Three-Dimensional S/CeO2/RGO Composites as Cathode Materials for Lithium–Sulfur Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
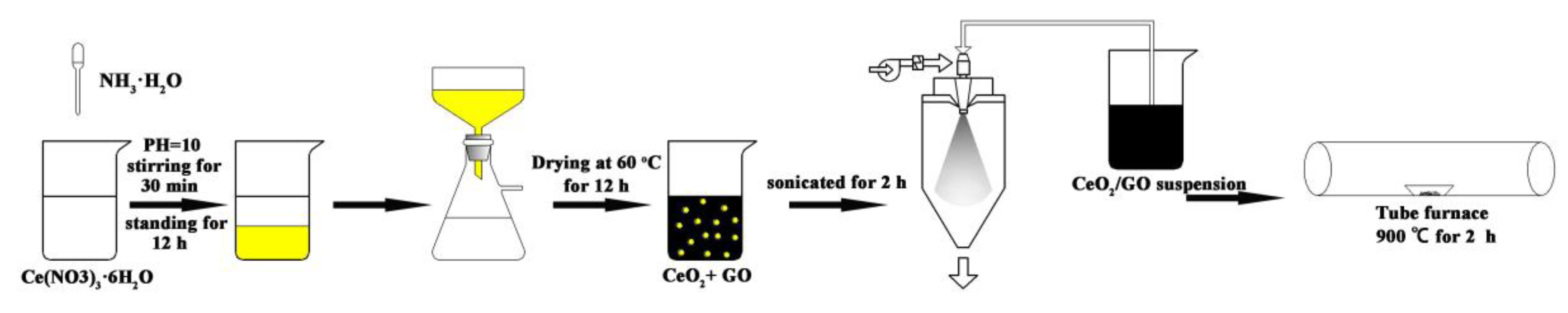
2.2. Sample Preparation
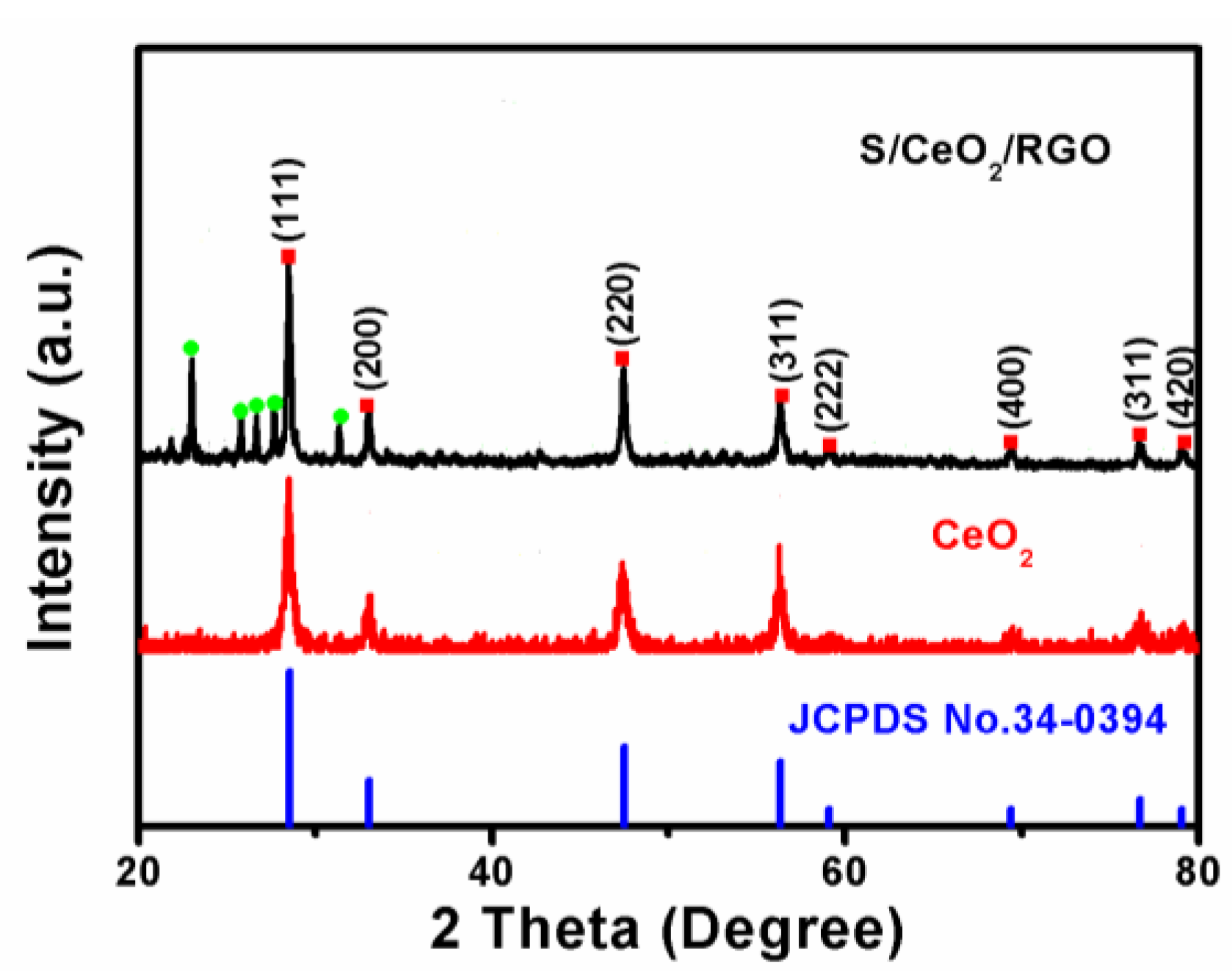
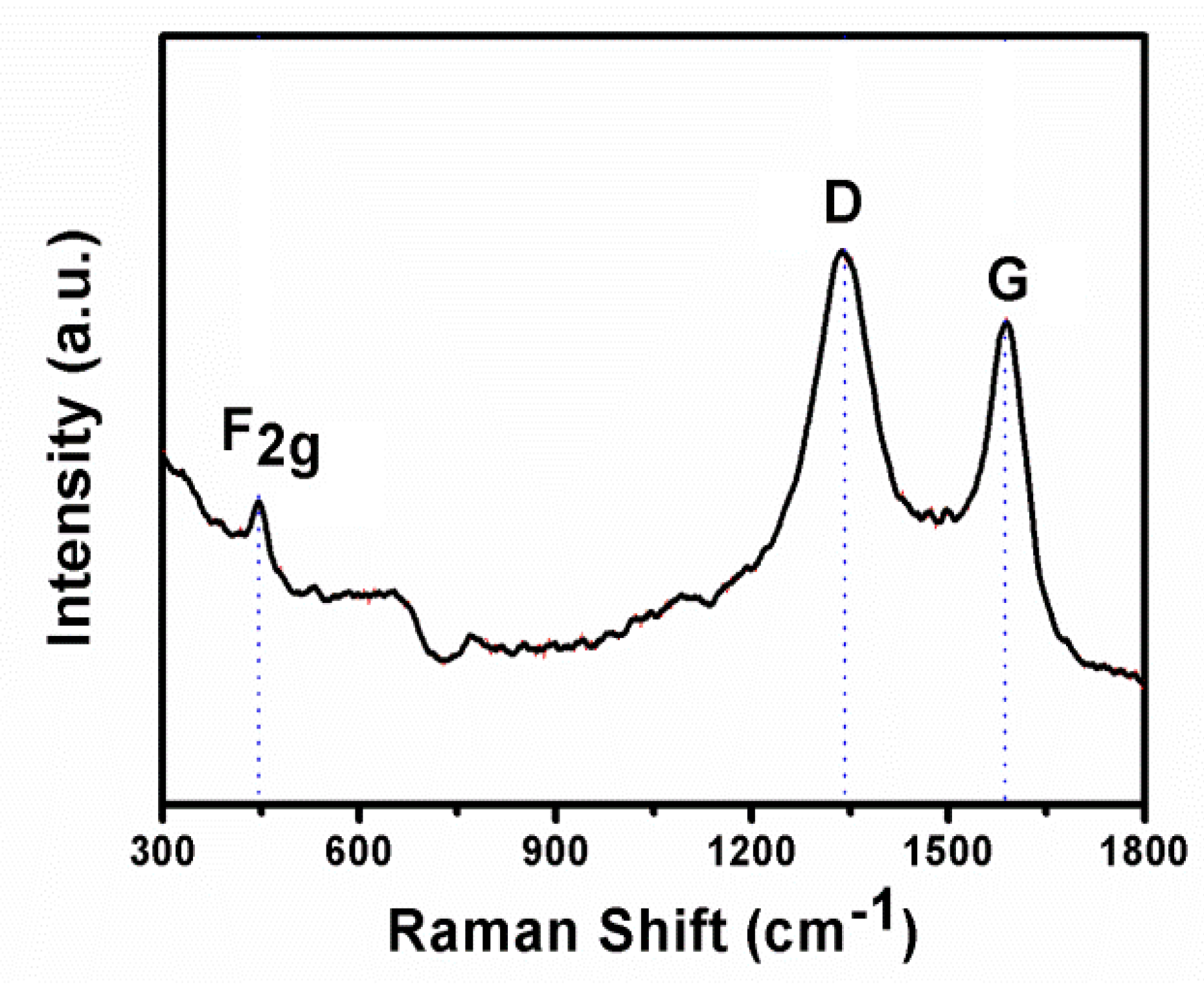
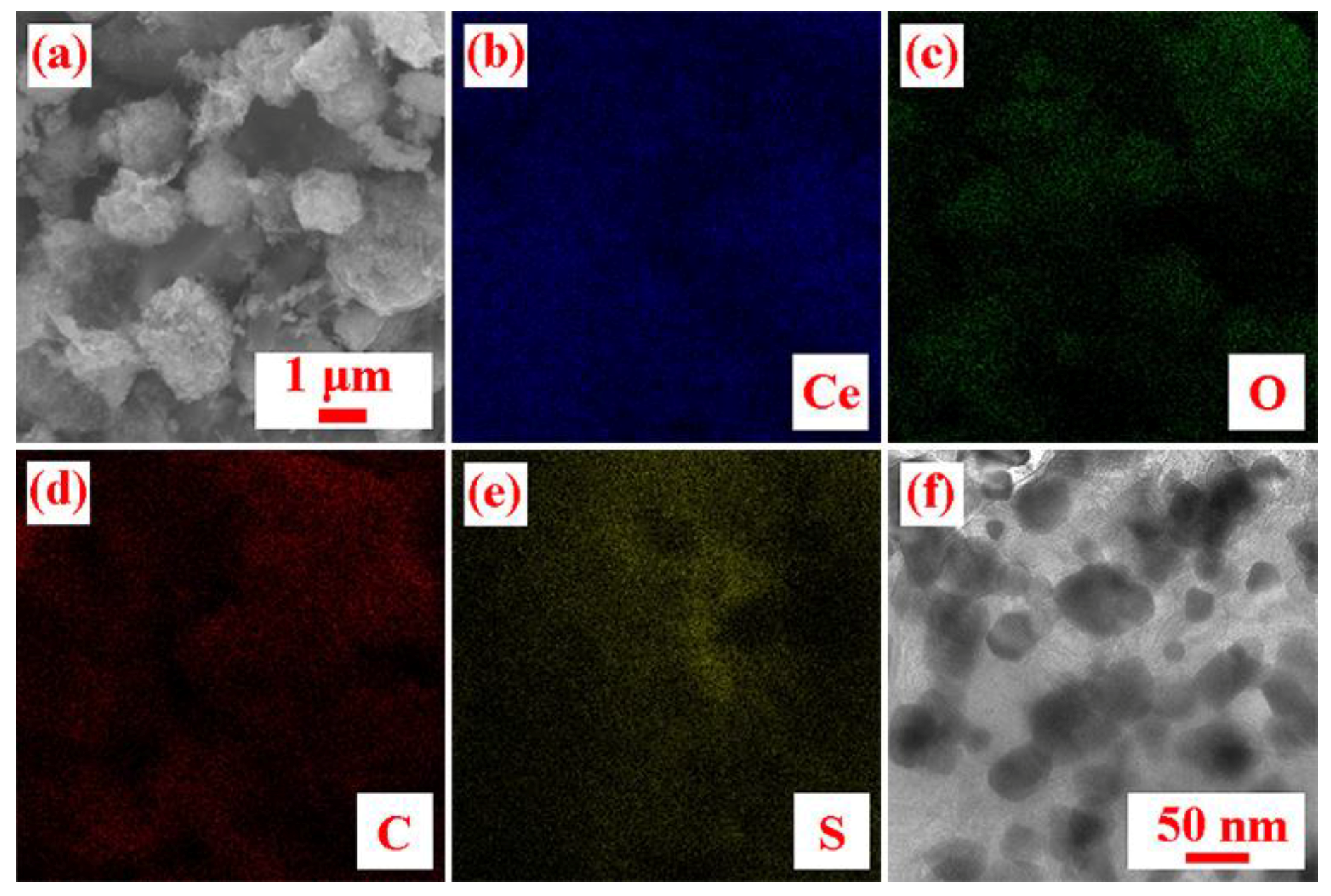
2.3. Characterization
2.4. Electrochemical Measurements
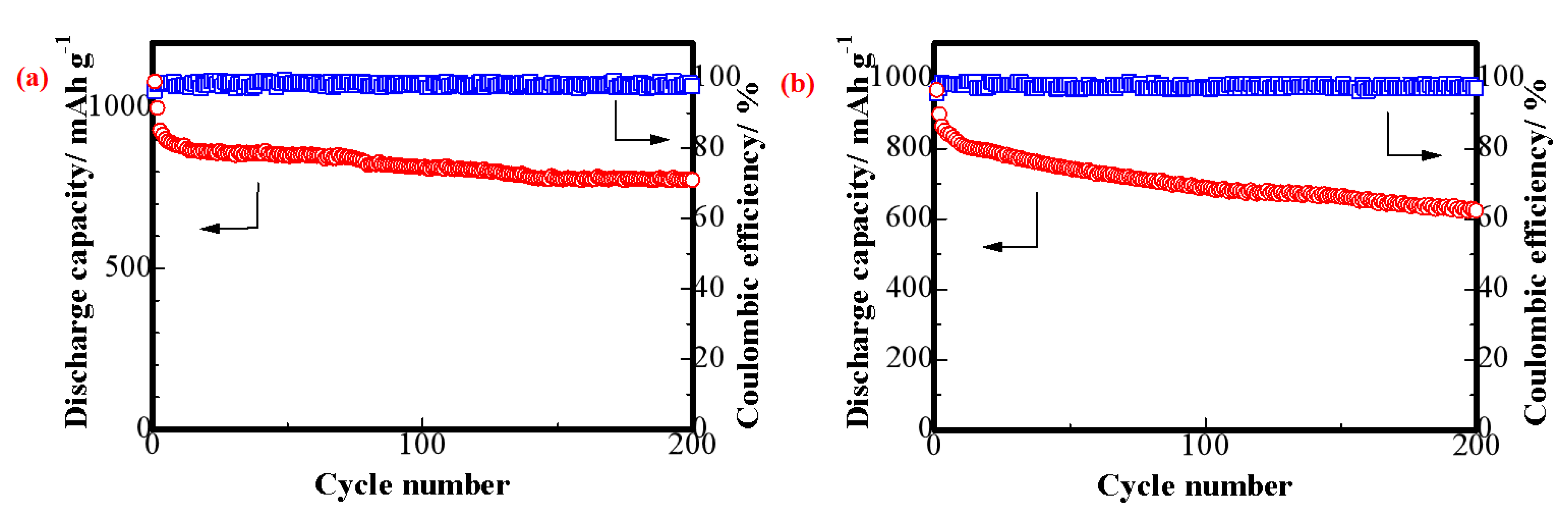
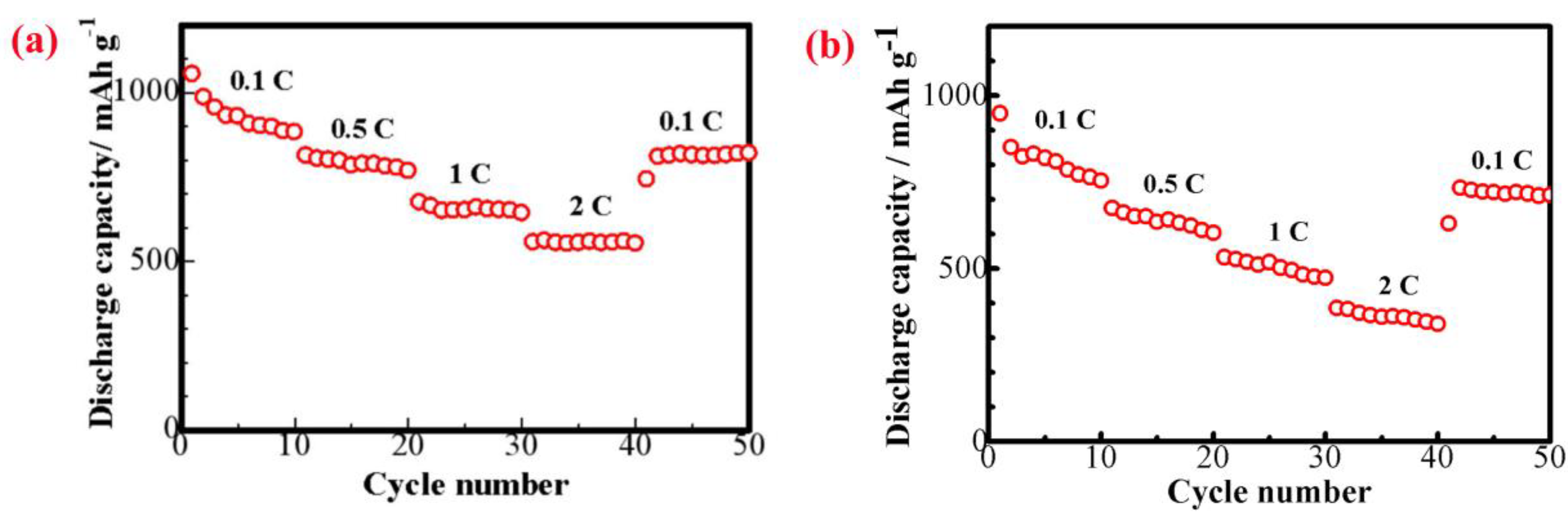
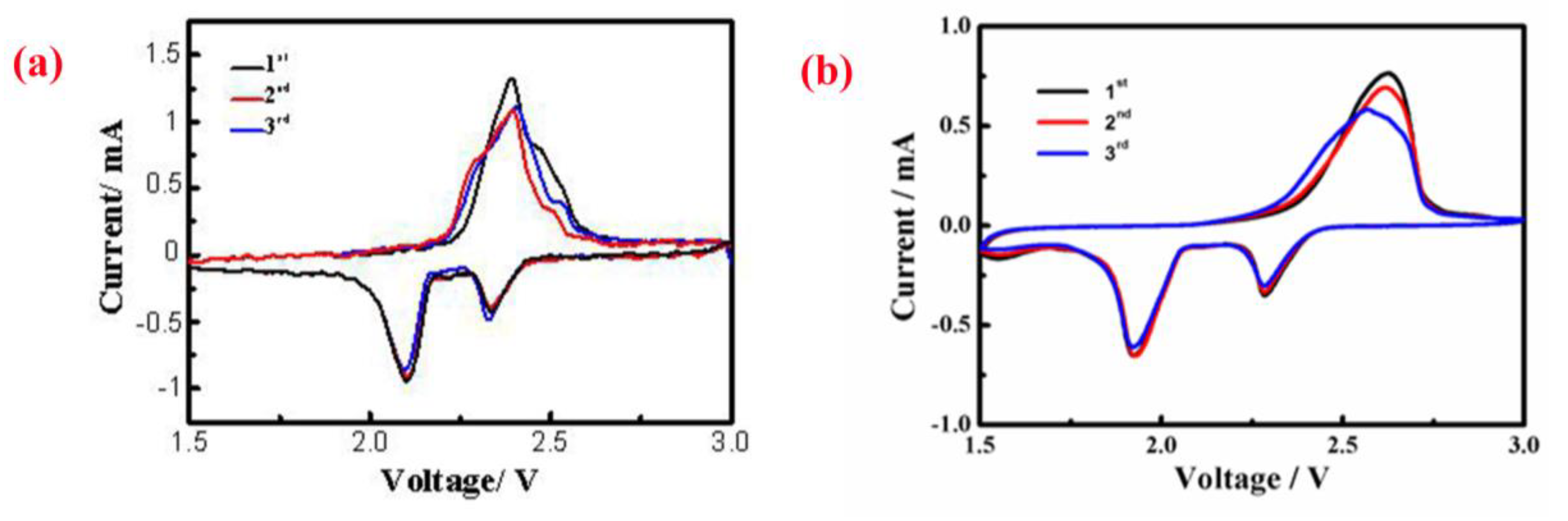
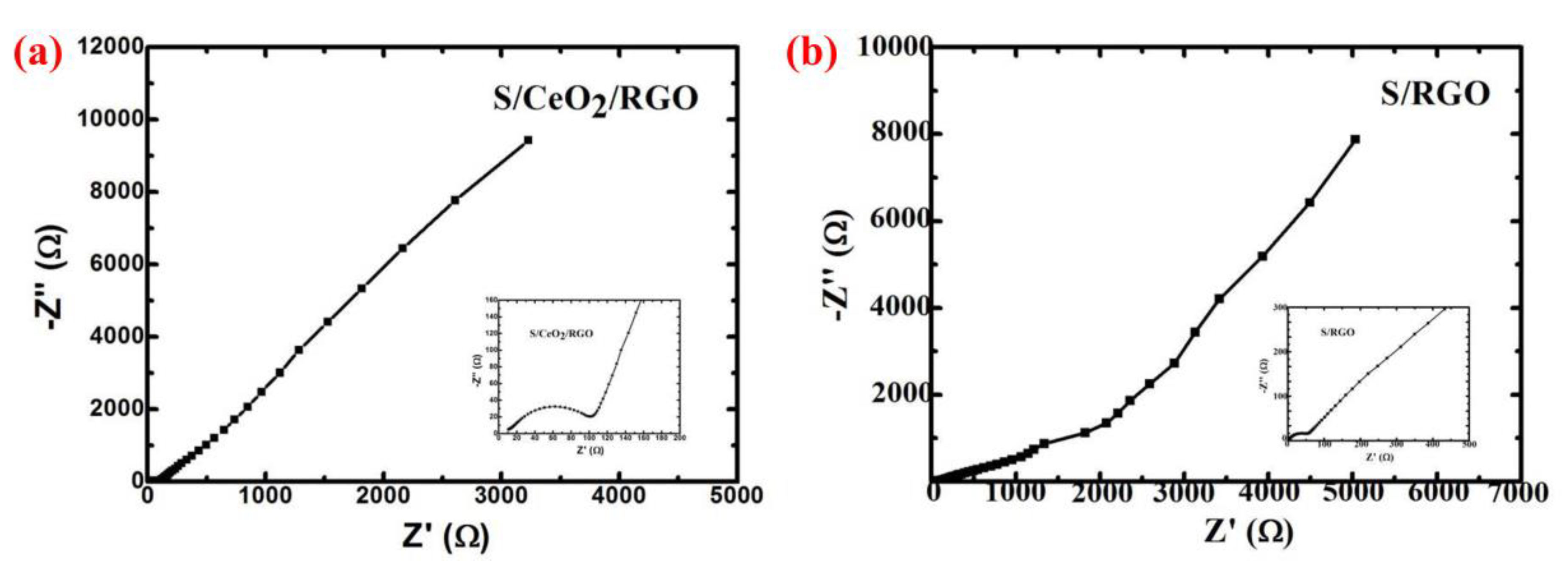
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Deng, Y.; Li, J.; Li, T.; Gao, X.; Yuan, C. Life cycle assessment of lithium sulfur battery for electricvehicles. J. Power Sources 2017, 343, 284–295. [Google Scholar] [CrossRef]
- Rosenman, A.; Markevich, E.; Salitra, G.; Aurbach, D.; Garsuch, A.; Chesneau, F.F. Review on Li-sulfur battery systems: An integral perspective. Adv. Energy Mater. 2015, 5, 1500212. [Google Scholar] [CrossRef]
- Yin, Y.X.; Xin, S.; Guo, Y.G.; Wan, L.J. Lithium-sulfur batteries: Electrochemistry, materials, andprospects. Angew. Chem. Int. Ed. 2013, 52, 13186–13200. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Gan, F.; Wang, P.; Chen, K.; Chen, S.; He, X. In situ synthesis of 3D sulfur-dopedgraphene/sulfur as a cathode material for lithium-sulfur batteries. J. Alloys Compd. 2018, 754, 64–71. [Google Scholar] [CrossRef]
