Nanosecond Laser Fabrication of Hydrophobic Stainless Steel Surfaces: The Impact on Microstructure and Corrosion Resistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of Hydrophobic Surfaces
2.3. Characterization
2.4. Wettability Measurements
2.5. Electrochemical Measurements
3. Results
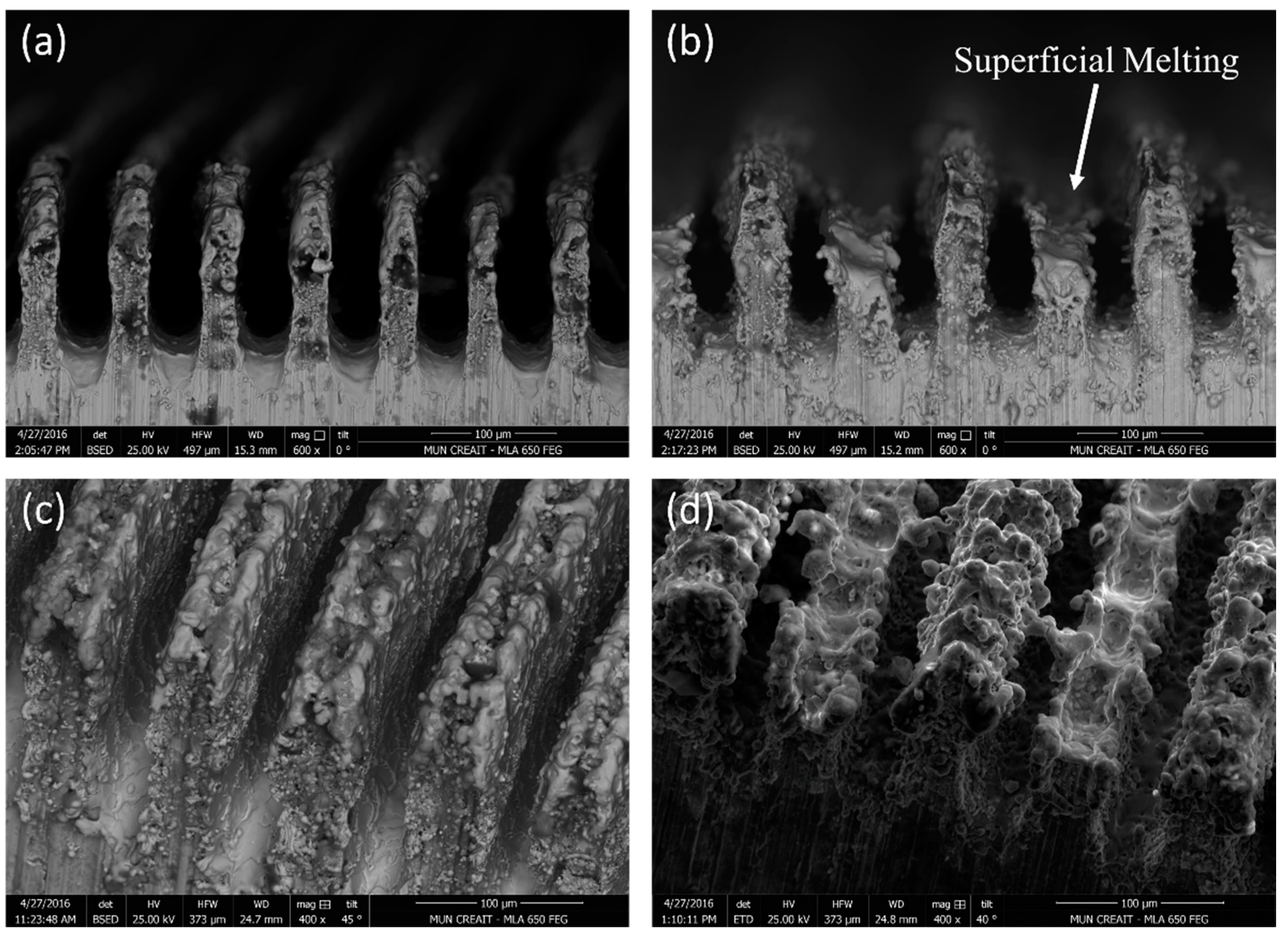
3.1. Surface Morphology
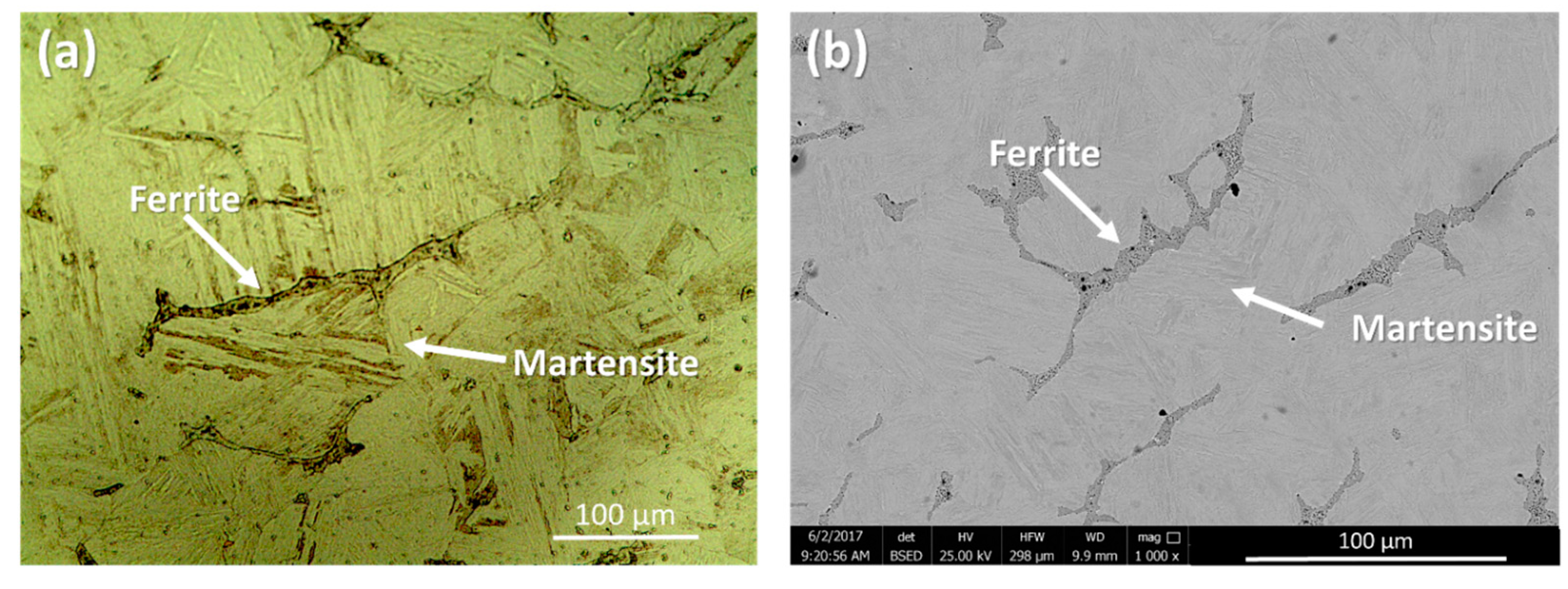
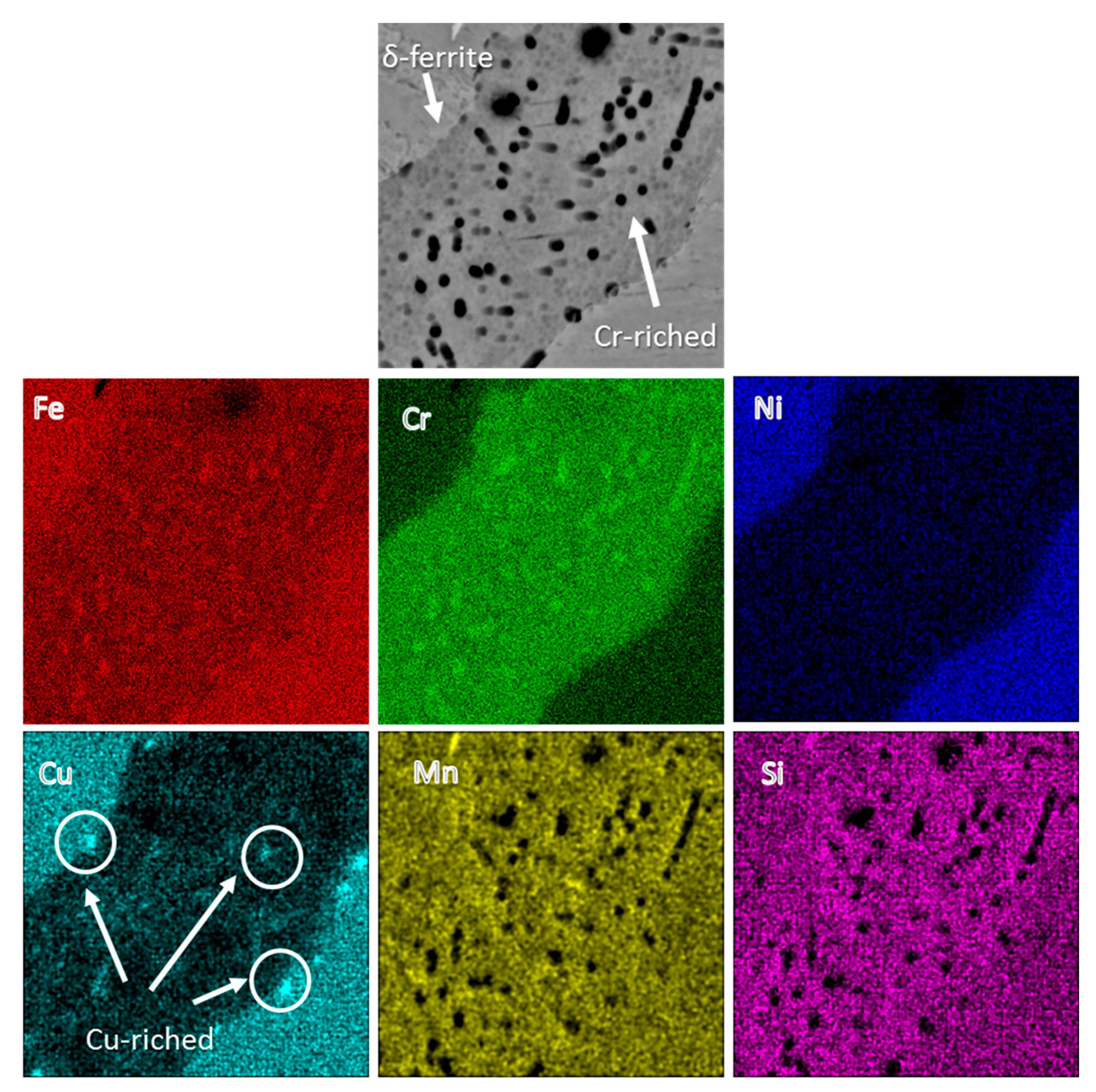
3.2. Microstructure
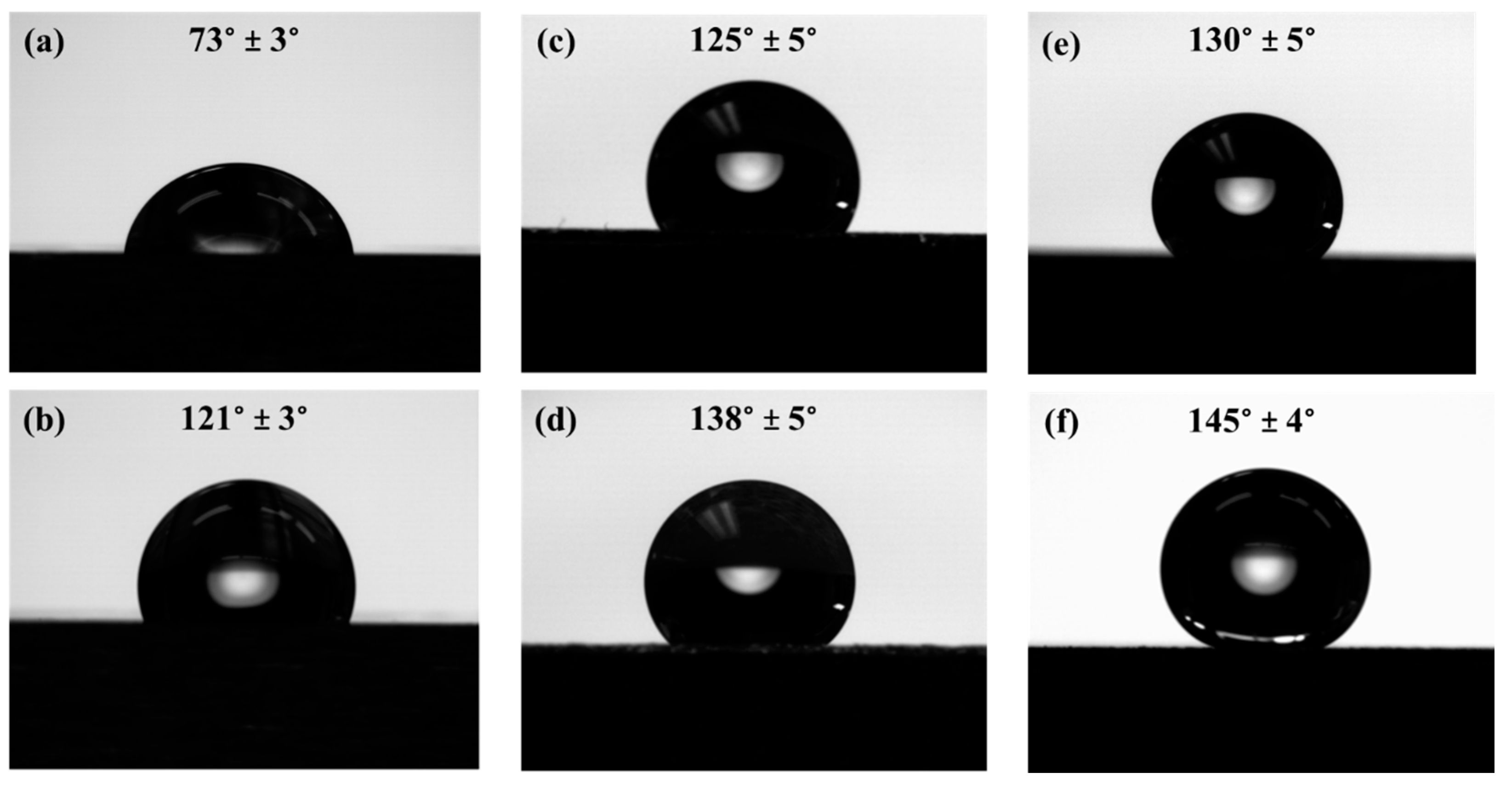
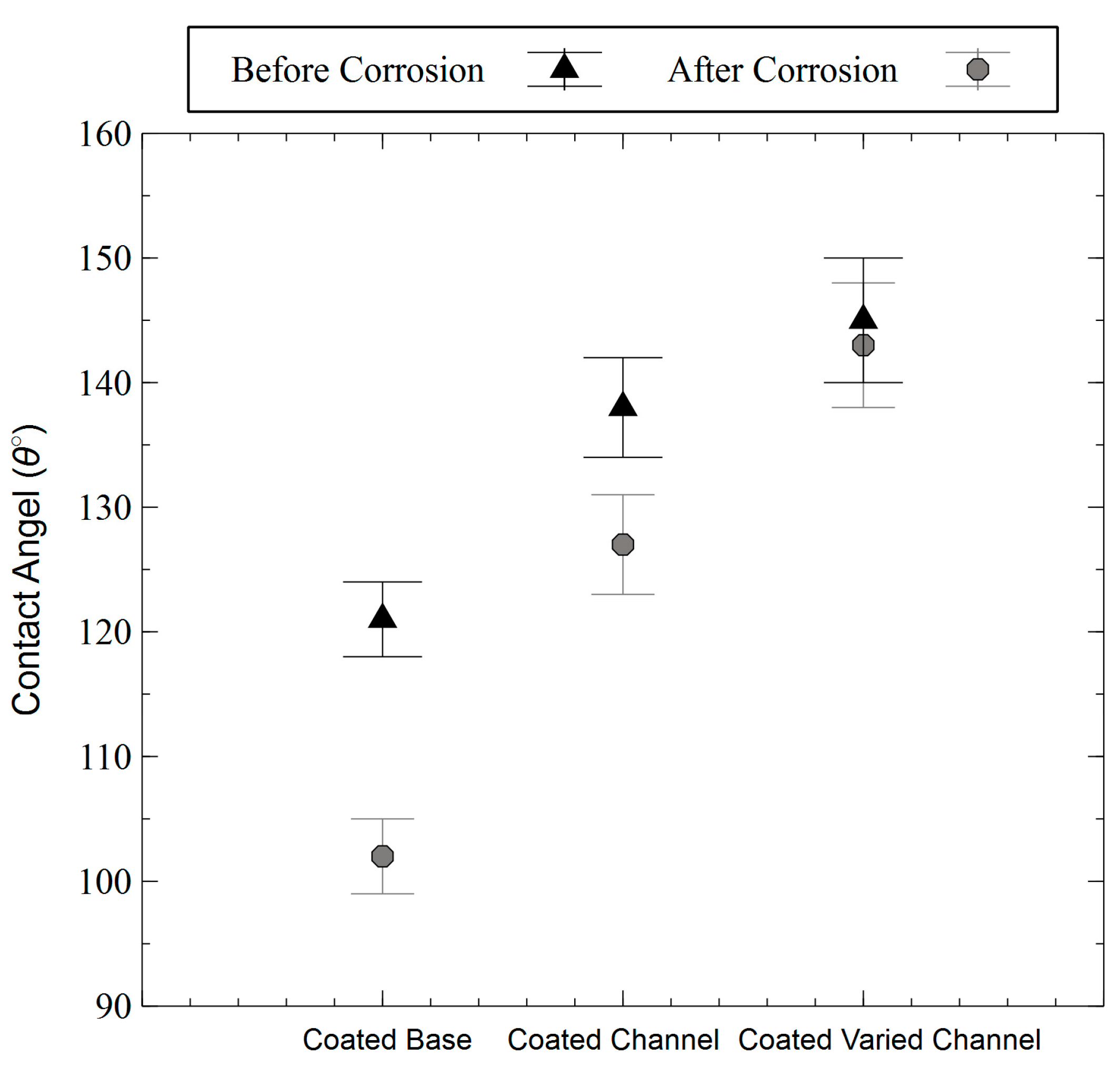
3.3. Wetting Behavior
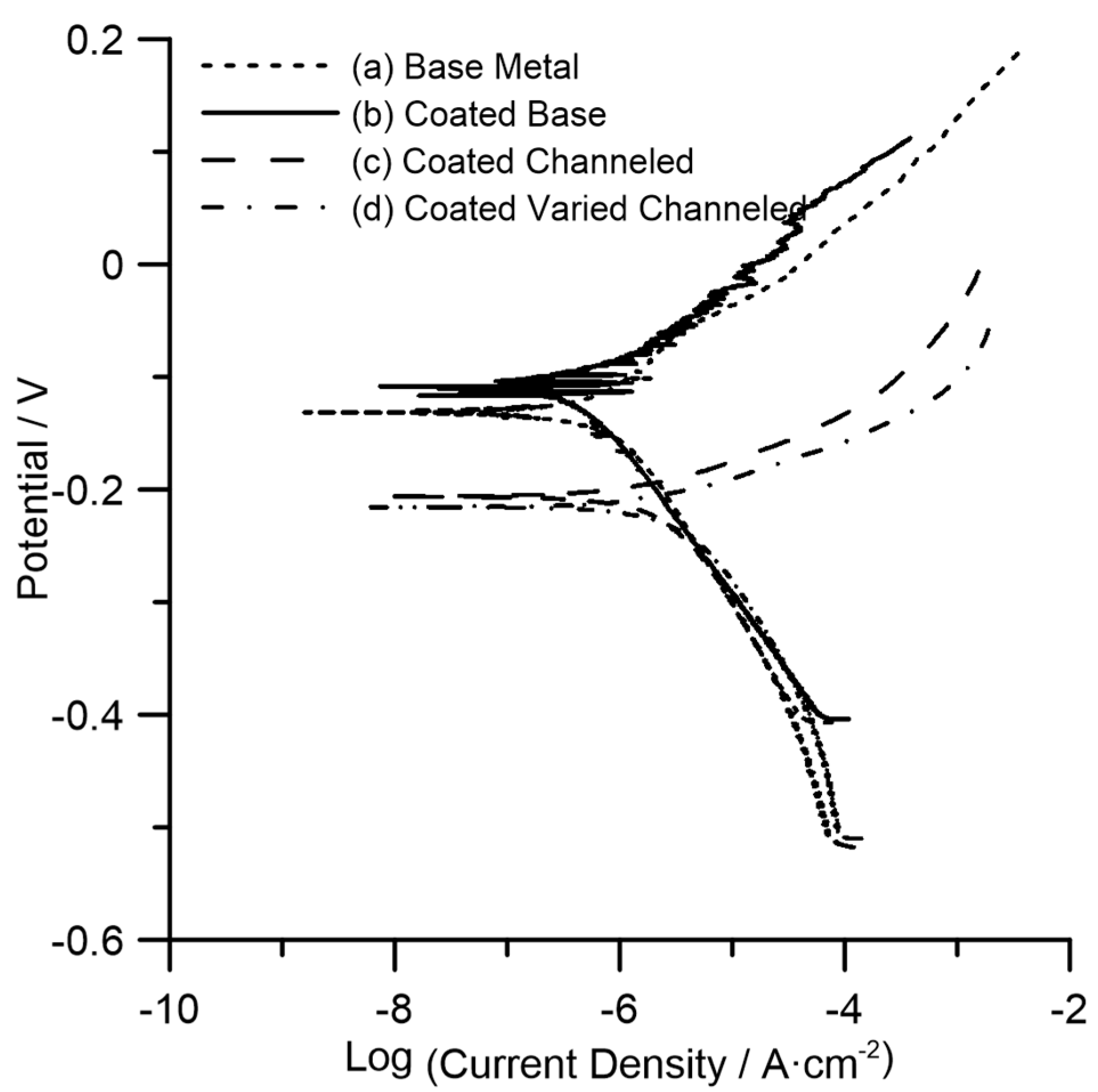
