Green Preparation of Straw Fiber Reinforced Hydrolyzed Soy Protein Isolate/Urea/Formaldehyde Composites for Biocomposite Flower Pots Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Biocomposite Flower Pot (BFP) Fabrication
2.3. Soil Culture Experiment
2.4. Mechanical Property and Weight Loss Tests after Biodegradation
2.5. Characterization
3. Results and Discussion
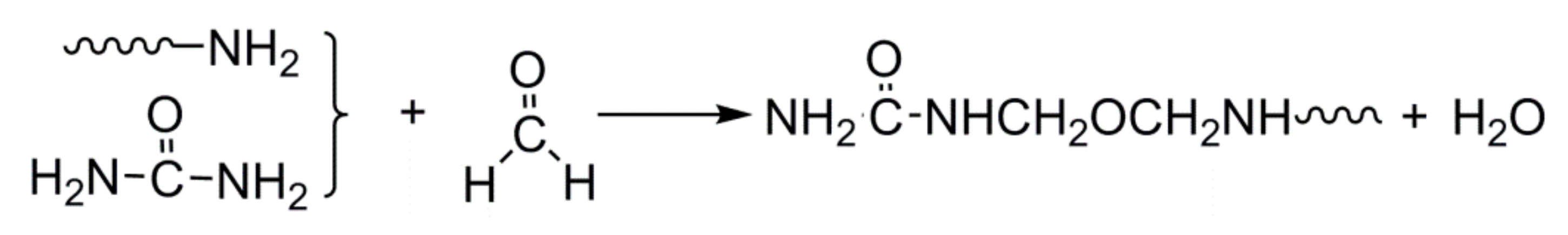
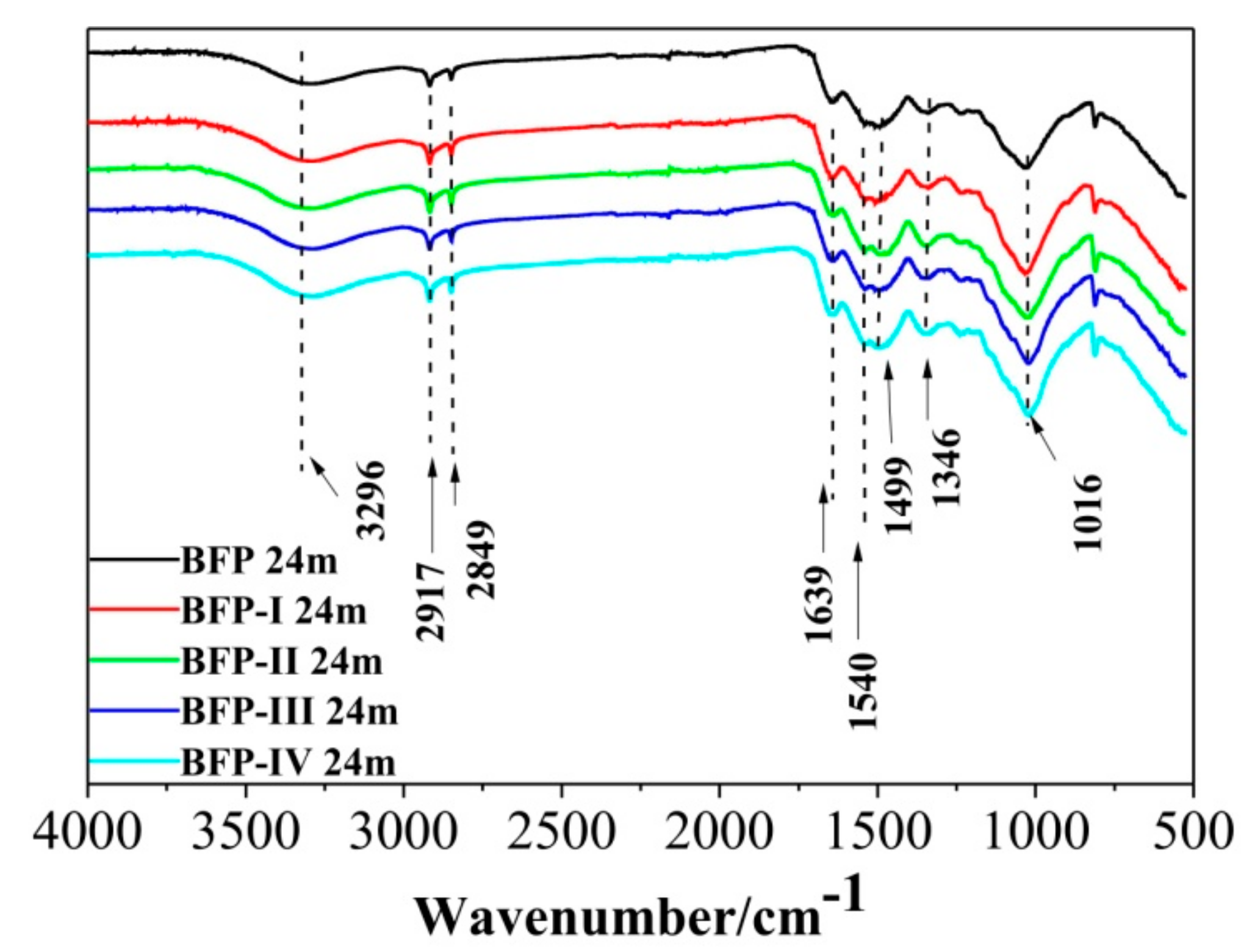
3.1. Chemical Structure
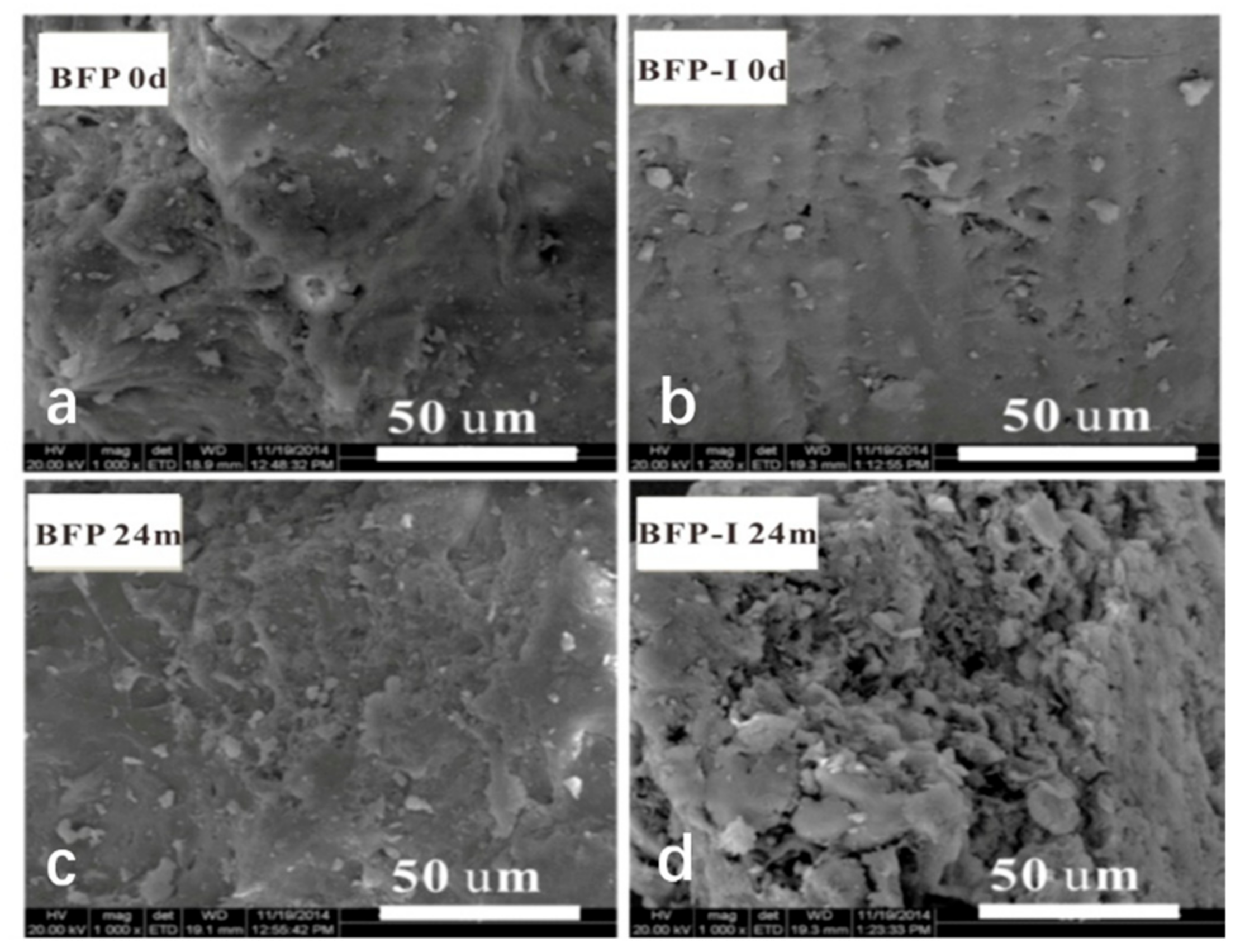
3.2. Morphology and Mechanical Tensile Property
3.3. Biodegradation in Soil
3.4. Thermal Property
3.5. X-ray Diffraction Analysis
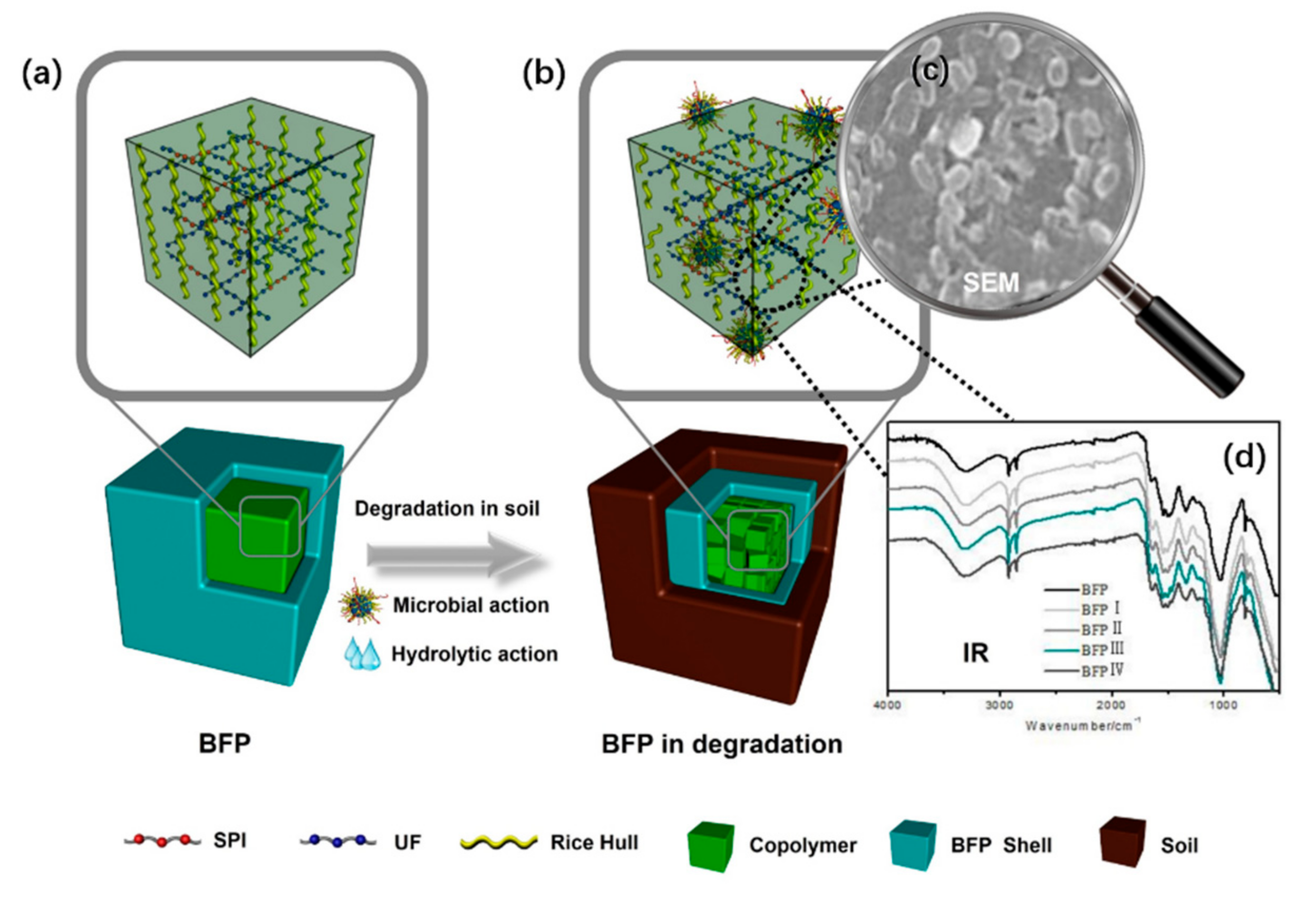
3.6. Degrading Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, P.; Liao, G. A Novel Fluorescent Biosensor for Adenosine Triphosphate Detection Based on a Metal–Organic Framework Coating Polydopamine Layer. Materials 2018, 11, 1616. [Google Scholar] [CrossRef] [PubMed]
- Khazaeian, A.; Ashori, A.; Dizaj, M.Y. Suitability of sorghum stalk fibers for production of particleboard. Carbohydr. Polym. 2015, 120, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.; Chen, J.; Zeng, W.; Yu, C.; Yi, C.; Xu, Z. Facile Preparation of Uniform Nanocomposite Spheres with Loading Silver Nanoparticles on Polystyrene-methyl Acrylic Acid Spheres for Catalytic Reduction of 4-Nitrophenol. J. Phys. Chem. C 2016, 120, 25935–25944. [Google Scholar] [CrossRef]
- Liao, G.; Li, Q.; Zhao, W.; Pang, Q.; Gao, H.; Xu, Z. In-situ construction of novel silver nanoparticle decorated polymeric spheres as highly active and stable catalysts for reduction of methylene blue dye. Appl. Catal. A Gen. 2018, 549, 102–111. [Google Scholar] [CrossRef]
- Yara-Varón, E.; Fabiano-Tixier, A.S.; Balcells, M.; Canela-Garayoa, R.; Bily, A.; Chemat, F. Is it possible to substitute hexane with green solvents for extraction of carotenoids? A theoretical versus experimental solubility study. RSC Adv. 2016, 6, 27750–27759. [Google Scholar] [CrossRef]
