Impact of Two-Dimensional Particle Size Distribution on Estimation of Water Vapor Diffusivity in Micrometric Size Cellulose Particles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
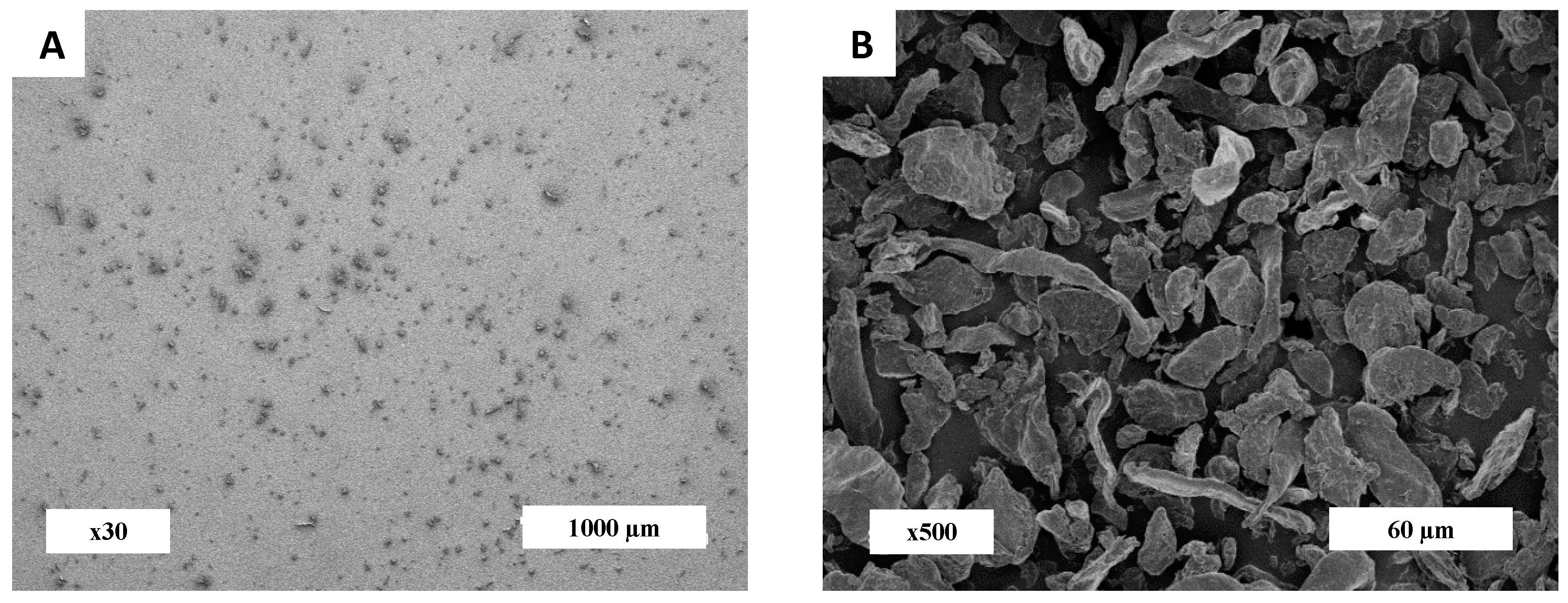
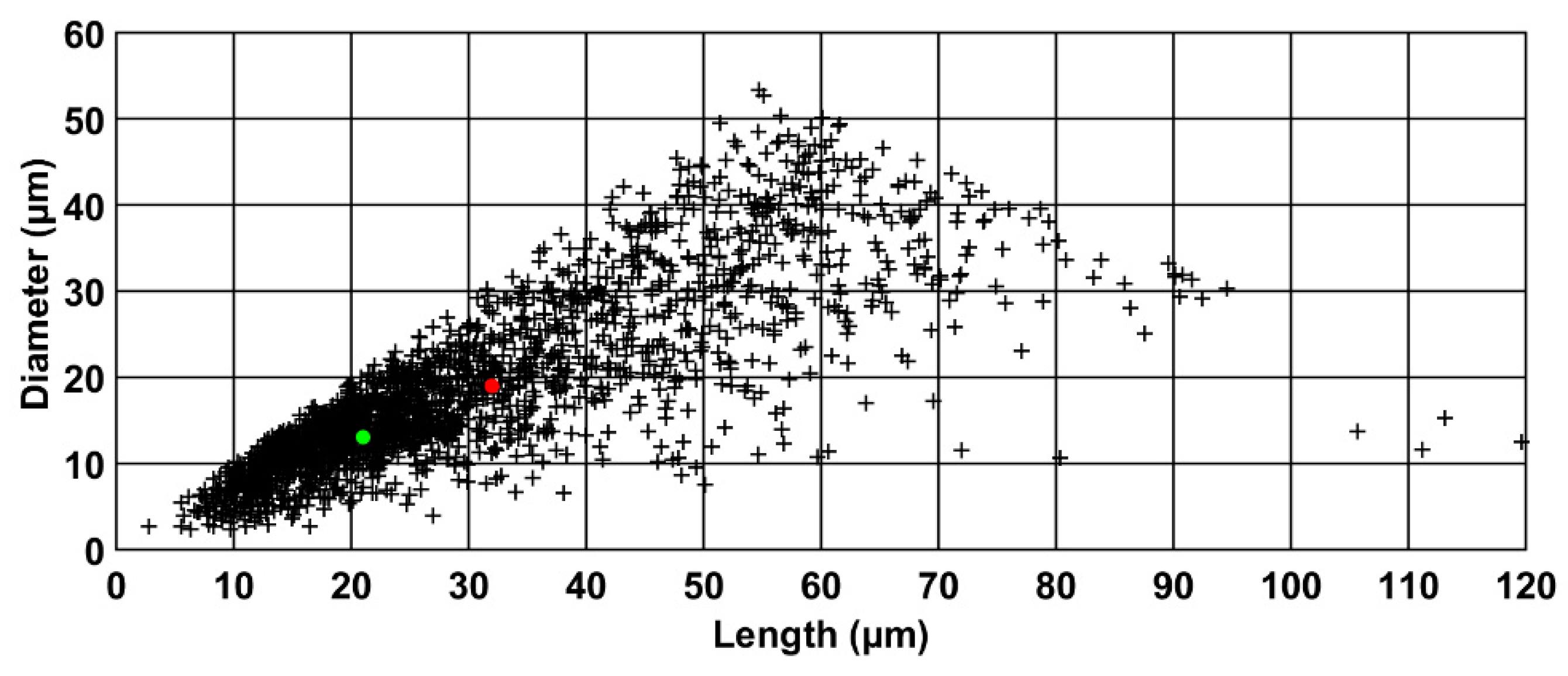
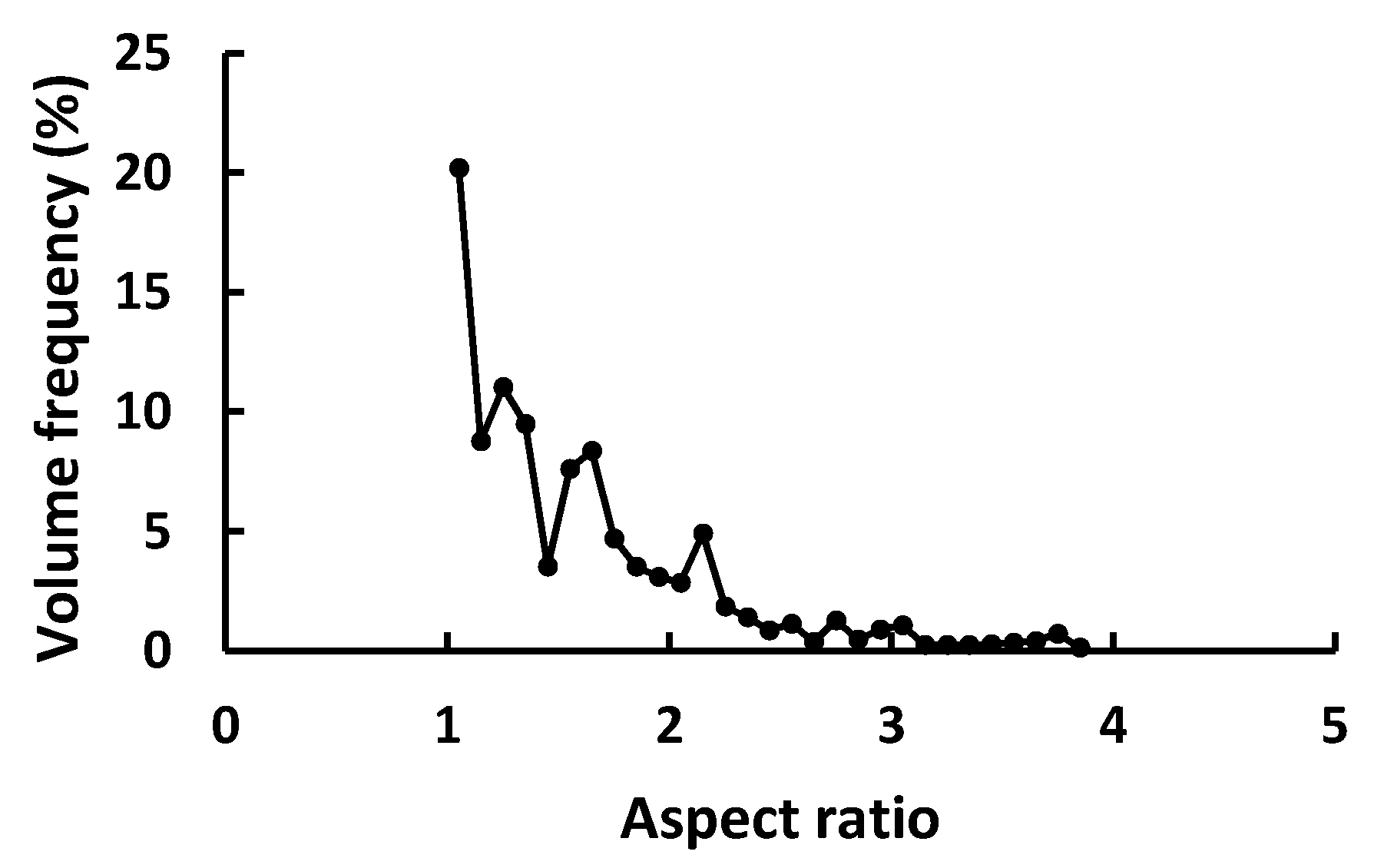
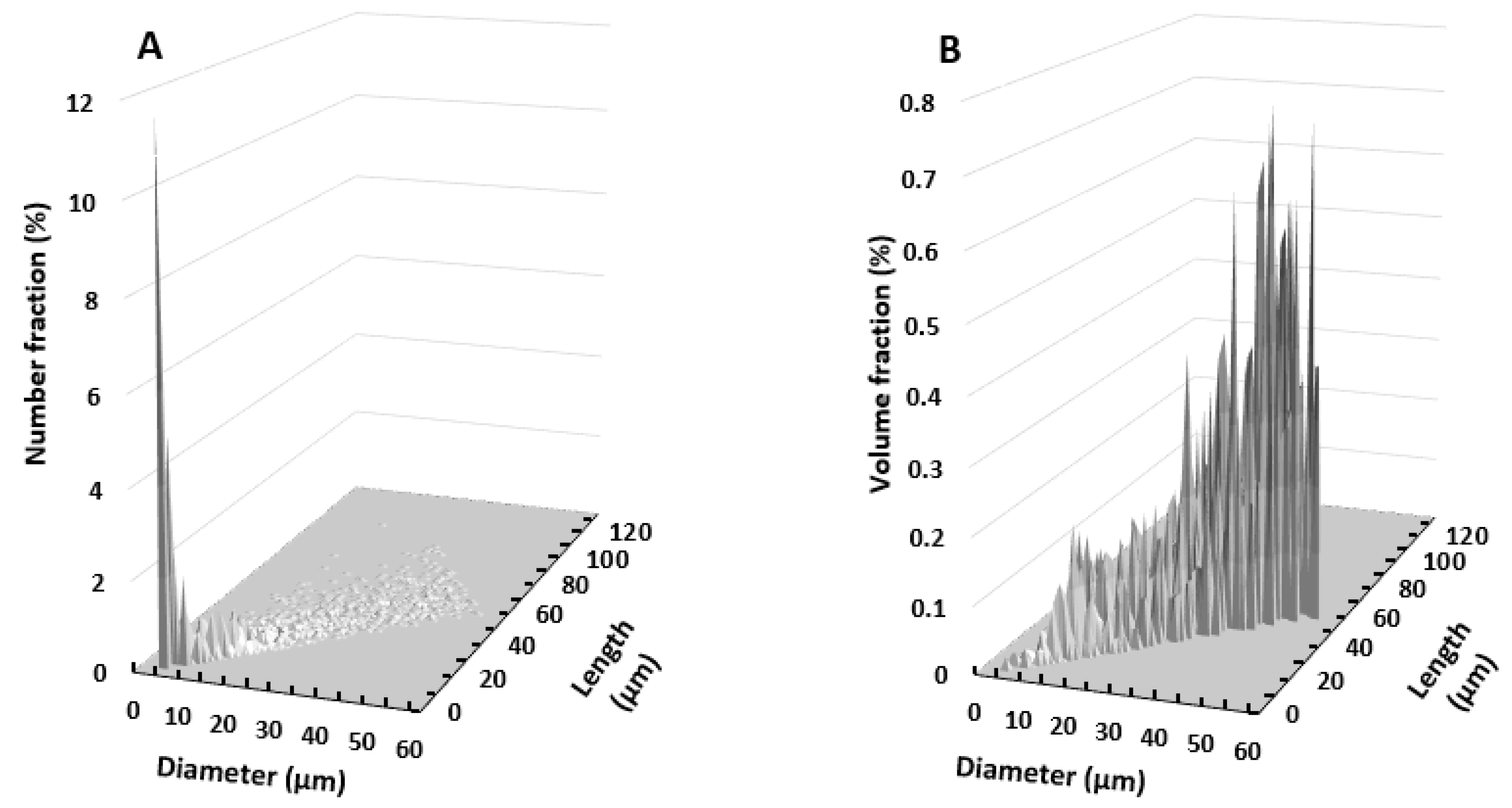
2.2.1. Particle Morphology
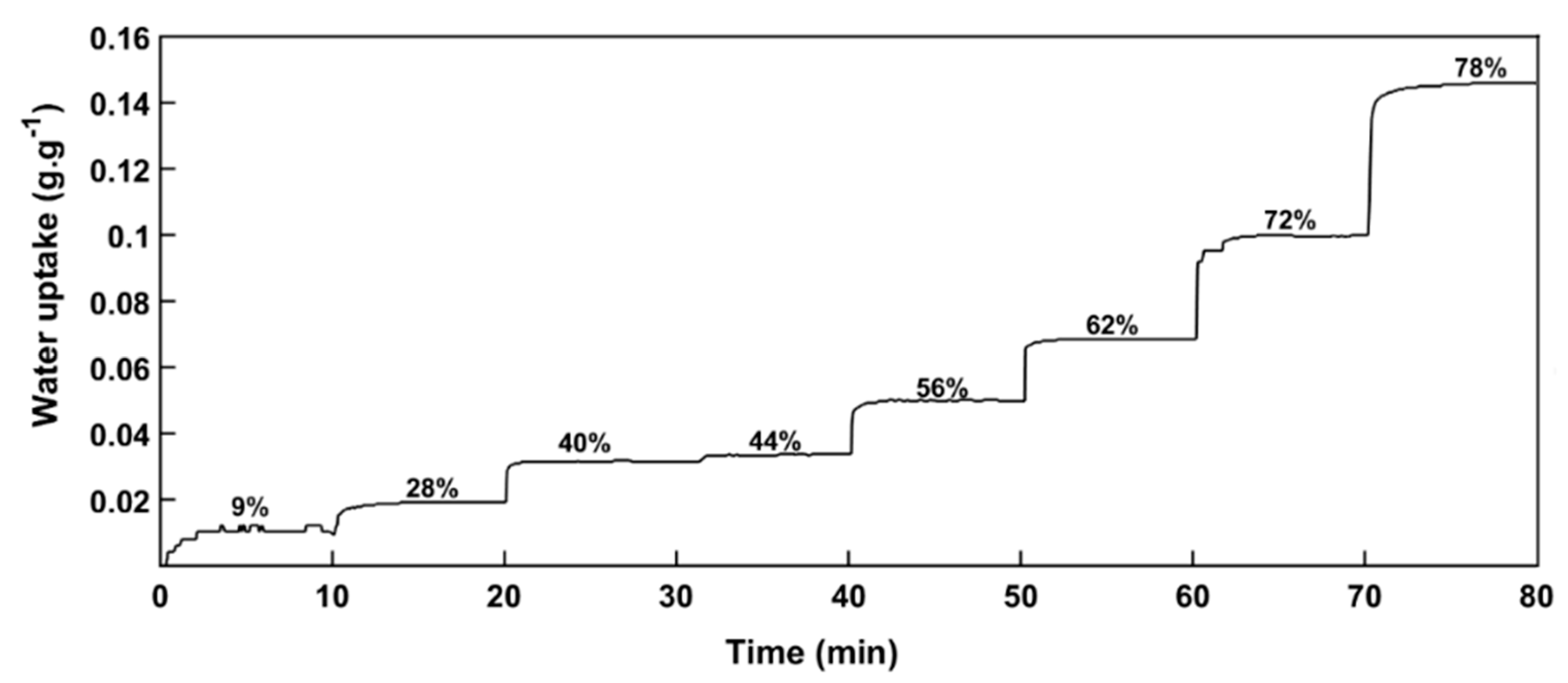
2.2.2. Water Vapor Sorption Kinetics
2.2.3. Modeling
2.2.4. Statistical Analysis
3. Results and Discussion
3.1. Morphological Analysis
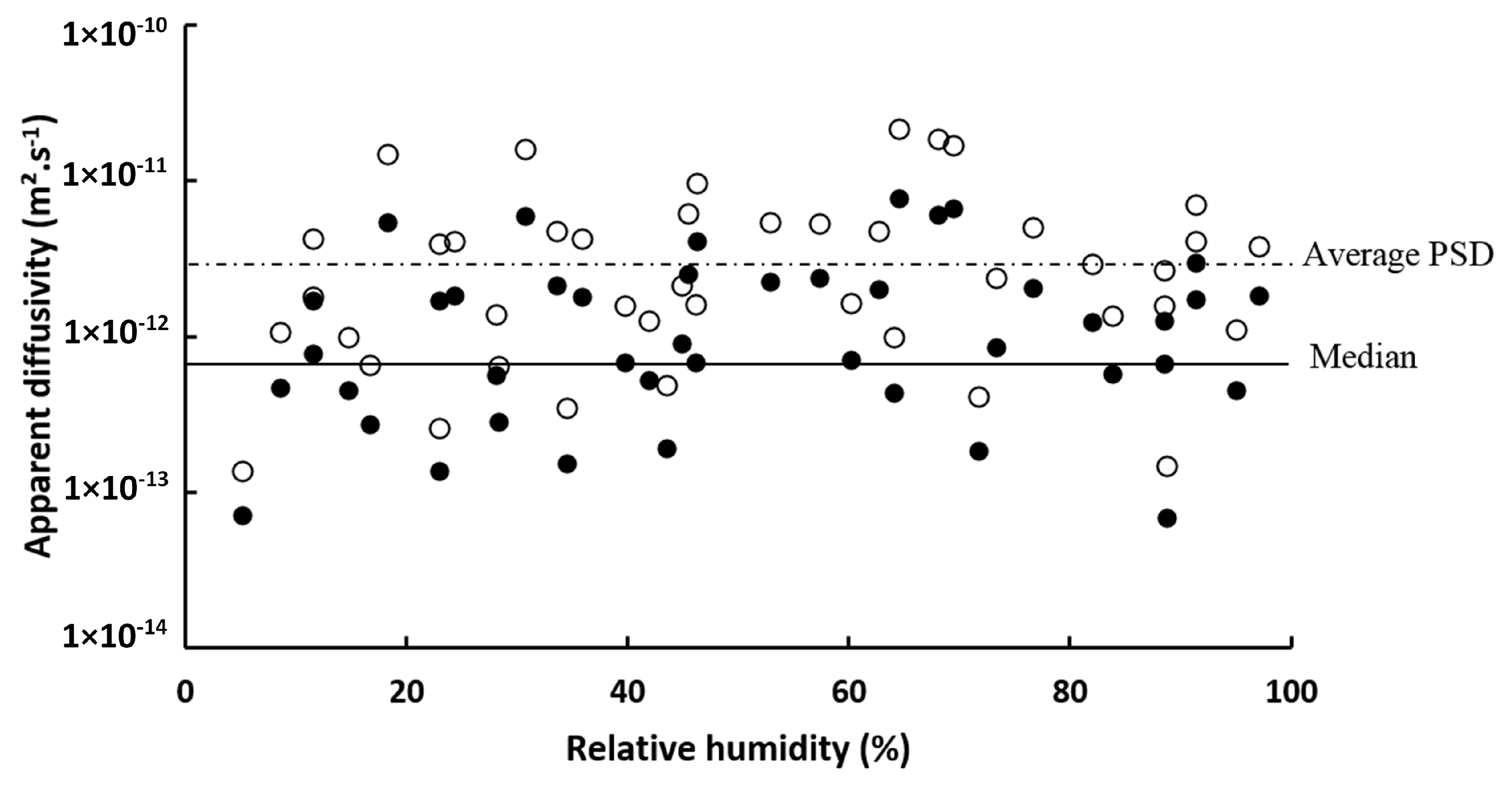
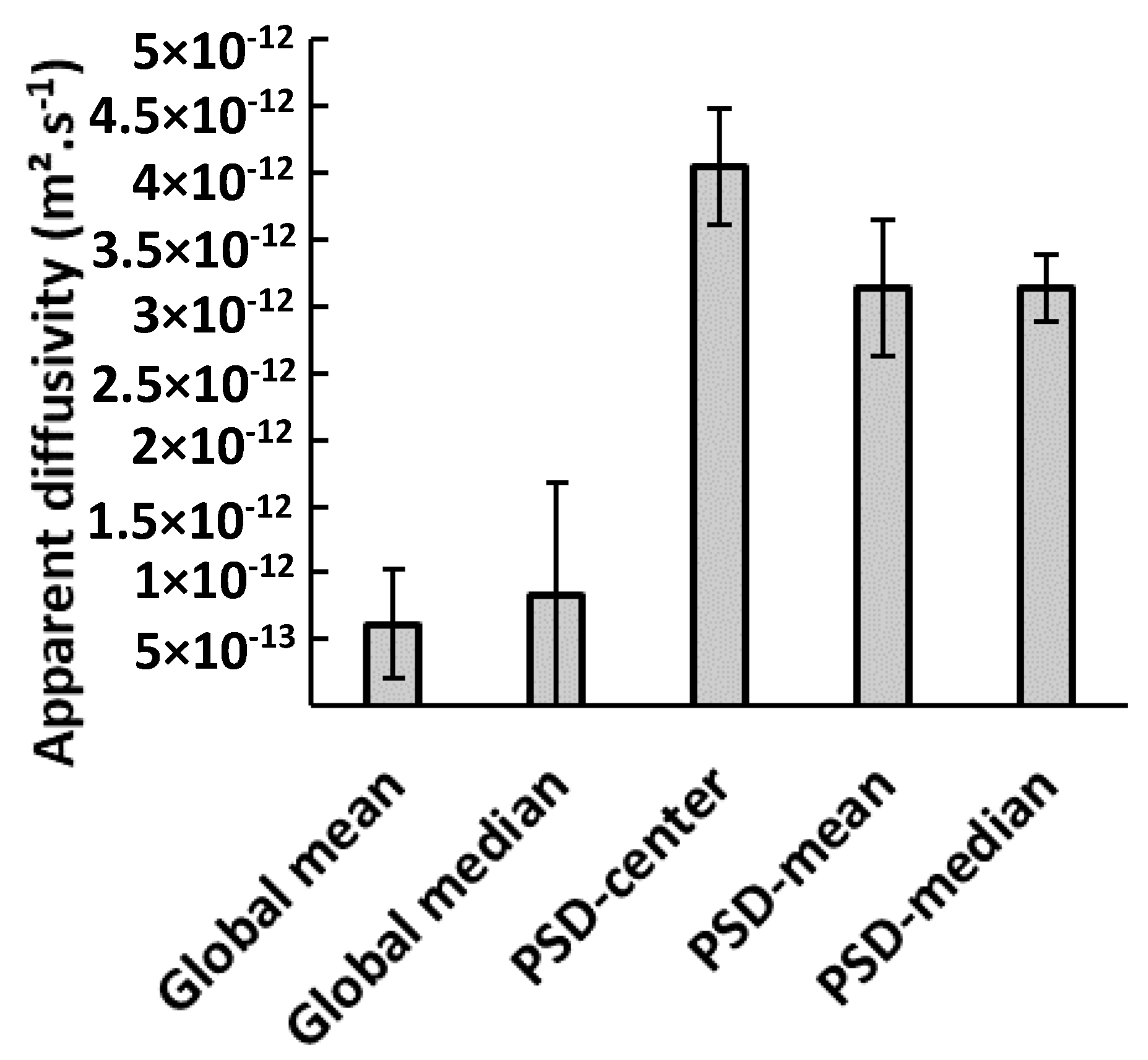
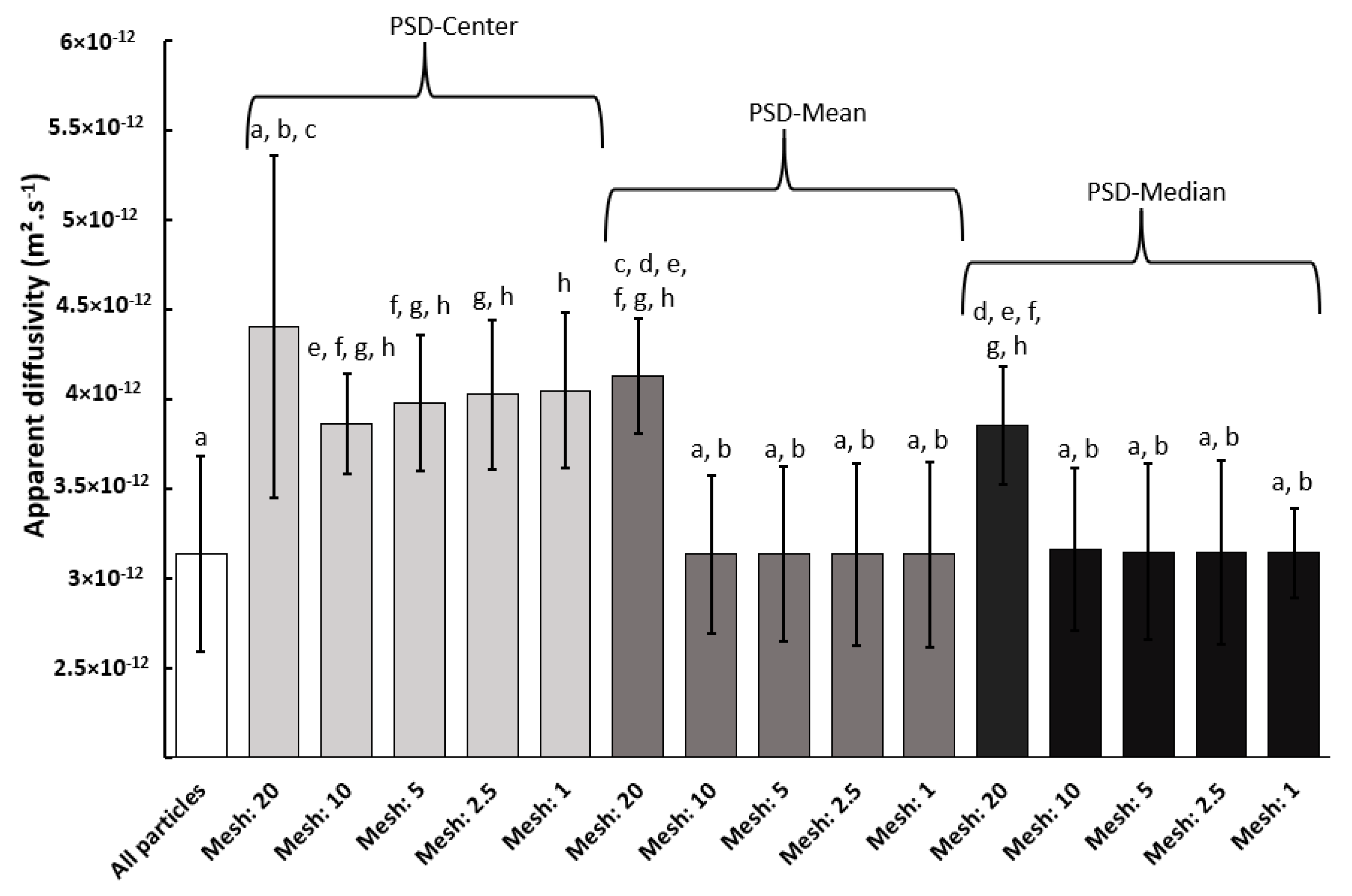
