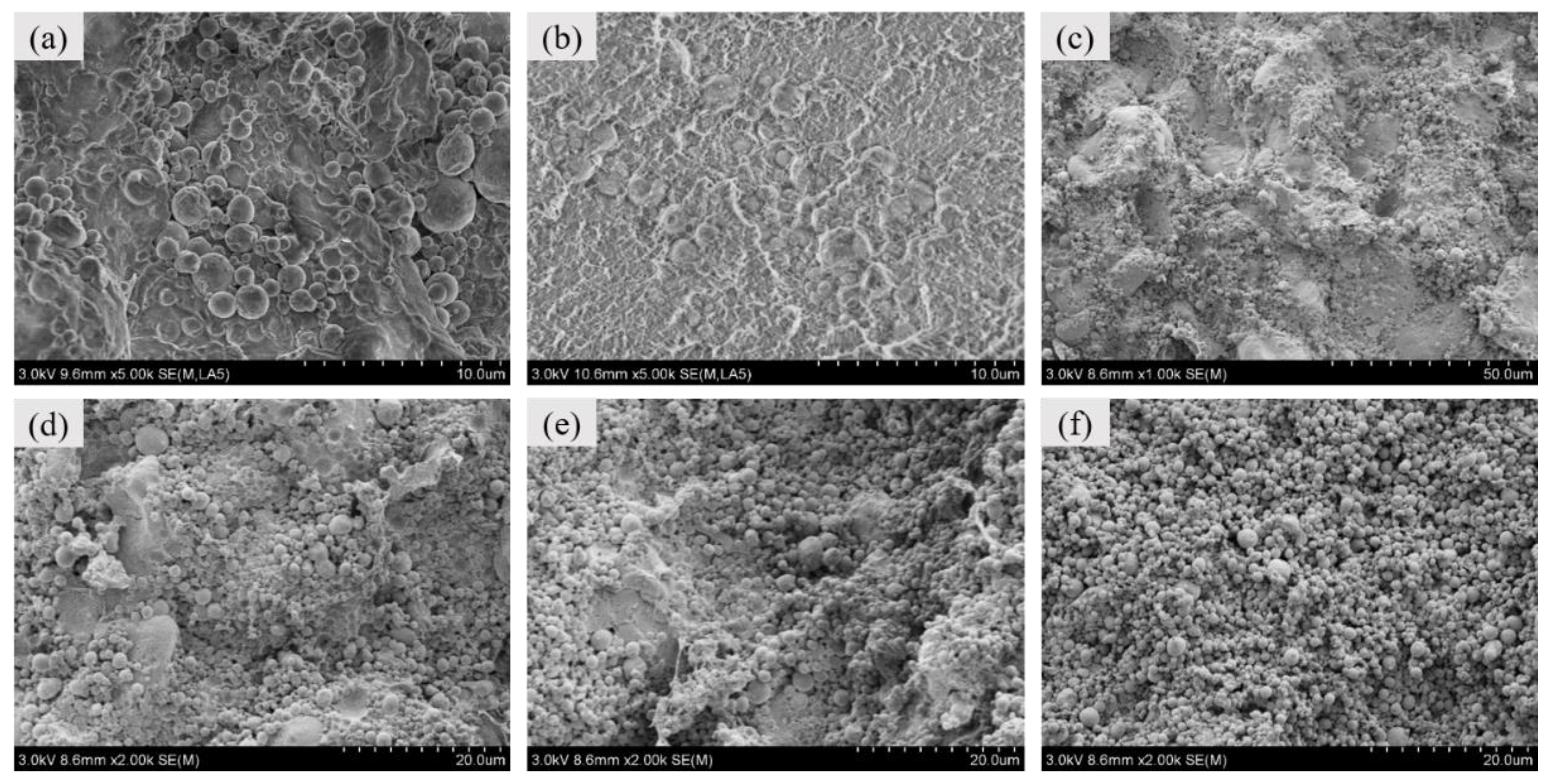
3.1. Mesoscale Characteristics
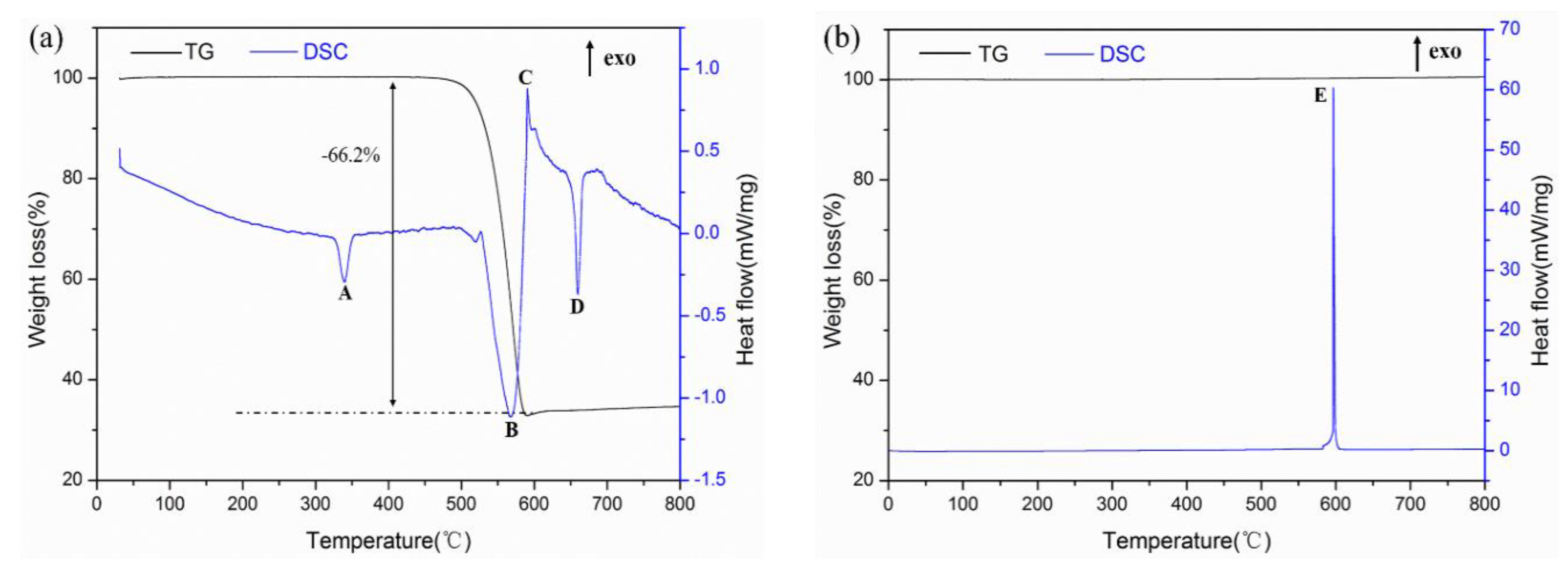
3.2. Thermal Behavior of Al-PTFE and Al-Ni Mixtures
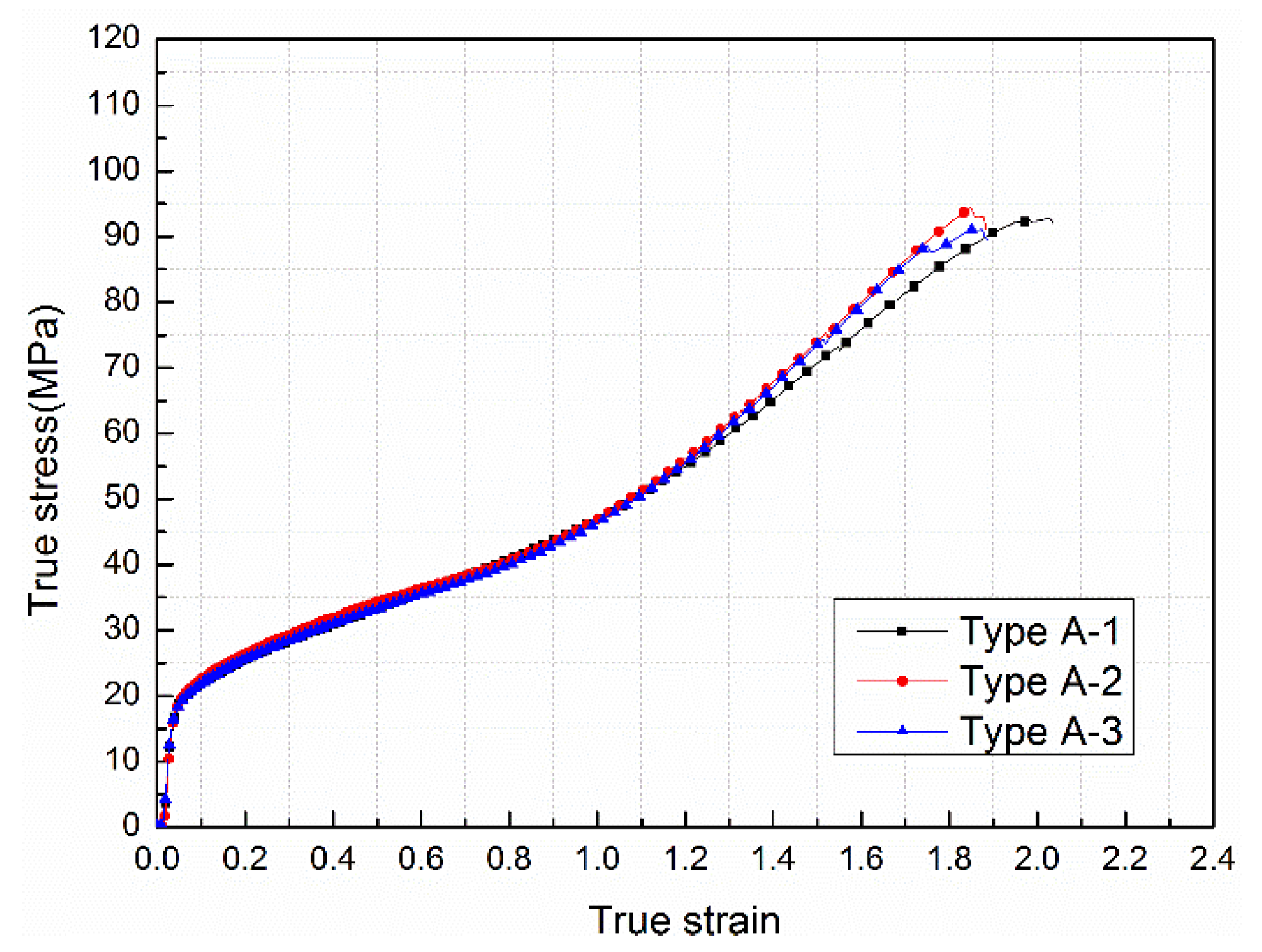
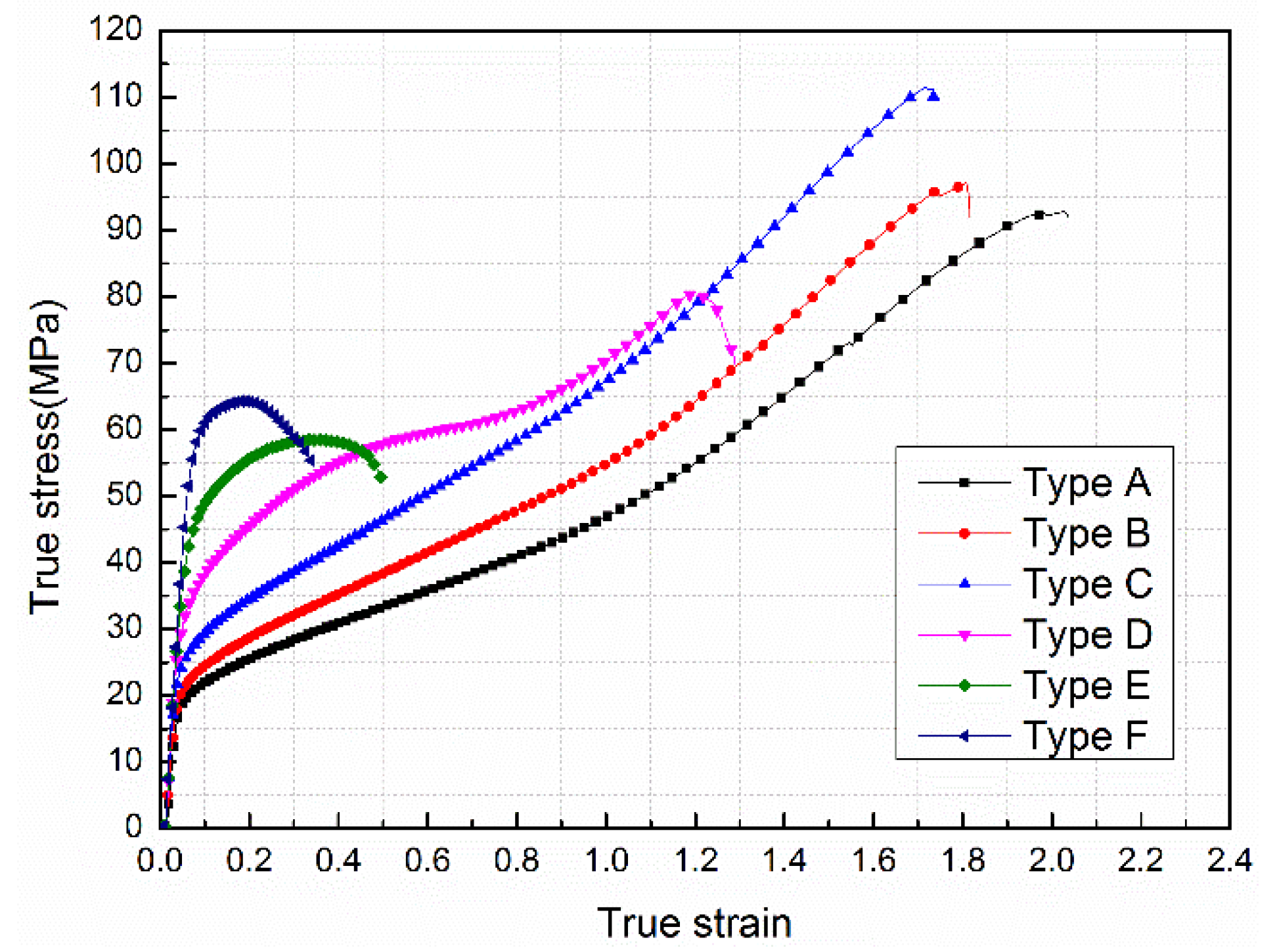
3.3. Mechanical Properties under Quasi-Static Compression
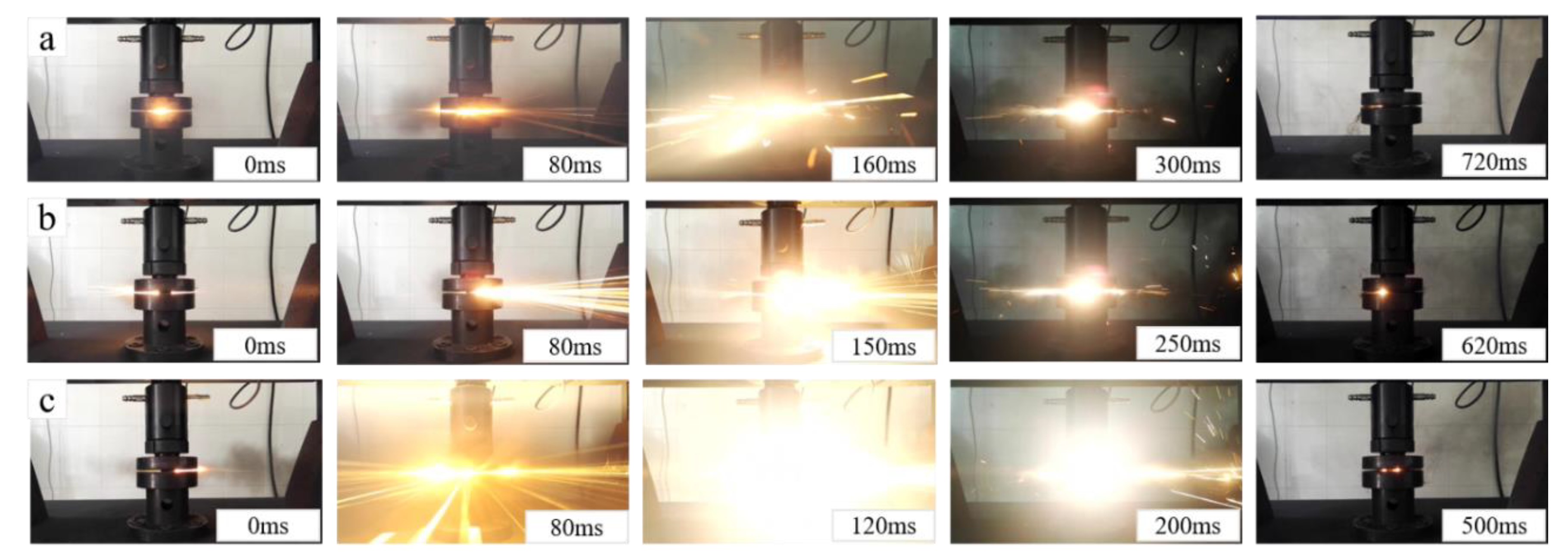
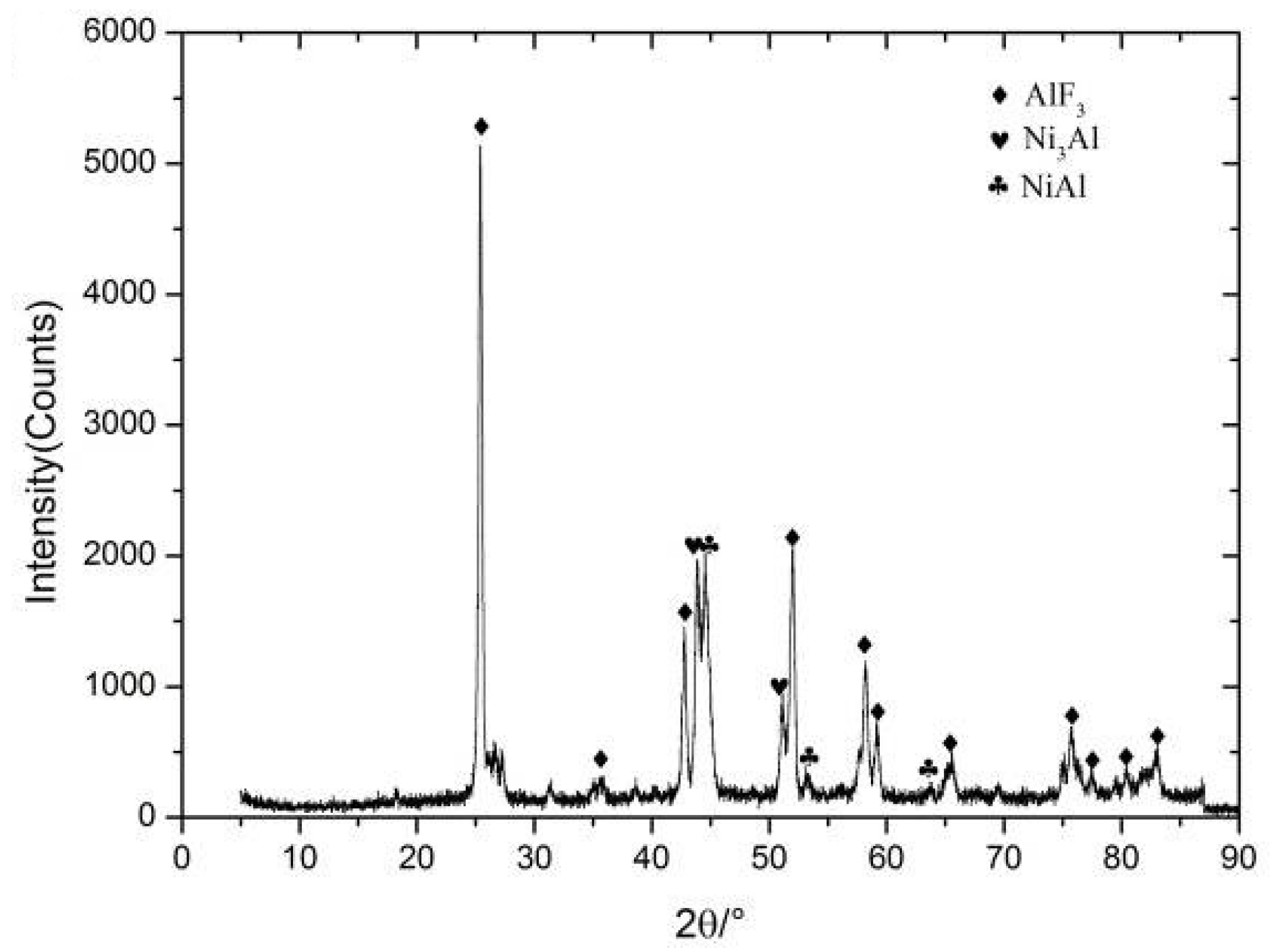
3.4. Reaction Characteristics under Quasi-Static Compression
4. Conclusions
- (1)
- The TG-DSC curves of Al-PTFE and Al-Ni mixtures illustrated that the reaction between Al and PTFE began at 587.6 °C, the reaction between Al and Ni started at 582.7 °C, and the reaction heat of Al-Ni (991.9 J/g) was 12.5 times higher than that of Al-PTFE (79.6 J/g).
- (2)
- The addition of Ni particles to Al-PTFE had a significant effect on the mechanical properties of the material. With the increase in Ni content, the material changed from ductile to brittle and the strain hardening modulus and compressive strength rose first and subsequently decreased, reaching a maximum of 51.35 MPa and 111.41 MPa respectively when the Ni volume fraction was 10%.