- Xia, T.; Huang, H.; Wu, G.; Sun, E.; Jin, X.; Tang, W. The characteristic changes of rice straw fibers in anaerobic digestion and its effect on rice straw-reinforced composites. Ind. Crops Prod. 2018, 121, 73–79. [Google Scholar] [CrossRef]
- Otoni, C.G.; Lodi, B.D.; Lorevice, M.V.; Leitão, R.C.; Ferreira, M.D.; de Moura, M.R.; Mattoso, L.H.C. Optimized and scaled-up production of cellulose-reinforced biodegradable composite films made up of carrot processing waste. Ind. Crops Prod. 2018, 121, 66–72. [Google Scholar] [CrossRef]
- Qu, P.; Huang, H.; Wu, G.; Sun, E.; Chang, Z. Hydrolyzed soy protein isolates modified urea–formaldehyde resins as adhesives and its biodegradability. J. Adhes. Sci. Technol. 2015, 29, 2381–2398. [Google Scholar] [CrossRef]
- Liao, G.; Gong, Y.; Yi, C.; Xu, Z. Soluble, Antibaterial, and Anticorrosion Studies of Sulfonated Polystyrene/Polyaniline/Silver Nanocomposites Prepared with the Sulfonated Polystyrene Template. Chin. J. Chem. 2017, 35, 1157–1164. [Google Scholar] [CrossRef]
- Liao, G.; Zhao, W.; Li, Q.; Pang, Q.; Xu, Z. Novel Poly(acrylic acid)-modified Tourmaline/Silver Composites for Adsorption Removal of Cu(II) ions and Catalytic Reduction of Methylene Blue in Water. Chem. Lett. 2017, 46, 1631–1634. [Google Scholar] [CrossRef]
- Sugiyama, M.; Takahashi, M.; Katsube, T.; Koyama, A.; Itamura, H. Effects of Applied Nitrogen Amounts on the Functional Components of Mulberry (Morus alba L.) Leaves. J. Agric. Food Chem. 2016, 64, 6923–6929. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lopez-Nieves, S.; Goldman, I.L.; Maeda, H.A. Limited Tyrosine Utilization Explains Lower Betalain Contents in Yellow than in Red Table Beet Genotypes. J. Agric. Food Chem. 2017, 65, 4305–4313. [Google Scholar] [CrossRef]
- Kale, G.; Kijchavengkul, T.; Auras, R.; Rubino, M.; Selke, S.E.; Singh, S.P. Compostability of bioplastic packaging materials: An overview. Macromol. Biosci. 2007, 7, 255–277. [Google Scholar] [CrossRef] [PubMed]