- Xu, T.; Song, J.; Gordin, M.L.; Sohn, H.; Yu, Z.; Chen, S.; Wang, D. Mesoporous carbon-carbon nanotube-sulfur composite microspheres for high-areal-capacity lithium-sulfur battery cathodes. ACS Appl. Mater. Interface 2013, 5, 11355–11362. [Google Scholar] [CrossRef] [PubMed]
- Kolosnitsyn, V.S.; Karaseva, E.V. Lithium-sulfur batteries: Problems and solutions. Russ. J. Electrochem. 2008, 44, 506–509. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Bakenov, Z. A novel lithium/sulfur battery based on sulfur/graphene nanosheet composite cathode and gel polymer electrolyte. Nanoscale Res. Lett. 2014, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Evers, S.; Nazar, L.F. New approaches for high energy density lithium-sulfur battery cathodes. Acc. Chem. Res. 2013, 46, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.R.; Zhai, Y.P.; Xu, G.L.; Jiang, Y.X.; Zhao, D.Y.; Li, J.T.; Huang, L.; Sun, S.G. Ordered mesoporous carbon/sulfur nanocomposite of high performances as cathode for lithium–sulfur battery. Electrochim. Acta 2011, 56, 9549–9555. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, S.; Yan, L.; Xiao, M.; Han, D.; Meng, Y. Mesoporous carbon materials prepared from litchi shell as sulfur encapsulator for lithium-sulfur battery application. J. Power Sources 2016, 324, 547–555. [Google Scholar] [CrossRef]
- Xu, Z.L.; Kim, J.K.; Kang, K. Carbon nanomaterials for advanced lithium sulfur batteries. Nano Today 2018, 19, 84–107. [Google Scholar] [CrossRef]