- (3)
- In the quasi-static compression test, no reaction was occurred for specimens with a Ni volume fraction of 20, 30 and 40%, while an exothermic reaction occurred for specimens with a Ni volume fraction of 0, 5 and 10%. As the reaction heat of Al-Ni was far higher than that of Al-PTFE, the reaction intensity and reaction speed took on an upward trend as the Ni content increased.
- (4)
- In the Al-Ni-PTFE system, the reaction between Al and PTFE preceded the reaction between Al and Ni and the feasibility of increasing the energy density and the damaging effect of the Al-Ni-PTFE reactive material by means of the Ni-Al reaction was proved.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nielson, D.B.; Truitt, R.M.; Ashcroft, B.N. Reactive Material Enhanced Projectiles and Related Methods. U.S. Patent 7603951, 20 October 2009. [Google Scholar]
- Hunt, E.M.; Malcolm, S.; Pantoya, M.L.; Davis, F. Impact ignition of nano and micron composite energetic materials. Int. J. Impact Eng. 2009, 36, 842–846. [Google Scholar] [CrossRef]
- Mock, W., Jr.; Holt, W.H. Impact Initiation of Rods of Pressed Polytetrafluoroethylene (PTFE) and Aluminum Powders. In Proceedings of the AIP Conference Proceedings, Albuquerque, NM, USA, 12–16 February 2006; American Institute of Physics: College Park, MD, USA, 2006; Volume 845, pp. 1097–1100. [Google Scholar]
- Zhang, X.F.; Shi, A.S.; Qiao, L.; Zhang, J.; Zhang, Y.G.; Guan, Z.W. Experimental study on impact-initiated characters of multifunctional energetic structural materials. J. Appl. Phys. 2013, 113, 083508. [Google Scholar] [CrossRef]
- Glavier, L.; Taton, G.; Ducéré, J.-M.; Baijot, V.; Pinon, S.; Calais, T.; Estève, A.; Rouhani, M.D.; Rossi, C. Nanoenergetics as pressure generator for nontoxic impact primers: Comparison of Al/Bi2O3, Al/CuO, Al/MoO3 nanothermites and Al/PTFE. Combust. Flame 2015, 162, 1813–1820. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, Y.; Yu, Q.; Liu, Z.; Yu, W. Impact-induced initiation and energy release behavior of reactive materials. J. Appl. Phys. 2011, 110, 074904. [Google Scholar]
- Sorensen, B. High-velocity impact of encased Al/PTFE projectiles on structural Aluminum armor. Procedia Eng. 2015, 103, 569–576. [Google Scholar] [CrossRef]