- Oguz, O.; Bilge, K.; Simsek, E.; Citak, M.K.; Wis, A.A.; Ozkoc, G.; Menceloglu, Y.Z. High-Performance Green Composites of Poly(lactic acid) and Waste Cellulose Fibers Prepared by High-Shear Thermokinetic Mixing. Ind. Eng. Chem. Res. 2017, 56, 8568–8579. [Google Scholar] [CrossRef]
- Xiao, L.; Zeng, W.; Liao, G.; Yi, C.; Xu, Z. Thermally and Chemically Stable Candle Soot Superhydrophobic Surface with Excellent Self-Cleaning Properties in Air and Oil. ACS Appl. Nano Mater. 2018, 1, 1204–1211. [Google Scholar] [CrossRef]
- Kasirajan, S.; Ngouajio, M. Polyethylene and biodegradable mulches for agricultural applications: A review. Agron. Sustain. Dev. 2012, 32, 501–529. [Google Scholar] [CrossRef]
- Brodhagen, M.; Peyron, M.; Miles, C.; Inglis, D.A. Biodegradable plastic agricultural mulches and key features of microbial degradation. Appl. Microbiol. Biotechnol. 2015, 99, 1039–1056. [Google Scholar] [CrossRef] [PubMed]
- McCullough, F.; Jones, S.; Vignali, D. The pot snack market—Are today’s consumers demanding health as well as convenience? Int. J. Consum. Stud. 2003, 27, 248. [Google Scholar] [CrossRef]
- Pelaez-Samaniego, M.R.; Hummel, R.L.; Liao, W.; Ma, J.; Jensen, J.; Kruger, C.; Frear, C. Approaches for adding value to anaerobically digested dairy fiber. Renew. Sustain. Energy Rev. 2017, 72, 254–268. [Google Scholar] [CrossRef]
- Gao, W.; Du, G.; Ma, H.; Li, J. Dynamic mechanical analysis of urea formaldehyde resin modified by ammonium pentaborate as wood adhesive. Polym. Compos. 2016, 37, 2404–2410. [Google Scholar] [CrossRef]
- Duan, H.; Qiu, T.; Guo, L.; Ye, J.; Li, X. The microcapsule-type formaldehyde scavenger: The preparation and the application in urea-formaldehyde adhesives. J. Hazard. Mater. 2015, 293, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Lv, M.; Yang, X. The effect of humidity on formaldehyde emission parameters of a medium-density fiberboard: Experimental observations and correlations. Build. Environ. 2016, 101, 110–115. [Google Scholar] [CrossRef]