- Liang, X.; Kwok, C.Y.; Lodi-Marzano, F.; Pang, Q.; Cuisinier, M.; Huang, H.; Hart, C.J.; Houtarde, D.; Kaup, K.; Sommer, H.; et al. Tuning transition metal oxide-sulfur interactions for long life lithium sulfur batteries: The “goldilocks” principle. Adv. Energy Mater. 2016, 6, 1501636. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Yermukhambetova, A.; Bakenov, Z.; Chen, P. Ternary sulfur/polyacrylonitrile/Mg0.6Ni0.4O composite cathodes for high performance lithium/sulfur batteries. J. Mater. Chem. A 2013, 1, 295–301. [Google Scholar] [CrossRef]
- Yin, F.; Liu, X.; Zhang, Y.; Zhao, Y.; Menbayeva, A.; Bakenov, Z.; Wang, X. Well-dispersed sulfur anchored on interconnected polypyrrole nanofiber network as high performance cathode for lithium-sulfur batteries. Solid State Sci. 2017, 66, 44–49. [Google Scholar] [CrossRef]
- Jeong, T.G.; Lee, Y.S.; Cho, B.W.; Kim, Y.T.; Jung, H.G.; Chung, K.Y. Improved performance of dual-conducting polymer-coated sulfur composite with high sulfur utilization for lithium-sulfur batteries. J. Alloys Compd. 2018, 742, 868–876. [Google Scholar] [CrossRef]
- Chen, D.; Yang, R.; Chen, L.; Zou, Y.; Ren, B.; Li, L.; Li, S.; Yan, Y.; Xu, Y. One-pot fabrication of nitrogen and sulfur dual-doped graphene/sulfur cathode via microwave assisted method for long cycle-life lithium-sulfur batteries. J. Alloys Compd. 2018, 746, 116–124. [Google Scholar] [CrossRef]