- Feng, B.; Fang, X.; Li, Y.C.; Wang, H.X.; Mao, Y.M.; Wu, S.Z. An initiation phenomenon of Al-PTFE under quasi-static compression. Chem. Phys. Lett. 2015, 637, 38–41. [Google Scholar] [CrossRef]
- Feng, B.; Li, Y.C.; Wu, S.Z.; Wang, H.X.; Tao, Z.M.; Fang, X. A crack-induced initiation mechanism of Al-PTFE under quasi-static compression and the investigation of influencing factors. Mater. Des. 2016, 108, 411–417. [Google Scholar] [CrossRef]
- Wu, J.X.; Fang, X.; Gao, Z.R.; Wang, H.X.; Huang, J.Y.; Wu, S.Z.; Li, Y.C. Investigation on Mechanical Properties and Reaction Characteristics of Al-PTFE Composites with different Al Particle Size. Adv. Mater. Sci. Eng. 2018, 2018, 2767563. [Google Scholar] [CrossRef]
- Cai, J.; Walley, S.; Hunt, R.; Proud, W.; Nesterenko, V.; Meyers, M. High-strain, high-strain-rate flow and failure in PTFE/Al/W granular composites. Mater. Sci. Eng. A 2008, 472, 308–315. [Google Scholar] [CrossRef]
- Herbold, E.; Nesterenko, V.; Benson, D.; Cai, J.; Vecchio, K.; Jiang, F.; Addiss, J.; Walley, S.; Proud, W. Particle size effect on strength, failure, and shock behavior in Polytetrafluoroethylene-Al-W granular composite materials. J. Appl. Phys. 2008, 104, 103903. [Google Scholar] [CrossRef]