- Ayrilmis, N.; Kaymakci, A. Reduction of formaldehyde emission from light MDF panels by adding chestnut shell flour. Holzforschung 2012, 66, 443–446. [Google Scholar] [CrossRef]
- Pirayesh, H.; Khanjanzadeh, H.; Salari, A. Effect of using walnut/almond shells on the physical, mechanical properties and formaldehyde emission of particleboard. Compos. Part B Eng. 2013, 45, 858–863. [Google Scholar] [CrossRef]
- Essawy, H.A.; Tawfik, M.E.; Elsayed, N.H. Effect of addition of glycolysis products of poly(ethyleneterephthalate) wastes to urea-formaldehyde resin on its adhesion performance to wood substrates and formaldehyde emission. J. Appl. Polym. Sci. 2012, 123, 2377–2383. [Google Scholar] [CrossRef]
- Yamanaka, S.; Magara, K.; Hirabayashi, Y.; Fujimoto, T.; Kuga, Y. Reduction of formaldehyde emission from plywood using composite resin composed of resorcinol–formaldehyde and urea-modified scallop shell nanoparticles. Wood Sci. Technol. 2016, 51, 297–308. [Google Scholar] [CrossRef] [Green Version]
- Wan, G.; Xia, H.; Wang, J.; Liu, J.; Song, B.; Yang, Y.; Bai, Q. Synthesis of SiO2@SiO2 core-shell microspheres using urea-formaldehyde polymers as the templates for fast separation of small solutes and proteins. Chin. Chem. Lett. 2018, 29, 213–216. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, N.; Liu, L.; Liu, Y. Surface sedimentation and adherence of Nano-SiO2 to improve thermal stability and flame resistance of melamine-formaldehyde foam via sol-gel method. Fire Mater. 2018, 42, 183–189. [Google Scholar] [CrossRef]
- Zeng, W.; Chen, J.; Yang, H.; Deng, L.; Liao, G.; Xu, Z. Robust coating with superhydrophobic and self-cleaning properties in either air or oil based on natural zeolite. Surf. Coat. Technol. 2017, 309, 1045–1051. [Google Scholar] [CrossRef]