- Hong, X.; Liang, J.; Tang, X.; Yang, H.; Li, F. Hybrid graphene album with polysulfides adsorption layer for Li-S batteries. Chem. Eng. Sci. 2018. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, F.; Gao, Y.; Wang, Y.; Wang, S.; Gao, Q.; Jiao, Z.; Zhao, B.; Chen, Z. Inhibiting the shuttle effect of Li-S battery with a graphene oxide coating separator: Performance improvement and mechanism study. J. Power Sources 2017, 342, 929–938. [Google Scholar] [CrossRef]
- Park, G.D.; Lee, J.; Piao, Y.; Kang, Y.C. Mesoporous graphitic carbon-TiO2 composite microspheres produced by a pilot-scale spray-drying process as an efficient sulfur host material for Li-S batteries. Chem. Eng. J. 2018, 335, 600–611. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, P.; Zhao, P.; Wang, M. Polar cross-linked polystyrene as polysulfides anchor enhanced cycle performance and coulombic efficiency for lithium sulfur batteries. J. Electroanal. Chem. 2018, 810, 171–175. [Google Scholar] [CrossRef]
- Ling, B.; Chen, A.; Liu, W.; Liu, K.; Hu, H.; Zhang, J. Simply and rapidly synthesized composites of MnO2 nanosheets anchoring on carbon nanotubes as efficient sulfur hosts for Li-S batteries. Mater. Lett. 2018, 218, 321–324. [Google Scholar] [CrossRef]
- Sun, F.; Wang, J.; Long, D.; Qiao, W.; Ling, L.; Lv, C.; Cai, R. A high-rate lithium–sulfur battery assisted by nitrogen-enriched mesoporous carbons decorated with ultrafine La2O3 nanoparticles. J. Mater. Chem. A 2013, 1, 13283–13289. [Google Scholar] [CrossRef]
- Ding, B.; Shen, L.; Xu, G.; Ping, N.; Zhang, X. Encapsulating sulfur into mesoporous TiO2 host as a high performance cathode for lithium–sulfur battery. Electrochim. Acta 2013, 107, 78–84. [Google Scholar] [CrossRef]