3.2. Water Vapor Diffusivity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mohanty, A.K.; Misra, M.; Drzal, L.T. (Eds.) Natural Fibers, Biopolymers, and Biocomposites; Taylor & Francis: Boca Raton, FL, USA, 2005; ISBN 978-0-8493-1741-5. [Google Scholar]
- Avérous, L.; Le Digabel, F. Properties of biocomposites based on lignocellulosic fillers. Carbohydr. Polym. 2006, 66, 480–493. [Google Scholar] [CrossRef]
- Azizi Samir, M.A.S.; Alloin, F.; Dufresne, A. Review of Recent Research into Cellulosic Whiskers, Their Properties and Their Application in Nanocomposite Field. Biomacromolecules 2005, 6, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Kamel, S. Nanotechnology and its applications in lignocellulosic composites, a mini review. Express Polym. Lett. 2007, 1, 546–575. [Google Scholar] [CrossRef]
- Minelli, M.; Giancinti Baschetti, M.; Doghieri, F.; Ankerfors, M.; Lindström, T.; Siro, I.; Plackett, D. Investigation of mass transport properties of MFC films. J. Membr. Sci. 2010, 358, 67–75. [Google Scholar] [CrossRef]
- Berthet, M.-A.; Angellier-Coussy, H.; Machado, D.; Hilliou, L.; Staebler, A.; Vicente, A.; Gontard, N. Exploring the potentialities of using lignocellulosic fibres derived from three food by-products as constituents of biocomposites for food packaging. Ind. Crops Prod. 2015, 69, 110–122. [Google Scholar] [CrossRef]
- Wolf, C.; Guillard, V.; Angellier-Coussy, H.; Silva, G.G.D.; Gontard, N. Water vapor sorption and diffusion in wheat straw particles and their impact on the mass transfer properties of biocomposites. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Wolf, C.; Angellier-Coussy, H.; Gontard, N.; Doghieri, F.; Guillard, V. How the shape of fillers affects the barrier properties of polymer/non-porous particles nanocomposites: A review. J. Membr. Sci. 2018, 556, 393–418. [Google Scholar] [CrossRef]
- Mazeau, K. The hygroscopic power of amorphous cellulose: A modeling study. Carbohydr. Polym. 2015, 117, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Kittle, J.D.; Du, X.; Jiang, F.; Qian, C.; Heinze, T.; Roman, M.; Esker, A.R. Equilibrium Water Contents of Cellulose Films Determined via Solvent Exchange and Quartz Crystal Microbalance with Dissipation Monitoring. Biomacromolecules 2011, 12, 2881–2887. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.-M.; Sin, L.T.; Tee, T.-T.; Bee, S.-T.; Hui, D.; Low, C.-Y.; Rahmat, A.R. Extraction of cellulose nanocrystals from plant sources for application as reinforcing agent in polymers. Compos. Part B Eng. 2015, 75, 176–200. [Google Scholar] [CrossRef]
- Popescu, C.-M.; Hill, C.A.S.; Curling, S.; Ormondroyd, G.; Xie, Y. The water vapour sorption behaviour of acetylated birch wood: How acetylation affects the sorption isotherm and accessible hydroxyl content. J. Mater. Sci. 2014, 49, 2362–2371. [Google Scholar] [CrossRef]
- Li, T.-Q.; Henriksson, U.; Klason, T.; Ödberg, L. Water diffusion in wood pulp cellulose fibers studied by means of the pulsed gradient spin-echo method. J. Colloid Interface Sci. 1992, 154, 305–315. [Google Scholar] [CrossRef]
- Hakalahti, M.; Faustini, M.; Boissière, C.; Kontturi, E.; Tammelin, T. Interfacial Mechanisms of Water Vapor Sorption into Cellulose Nanofibril