- Ayrilmis, N.; Lee, Y.-K.; Kwon, J.H.; Han, T.-H.; Kim, H.-J. Formaldehyde emission and VOCs from LVLs produced with three grades of urea-formaldehyde resin modified with nanocellulose. Build. Environ. 2016, 97, 82–87. [Google Scholar] [CrossRef]
- Moubarik, A.; Mansouri, H.R.; Pizzi, A.; Allal, A.; Charrier, F.; Badia, M.A.; Charrier, B. Evaluation of mechanical and physical properties of industrial particleboard bonded with a corn flour–urea formaldehyde adhesive. Compos. Part B Eng. 2013, 44, 48–51. [Google Scholar] [CrossRef]
- Otake, Y.; Kobayashi, T.; Asabe, H.; Murakami, N.; Ono, K. Biodegradation of low-density polyethylene, polystyrene, polyvinyl chloride, and urea formaldehyde resin buried under soil for over 32 years. J. Appl. Polym. Sci. 1995, 56, 1789–1796. [Google Scholar] [CrossRef]
- Qu, P.; Huang, H.; Wu, G.; Sun, E.; Chang, Z. Effects of hydrolysis degree of soy protein isolate on the structure and performance of hydrolyzed soy protein isolate/urea/formaldehyde copolymer resin. J. Appl. Polym. Sci. 2015, 132, 41469–41477. [Google Scholar] [CrossRef]
- Xuan, L.; Hui, D.; Cheng, W.; Wong, A.H.H.; Han, G.; Tan, W.K.; Tawi, C.A.D. Effect of Preservative Pretreatment on the Biological Durability of Corn Straw Fiber/HDPE Composites. Materials 2017, 10, 789. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, M.; Liu, H.M. Emulsifying and physicochemical properties of soy hull hemicelluloses-soy protein isolate conjugates. Carbohydr. Polym. 2017, 163, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Qu, P.; Huang, H.; Wu, G.; Sun, E.; Chang, Z. The effect of hydrolyzed soy protein isolate on the structure and biodegradability of urea–formaldehyde adhesives. J. Adhes. Sci. Technol. 2015, 29, 502–517. [Google Scholar] [CrossRef]
- Wu, C.-S.; Liao, H.-T.; Cai, Y.-X. Characterisation, biodegradability and application of palm fibre-reinforced polyhydroxyalkanoate composites. Polym. Degrad. Stab. 2017, 140, 55–63. [Google Scholar] [CrossRef]