- Yu, Z.S.; Fang, X.; Gao, Z.R.; Wang, H.X.; Huang, J.Y.; Yao, M.; Li, Y.C. Mechanical and Reaction Properties of Al/TiH2/PTFE under Quasi-Static Compression. Adv. Eng. Mater. 2018, 20, 1800019. [Google Scholar] [CrossRef]
- Ge, C.; Maimaitituersun, W.; Dong, Y.X.; Tian, C. A Study on the Mechanical Properties and Impact-Induced Initiation Characteristics of Brittle PTFE/Al/W Reactive Materials. Materials 2017, 10, 452. [Google Scholar]
- Zhang, X.F.; Zhang, J.; Qiao, L.; Shi, A.S.; Zhang, Y.G.; He, Y.; Guan, Z.W. Experimental study of the compression properties of Al/W/PTFE granular composites under elevated strain rates. Mater. Sci. Eng. A 2013, 581, 48–55. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J.X.; Li, S.K.; Zhang, X.B. Investigation on reaction energy, mechanical behavior and impact insensitivity of W–PTFE–Al composites with different W percentage. Mater. Des. 2016, 92, 397–404. [Google Scholar] [CrossRef]
- Eakins, D.; Thadhani, N.N. Discrete particle simulation of shock wave propagation in a binary Ni + Al powder mixture. J. Appl. Phys. 2007, 101, 043508. [Google Scholar] [CrossRef]
- Eakins, D.; Thadhani, N.N. Shock-induced reaction in a flake nickel + spherical aluminum powder mixture. J. Appl. Phys. 2006, 100, 113521, reactive material enhanced projectiles and related methods. [Google Scholar] [CrossRef]
- Eakins, D.E.; Thadhani, N.N. Mesoscale simulation of the configuration-dependent shock-compression response of Ni + Al powder mixtures. Acta Mater. 2008, 56, 1496–1510. [Google Scholar] [CrossRef]
- Nielson, D.B.; Truitt, R.M.; Ashcroft, B.N. Reactive Material Enhanced Projectiles and Related Methods. U.S. Patent 2008/0035007 A1, 14 February 2008. [Google Scholar]
- Jordan, J.L.; Siviour, C.R.; Foley, J.R.; Brown, E.N. Compressive Properties of Extruded Polytetrafluoroethylene. Polymer 2007, 48, 4184–4195. [Google Scholar] [CrossRef]

| Type | Volume Fraction (vol %) | Mass Fraction (wt %) | TMD (g/cm3) | ||||
|---|---|---|---|---|---|---|---|
| Al | Ni | PTFE | Al | Ni | PTFE | ||
| A | 22% | 0 | 78% | 26.5% | 0 | 73.5% | 2.31 |
| B | 22% | 5% | 73% | 22.8% | 17.1% | 60.2% | 2.61 |