Films as Revealed by Quantitative Models. Biomacromolecules 2017, 18, 2951–2958. [Google Scholar] [CrossRef] [PubMed]
- Mihranyan, A.; Llagostera, A.P.; Karmhag, R.; Strømme, M.; Ek, R. Moisture sorption by cellulose powders of varying crystallinity. Int. J. Pharm. 2004, 269, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Driemeier, C.; Mendes, F.M.; Oliveira, M.M. Dynamic vapor sorption and thermoporometry to probe water in celluloses. Cellulose 2012, 19, 1051–1063. [Google Scholar] [CrossRef]
- Strömme, M.; Mihranyan, A.; Ek, R.; Niklasson, G.A. Fractal dimension of cellulose powders analyzed by multilayer BET adsorption of water and nitrogen. J. Phys. Chem. B 2003, 107, 14378–14382. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Oxford University Press: Oxford, UK, 1975; ISBN 978-0-19-853411-2. [Google Scholar]
- Bessadok, A.; Langevin, D.; Gouanvé, F.; Chappey, C.; Roudesli, S.; Marais, S. Study of water sorption on modified agave fibres. Carbohydr. Polym. 2009, 76, 74–85. [Google Scholar] [CrossRef]
- Célino, A.; Fréour, S.; Jacquemin, F.; Casari, P. Characterization and modeling of the moisture diffusion behavior of natural fibers. J. Appl. Polym. Sci. 2013, 130, 297–306. [Google Scholar] [CrossRef]
- Tiaya Mbou, E.; Njeugna, E.; Kemajou, A.; Sikame, N.R.T.; Ndapeu, D. Modelling of the Water Absorption Kinetics and Determination of the Water Diffusion Coefficient in the Pith of Raffia vinifera of Bandjoun, Cameroon. Available online: https://www.hindawi.com/journals/amse/2017/1953087/ (accessed on 14 September 2017).