- Phokha, S.; Hunpratub, S.; Usher, B.; Pimsawat, A.; Chanlek, N.; Maensiri, S. Effects of CeO2 nanoparticles on electrochemical properties of carbon/CeO2 composites. Appl. Sur. Sci. 2018, 446, 36–46. [Google Scholar] [CrossRef]
- Zhu, P.; Zang, J.; Zhu, J.; Lu, Y.; Chen, C.; Jiang, M.; Yan, C.; Dirican, M.; Selvan, R.K.; Kim, D.; et al. Effect of reduced graphene oxide reduction degree on the performance of polysulfide rejection in lithium-sulfur batteries. Carbon 2018, 126, 594–600. [Google Scholar] [CrossRef]
- Qian, X.; Jin, L.; Zhu, L.; Yao, S.; Rao, D.; Shen, X.; Xi, X.; Xiao, K.; Qin, S. CeO2 nanodots decorated ketjen black for high performance lithium–sulfur batteries. RSC Adv. 2016, 6, 111190–111196. [Google Scholar] [CrossRef]
- Ma, M.; Wang, H.; Liang, S.; Guo, S.; Zhang, Y.; Du, X. Porous carbon-wrapped cerium oxide hollow spheres synthesized via microwave hydrothermal for long-cycle and high-rate lithium-ion batteries. Electrochim. Acta 2017, 256, 110–118. [Google Scholar] [CrossRef]
- Tessonnier, J.P.; Rosenthal, D.; Hansen, T.W.; Hess, C.; Schuster, M.E.; Blume, R.; Girgsdies, F.; Pfänder, N.; Timpe, O.; Su, D.S. Analysis of the structure and chemical properties of some commercial carbon nanostructures. Carbon 2009, 47, 1779–1798. [Google Scholar] [CrossRef]