| C | 22% | 10% | 68% | 20.2% | 30.2% | 49.6% | 2.95 |
| D | 22% | 20% | 58% | 16.4% | 49.2% | 34.4% | 3.62 |
| E | 22% | 30% | 48% | 13.8% | 62.2% | 24.0% | 4.30 |
| F | 22% | 40% | 38% | 11.9% | 71.6% | 16.4% | 4.97 |
| No. | Onset Temperature/(°C) | Peak Temperature/(°C) | End Temperature/(°C) | Heat Release/(J/g) |
|---|---|---|---|---|
| Endo-peak-A | 328.9 | 339.5 | 349.6 | −45.4 |
| Endo-peak-B | 502.2 | 566.8 | 585.7 | −389.2 |
| Exo-peak-C | 587.6 | 590.2 | 642.1 | 79.6 |
| Endo-peak-D | 649.1 | 660.0 | 667.3 | −73.5 |
| Exo-peak-E | 582.7 | 597.3 | 605.5 | 991.9 |
| Type | Yield Strength/MPa | Elastic Modulus/MPa | Hardening Modulus/MPa | Compressive Strength/MPa | Failure Strain |
|---|---|---|---|---|---|
| A | 19.92 | 358.86 | 37.64 | 92.77 | 2.04 |
| B | 21.14 | 360.48 | 43.26 | 96.67 | 1.81 |
| C | 27.13 | 363.02 | 51.35 | 111.41 | 1.74 |
| D | 36.67 | 364.09 | 39.77 | 80.36 | 1.29 |
| E | 46.75 | 366.52 | - | 58.42 | 0.50 |
| F | 57.83 | 367.01 | - | 64.04 | 0.35 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Wang, H.; Fang, X.; Li, Y.; Mao, Y.; Yang, L.; Yin, Q.; Wu, S.; Yao, M.; Song, J. Investigation