- Nakasaki, K.; Hirai, H. Temperature control strategy to enhance the activity of yeast inoculated into compost raw material for accelerated composting. Waste Manag. 2017, 65, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Georgopoulos, S.T.; Tarantili, P.A.; Avgerinos, E.; Andreopoulos, A.G.; Koukios, E.G. Thermoplastic polymers reinforced with fibrous agricultural residues. Polym. Degrad. Stab. 2005, 90, 303–312. [Google Scholar] [CrossRef]
- Sung, G.; Kim, J.H. Influence of filler surface characteristics on morphological, physical, acoustic properties of polyurethane composite foams filled with inorganic fillers. Compos. Sci. Technol. 2017, 146, 147–154. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, Z.; Huang, Z.L.; Dong, M.; Yu, X.L.; Ning, P. A new strategy for co-composting dairy manure with rice straw: Addition of different inocula at three stages of composting. Waste Manag. 2015, 40, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Jurado, M.M.; Suarez-Estrella, F.; Lopez, M.J.; Vargas-Garcia, M.C.; Lopez-Gonzalez, J.A.; Moreno, J. Enhanced turnover of organic matter fractions by microbial stimulation during lignocellulosic waste composting. Bioresour. Technol. 2015, 186, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liao, G.; Zhang, S.; Pang, L.; Tong, H.; Zhao, W.; Xu, Z. Effect of adjustable molecular chain structure and pure silica zeolite nanoparticles on thermal, mechanical, dielectric, UV-shielding and hydrophobic properties of fluorinated copolyimide composites. Appl. Surf. Sci. 2017, 427, 437–450. [Google Scholar] [CrossRef]
- Li, Q.; Liao, G.; Tian, J.; Xu, Z. Preparation of Novel Fluorinated Copolyimide/Amine-Functionalized Sepia Eumelanin Nanocomposites with Enhanced Mechanical, Thermal, and UV-Shielding Properties. Macromol. Mater. Eng. 2018, 303, 1700407. [Google Scholar] [CrossRef]
- Xiao, L.; Deng, M.; Zeng, W.; Zhang, B.; Xu, Z.; Yi, C.; Liao, G. Novel Robust Superhydrophobic Coating with Self-Cleaning Properties in Air and Oil Based on Rare Earth Metal Oxide. Ind. Eng. Chem. Res. 2017, 56, 12354–12361. [Google Scholar] [CrossRef]