- Chakrabartty, S.; Mondal, A.; Chakrabarti, P.; Singh, S.K.; Saha, A.K.; Singh, P. Synthesis of biocompatible TiO2 nanodots: Glancing angle deposition technique. J. Nanosci. Nanotechnol. 2016, 16, 8705–8710. [Google Scholar] [CrossRef]
- Senkevich, J.J.; Yang, G.R.; Tang, F.; Wang, G.C.; Lu, T.M.; Cale, T.S.; Jezewski, C.; Lanford, W.A. Substrate-independent sulfur-activated dielectric and barrier-layer surfaces to promote the chemisorption of highly polarizable metallorganics. Appl. Phys. A 2004, 79, 1789–1796. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.B.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Li, H.; Sun, L.; Zhang, Y.; Tan, T.; Wang, G.; Bakenov, Z. Enhanced cycle performance of Li/S battery with the reduced graphene oxide/activated carbon functional interlayer. J. Energy Chem. 2017, 26, 1276–1281. [Google Scholar] [CrossRef]
- Han, S.; Pu, X.; Li, X.; Liu, M.; Li, M.; Feng, N.; Dou, S.; Hu, W. High areal capacity of Li-S batteries enabled by freestanding CNF/rGO electrode with high loading of lithium polysulfide. Electrochim. Acta 2017, 241, 406–413. [Google Scholar] [CrossRef]
- Li, X.; Pan, L.; Wang, Y.; Xu, C. High efficiency immobilization of sulfur on Ce-doped carbon aerogel for high performance lithium-sulfur batteries. Electrochim. Acta 2016, 190, 548–555. [Google Scholar] [CrossRef]
- Qian, X.; Zhao, D.; Jin, L.; Yao, S.; Rao, D.; Shen, X.; Zhou, Y.; Xi, X. Separator modified by spray-dried hollow spherical cerium oxide and its application in lithium sulfur batteries. RSC Adv. 2016, 6, 114989–114996. [Google Scholar] [CrossRef]