on the Thermal Behavior, Mechanical Properties and Reaction Characteristics of Al-PTFE Composites Enhanced by Ni Particle. Materials 2018, 11, 1741. https://doi.org/10.3390/ma11091741
Wu J, Wang H, Fang X, Li Y, Mao Y, Yang L, Yin Q, Wu S, Yao M, Song J. Investigation on the Thermal Behavior, Mechanical Properties and Reaction Characteristics of Al-PTFE Composites Enhanced by Ni Particle. Materials. 2018; 11(9):1741. https://doi.org/10.3390/ma11091741
Chicago/Turabian StyleWu, Jiaxiang, Huaixi Wang, Xiang Fang, Yuchun Li, Yiming Mao, Li Yang, Qin Yin, Shuangzhang Wu, Miao Yao, and Jiaxing Song. 2018. "Investigation on the Thermal Behavior, Mechanical Properties and Reaction Characteristics of Al-PTFE Composites Enhanced by Ni Particle" Materials 11, no. 9: 1741. https://doi.org/10.3390/ma11091741
APA StyleWu, J., Wang, H., Fang, X., Li, Y., Mao, Y., Yang, L., Yin, Q., Wu, S., Yao, M., & Song, J. (2018). Investigation on the Thermal Behavior, Mechanical Properties and Reaction Characteristics of Al-PTFE Composites Enhanced by Ni Particle. Materials, 11(9), 1741. https://doi.org/10.3390/ma11091741