- Khazaei, J. Water absorption characteristics of three wood varieties. Cercetări Agronomice în Moldova 2008, 41, 134. [Google Scholar]
- Thoury-Monbrun, V.; Gaucel, S.; Rouessac, V.; Guillard, V.; Angellier-Coussy, H. Assessing the potential of quartz crystal microbalance to estimate water vapor transfer in micrometric size cellulose particles. Carbohydr. Polym. 2018, 190, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Gianfrancesco, A.; Vuataz, G.; Mesnier, X.; Meunier, V. New methods to assess water diffusion in amorphous matrices during storage and drying. Food Chem. 2012, 132, 1664–1670. [Google Scholar] [CrossRef]
- Carta, G.; Ubiera, A. Particle-size distribution effects in batch adsorption. AIChE J. 2003, 49, 3066–3073. [Google Scholar] [CrossRef]
- Kaczmarski, K.; Bellot, J.-C. Influence of particle diameter distribution on protein recovery in the expanded bed adsorption process. J. Chromatogr. A 2005, 1069, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Angellier-Coussy, H.; Gastaldi, E.; Da Silva, F.C.; Gontard, N.; Guillard, V. Nanoparticle size and water diffusivity in nanocomposite agro-polymer based films. Eur. Polym. J. 2013, 49, 299–306. [Google Scholar] [CrossRef]
- Matthews, J.; Walker, R.L. Mathematical Methods of Physics; W.A. Benjamin: San Francisco, CA, USA, 1970. [Google Scholar]
- Stockbridge, C.D.; Warner, A.W.; Behrndt, K.H. Vacuum Microbalance Techniques; Proceedings of the Los Angeles Conference; Plenum Press: New York, NY, USA, 1963; Volume 3. [Google Scholar]
- Wilson, B.P.; Yliniemi, K.; Gestranius, M.; Hakalahti, M.; Putkonen, M.; Lundström, M.; Karppinen, M.; Tammelin, T.; Kontturi, E. Structural distinction due to deposition method in ultrathin films of cellulose nanofibres. Cellulose 2018, 25, 1715–1724. [Google Scholar] [CrossRef]
- Villares, A.; Moreau, C.; Dammak, A.; Capron, I.; Cathala, B. Kinetic aspects of the adsorption of xyloglucan onto cellulose nanocrystals. Soft Matter 2015, 11, 6472–6481. [Google Scholar] [CrossRef] [PubMed]
- Fuchiwaki, Y.; Tanaka, M.; Makita, Y.; Ooie, T. New Approach to a Practical Quartz Crystal Microbalance Sensor Utilizing an Inkjet Printing System. Sensors 2014, 14, 20468–20479. [Google Scholar] [CrossRef] [PubMed]
- Bedane, A.H.; Eić, M.; Farmahini-Farahani, M.; Xiao, H. Theoretical modeling of water vapor transport in cellulose-based materials. Cellulose 2016, 23, 1537–1552. [Google Scholar] [CrossRef]
| Diffusivity (×10−12 m2·s−1 ) | |
|---|---|
| Median | 0.8 ± 1.0 |
| All particles | 3.1 ± 2.3 |
| Center, mesh 20 | 4.4 ± 2.9 |
| Mean: mesh 20 | 4.1 ± 2.3 |
| Median: mesh 20 | 3.8 ± 2.3 |
| Center, mesh 10 | 3.9 ± 3.3 |
| Mean: mesh 10 | 3.1 ± 2.4 |
| Median: mesh 10 | 3.2 ± 2.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thoury-Monbrun, V.; Angellier-Coussy, H.; Guillard, V.; Legland, D.; Gaucel, S. Impact of Two-Dimensional Particle Size Distribution on Estimation of Water Vapor Diffusivity in Micrometric Size Cellulose Particles. Materials 2018, 11, 1712. https://doi.org/10.3390/ma11091712
Thoury-Monbrun V, Angellier-Coussy H, Guillard V, Legland D, Gaucel S. Impact of Two-Dimensional Particle Size Distribution on Estimation of Water Vapor Diffusivity in Micrometric Size Cellulose Particles. Materials. 2018; 11(9):1712. https://doi.org/10.3390/ma11091712
Chicago/Turabian StyleThoury-Monbrun, Valentin, Hélène Angellier-Coussy, Valérie Guillard, David Legland, and Sébastien Gaucel. 2018. "Impact of Two-Dimensional Particle Size Distribution on Estimation of Water Vapor Diffusivity in Micrometric Size Cellulose Particles" Materials 11, no. 9: 1712. https://doi.org/10.3390/ma11091712
APA StyleThoury-Monbrun, V., Angellier-Coussy, H., Guillard, V., Legland, D., & Gaucel, S. (2018). Impact of Two-Dimensional Particle Size Distribution on Estimation of Water Vapor Diffusivity in Micrometric Size Cellulose Particles. Materials, 11(9), 1712. https://doi.org/10.3390/ma11091712