- Li, Q.; Li, J.; Liao, G.; Xu, Z. The preparation of heparin-like hyperbranched polyimides and their antithrombogenic, antibacterial applications. J. Mater. Sci. Mater. Med. 2018, 29, 126. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Jin, H.; Sun, F.; Dai, X.; Xu, Z.; Yang, S.; Liao, G. Design and Synthesis of a Lead Sulfide Based Nanotheranostic Agent for Computer Tomography/Magnetic Resonance Dual-Mode-Bioimaging-Guided Photothermal Therapy. ACS Appl. Nano Mater. 2018, 1, 2294–2305. [Google Scholar] [CrossRef]
- Gnanasekaran, D.; Shanavas, A.; Focke, W.W.; Sadiku, R. Polyhedral oligomeric silsesquioxane/polyamide bio-nanocomposite membranes: Structure-gas transport properties. RSC Adv. 2015, 5, 11272–11283. [Google Scholar] [CrossRef]
- Rojo, E.; Alonso, M.V.; Domínguez, J.C.; Saz-Orozco, B.D.; Oliet, M.; Rodriguez, F. Alkali treatment of viscose cellulosic fibers from eucalyptus wood: Structural, morphological, and thermal analysis. J. Appl. Polym. Sci. 2013, 130, 2198–2204. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, H. X-ray diffraction study of bamboo fibers treated with NaOH. Fibers Polym. 2009, 9, 735–739. [Google Scholar] [CrossRef]
- Sebe, G.; Ham-Pichavant, F.; Ibarboure, E.; Koffi, A.L.; Tingaut, P. Supramolecular structure characterization of cellulose II nanowhiskers produced by acid hydrolysis of cellulose I substrates. Biomacromolecules 2012, 13, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Asada, C.; Sasaki, C.; Hirano, T.; Nakamura, Y. Chemical characteristics and enzymatic saccharification of lignocellulosic biomass treated using high-temperature saturated steam: Comparison of softwood and hardwood. Bioresour. Technol. 2015, 182, 245–250. [Google Scholar] [CrossRef] [PubMed]


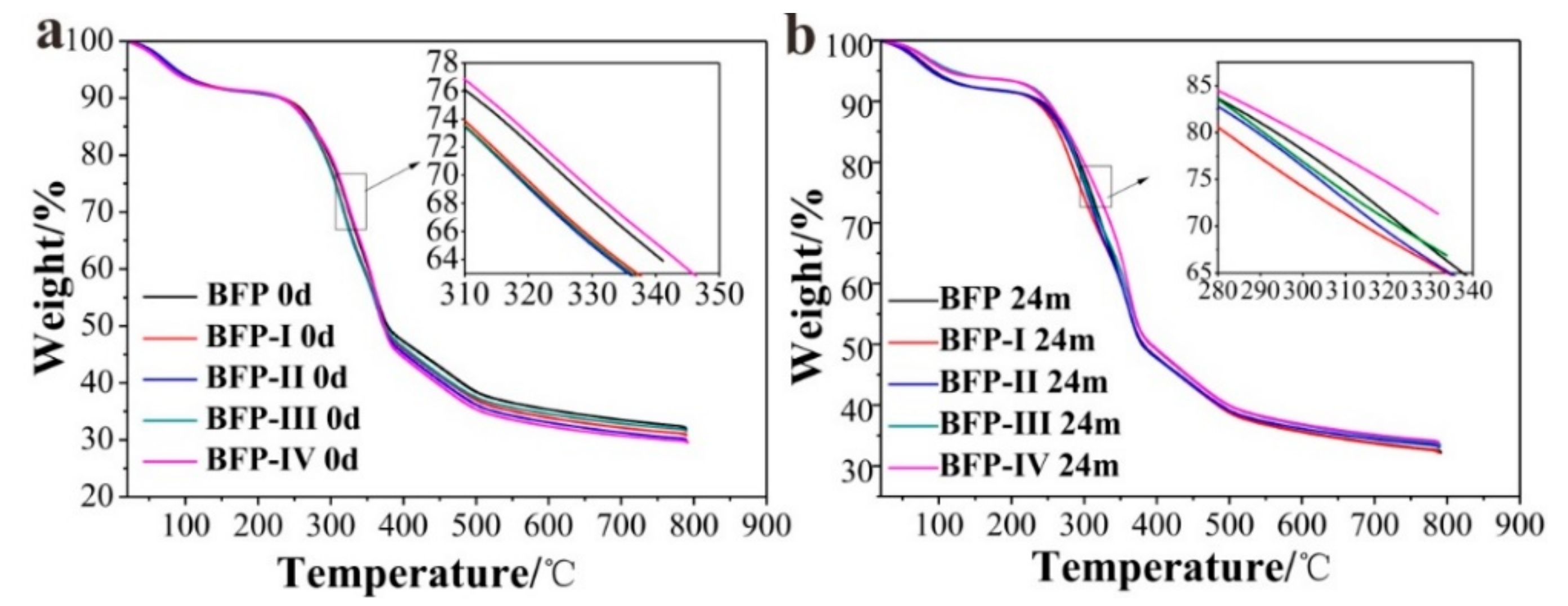

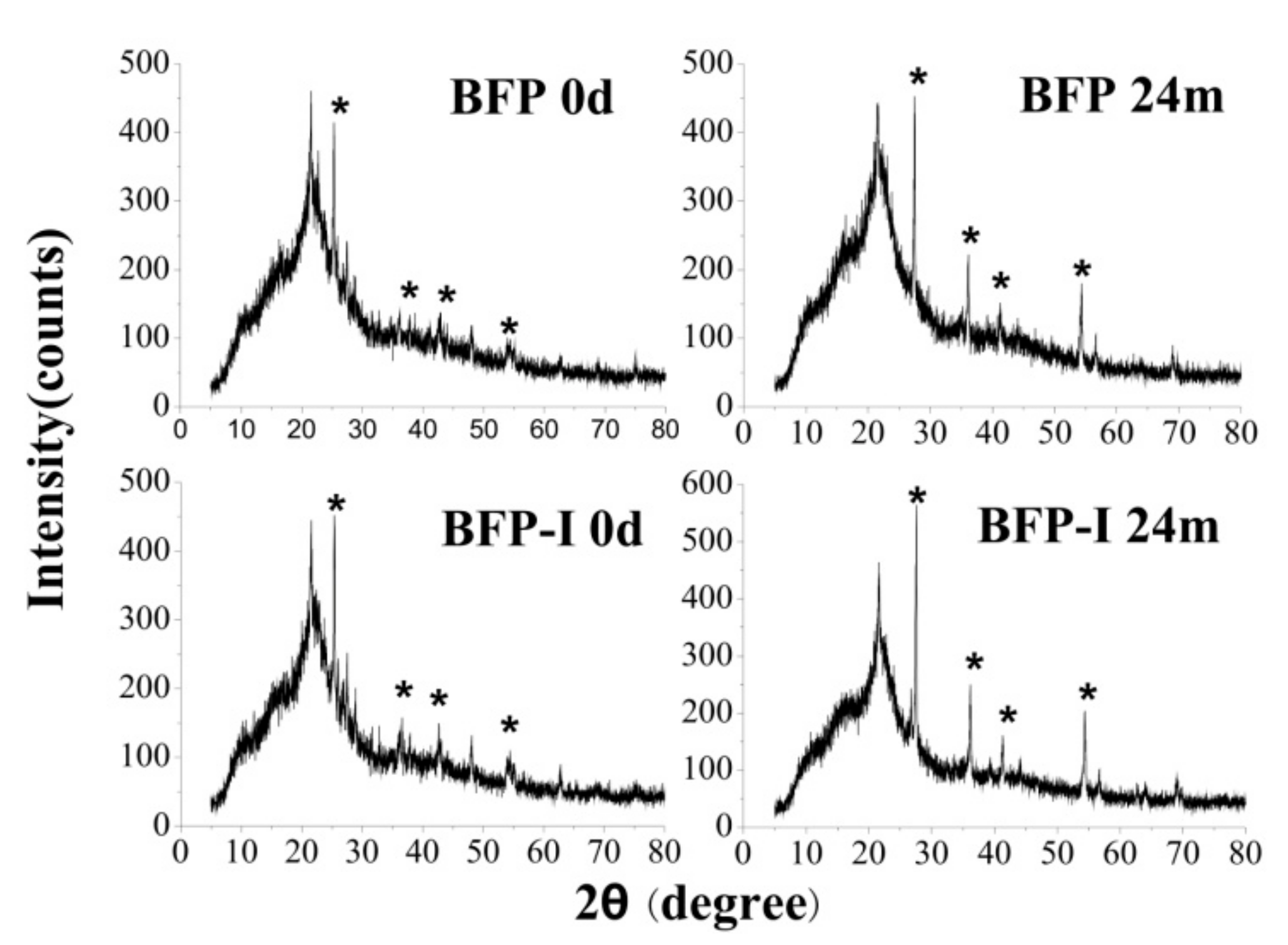
| Sample | HSPI Content (%) | Td, 30% (°C) | Rwc (wt %) | ||