- Han, P.; Manthiram, A. Boron- and nitrogen-doped reduced graphene oxide coated separators for high-performance Li-S batteries. J. Power Sources 2017, 369, 87–94. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, Q.; Jiang, L.; Peng, J.; Gao, Y.; Duan, Z.; Lu, X. Preparation of SnO2@rGO/CNTs/S composite and application for lithium-sulfur battery cathode material. Appl. Surf. Sci. 2018, 462, 393–398. [Google Scholar] [CrossRef]
- Gu, X.; Tong, C.; Wen, B.; Liu, L.; Lai, C.; Zhang, S. Ball-milling synthesis of ZnO@sulphur/carbon nanotubes and Ni(OH)2@sulphur/carbon nanotubes composites for high-performance lithium-sulphur batteries. Electrochim. Acta 2016, 196, 369–376. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Lou, X. Hollow Carbon Nanofibers Filled with MnO2 Nanosheets as Efficient Sulfur Hosts for Lithium–Sulfur Batteries. Angew. Chem. 2015, 127, 13078–13082. [Google Scholar] [CrossRef]
- Ponraj, R.; Kannan, A.; Ahn, J.; Kim, D. Improvement of cycling performance of lithium-sulfur batteries by using magnesium oxide as a functional additive for trapping lithium polysulfide. ACS Appl. Mater. Interfaces 2016, 8, 4000–4006. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Yin, L.; Wang, D.; Li, L.; Pei, S.; Gentle, I.; Li, F.; Cheng, H. Fibrous Hybrid of Graphene and Sulfur Nanocrystals for High-Performance Lithium Sulfur Batteries. ACS Nano 2013, 7, 5367–5375. [Google Scholar] [CrossRef] [PubMed]

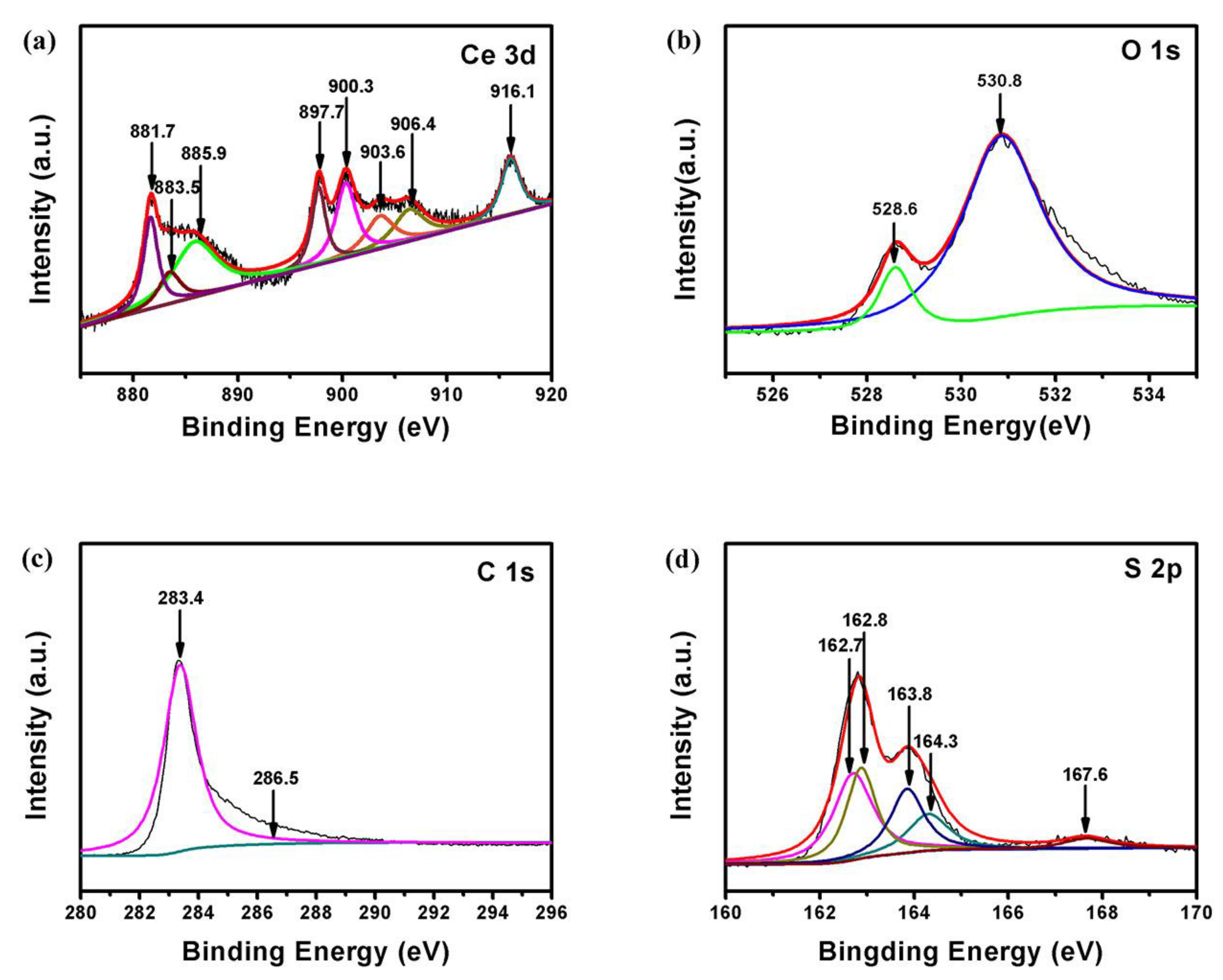