|---|---|---|---|---|---|
| Td, 30% (0 m) a | Td, 30% (24 m) b | Rw (0 m) a | Rw (24 m) b | ||
| BFP | 0 | 325.42 | 323.48 | 31.77 | 32.10 |
| BFP-I | 6 | 315.60 | 316.01 | 30.72 | 32.14 |
| BFP-II | 9 | 317.25 | 318.09 | 29.87 | 33.04 |
| BFP-III | 18 | 318.38 | 322.14 | 31.49 | 33.36 |
| BFP-IV | 25 | 326.98 | 336.04 | 29.46 | 33.81 |
| Sample | HSPI Content (%) | Onset Temperature (°C) | Peak Temperature (°C) | ΔHf (J/g) | |||
|---|---|---|---|---|---|---|---|
| OT (0 m) | OT (24 m) | PT (0 m) | PT (24 m) | ΔHf (0 m) | ΔHf (24 m) | ||
| BFP | 0 | 49.71 | 37.42 | 121.27 | 98.46 | 184.3 | 141.1 |
| BFP-I | 6 | 53.48 | 76.17 | 115.71 | 112.50 | 143.2 | 136.5 |
| BFP-II | 9 | 52.56 | 75.33 | 114.46 | 112.22 | 139.8 | 137.2 |
| BFP-III | 18 | 50.25 | 53.95 | 114.41 | 113.80 | 132.1 | 126.1 |
| BFP-IV | 25 | 57.86 | 60.52 | 115.71 | 113.62 | 105.1 | 119.7 |
| Samples | CrI (%) | Increase Rate of CrI (%) | |
|---|---|---|---|
| CrI (0 m) | CrI (24 m) | ||
| BFP | 49.45 | 53.09 | 7.36 |
| BFP-I | 41.25 | 50.74 | 23.01 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, E.; Liao, G.; Zhang, Q.; Qu, P.; Wu, G.; Xu, Y.; Yong, C.; Huang, H. Green Preparation of Straw Fiber Reinforced Hydrolyzed Soy Protein Isolate/Urea/Formaldehyde Composites for Biocomposite Flower Pots Application. Materials 2018, 11, 1695. https://doi.org/10.3390/ma11091695
Sun E, Liao G, Zhang Q, Qu P, Wu G, Xu Y, Yong C, Huang H. Green Preparation of Straw Fiber Reinforced Hydrolyzed Soy Protein Isolate/Urea/Formaldehyde Composites for Biocomposite Flower Pots Application. Materials. 2018; 11(9):1695. https://doi.org/10.3390/ma11091695
Chicago/Turabian StyleSun, Enhui, Guangfu Liao, Qian Zhang, Ping Qu, Guofeng Wu, Yueding Xu, Cheng Yong, and Hongying Huang. 2018. "Green Preparation of Straw Fiber Reinforced Hydrolyzed Soy Protein Isolate/Urea/Formaldehyde Composites for Biocomposite Flower Pots Application" Materials 11, no. 9: 1695. https://doi.org/10.3390/ma11091695