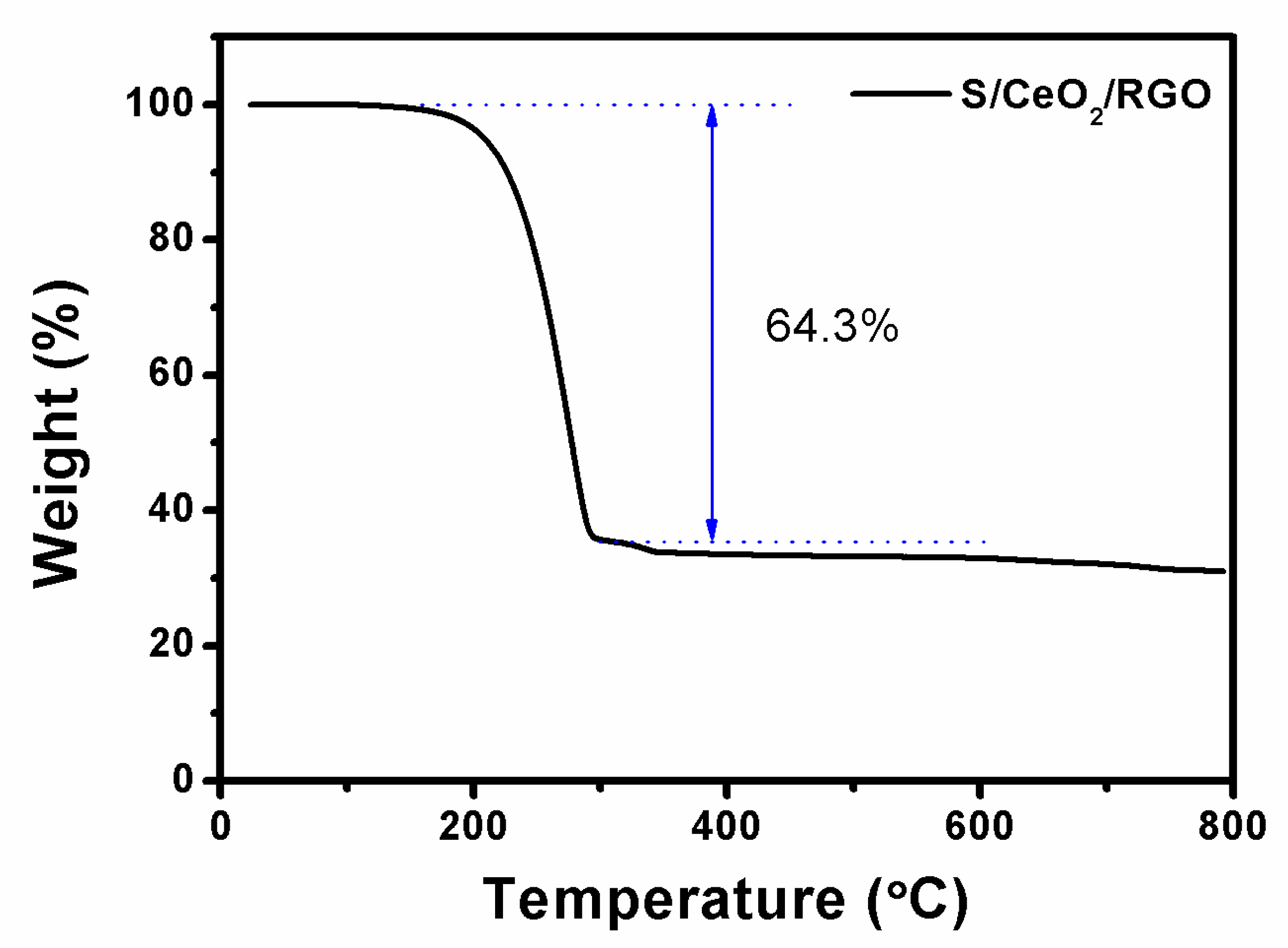
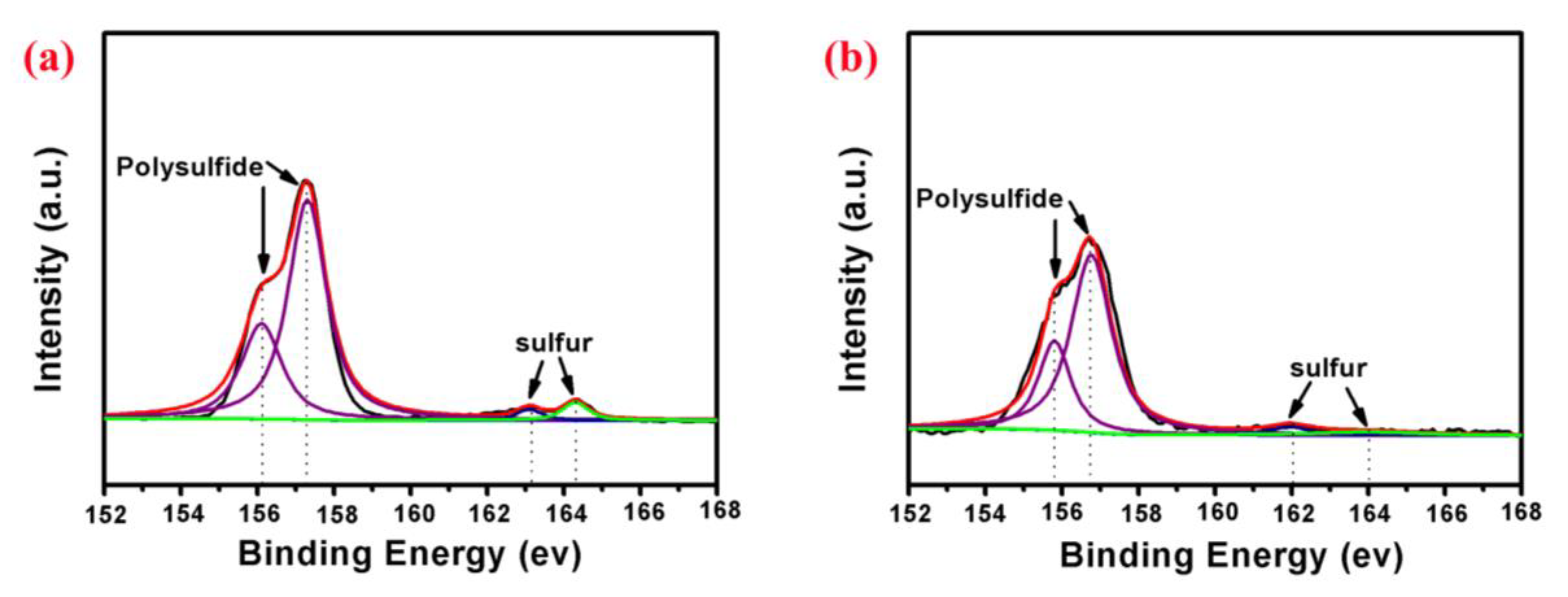
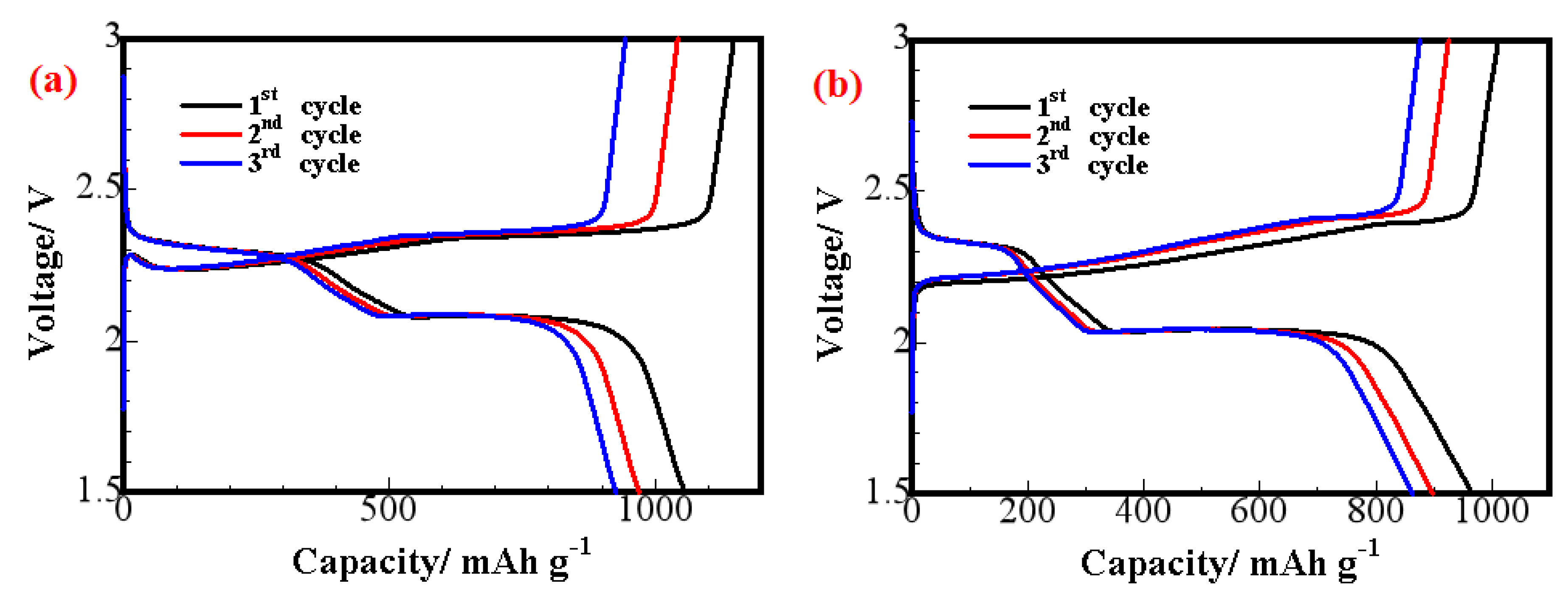
| Cathodes | Current Density (discharge) | Initial Discharge Capacity (mAh/g) | Discharge Capacity (after n th) (mAh/g) | Reference |
|---|---|---|---|---|
| SnO2@rGO/S | 0.1 C | 859 | 671 (50) | [37] |
| ZnO@S/CNT | 0.16 A/g | 988 | 942 (70) | [38] |
| MnO2@HCF/S | 0.5 C | 890 | 662 (300) | [39] |
| MgO@S | 0.2 C | 940 | 620 (100) | [40] |
| Fibrous rGO/S | 0.75 A/g | 710 | 541 (100) | [41] |
| 3D S/CeO2/RGO | 0.1 C | 1054 | 792 (200) | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Q.; Cui, G.; Tian, Y.; Tan, T.; Zhang, Y. Three-Dimensional S/CeO2/RGO Composites as Cathode Materials for Lithium–Sulfur Batteries. Materials 2018, 11, 1720. https://doi.org/10.3390/ma11091720
Hao Q, Cui G, Tian Y, Tan T, Zhang Y. Three-Dimensional S/CeO2/RGO Composites as Cathode Materials for Lithium–Sulfur Batteries. Materials. 2018; 11(9):1720. https://doi.org/10.3390/ma11091720
Chicago/Turabian StyleHao, Qiuyan, Guoliang Cui, Yuan Tian, Taizhe Tan, and Yongguang Zhang. 2018. "Three-Dimensional S/CeO2/RGO Composites as Cathode Materials for Lithium–Sulfur Batteries" Materials 11, no. 9: 1720. https://doi.org/10.3390/ma11091720
APA StyleHao, Q., Cui, G., Tian, Y., Tan, T., & Zhang, Y. (2018). Three-Dimensional S/CeO2/RGO Composites as Cathode Materials for Lithium–Sulfur Batteries. Materials, 11(9), 1720. https://doi.org/10.3390/ma11091720