3.4. Corrosion Behavior
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thirumoolan, D.; Siva, T.; Vetrivel, K.; Sathiyanarayanan, S.; Basha, K.A. Corrosion resistant performance of hydrophobic poly(N-vinyl imidazole-co-ethyl methacrylate) coating on mild steel. Prog. Org. Coat. 2015, 89, 181–191. [Google Scholar] [CrossRef]
- Mohamed, A.M.A.; Abdullah, A.M.; Younan, N.A. Corrosion behavior of superhydrophobic surfaces: A review. Arab. J. Chem. 2015, 8, 749–765. [Google Scholar] [CrossRef]
- Zakowski, K.; Narozny, M.; Szocinski, M.; Darowicki, K. Influence of water salinity on corrosion risk-the case of the southern Baltic Sea coast. Environ. Monit. Assess. 2014, 186, 4871–4879. [Google Scholar] [CrossRef] [PubMed]
- Ladan Khaksar, G.W.; Shirokoff, J. Electrochemical and microstructural analysis of FeS films from acidic chemical bath at varying temperatures, pH, and immersion time. Int. J. Corros. 2016, 2016, 9. [Google Scholar] [CrossRef]
- Wang, X.T.; Hou, B.R. Effect of sulphide pollutants on mild steel corrosion in 3.5% NaCl solutions. Corros. Eng. Sci. Technol. 2010, 45, 57–60. [Google Scholar] [CrossRef]
- Xiong, J.; Tan, M.Y.; Forsyth, M. The corrosion behaviors of stainless steel weldments in sodium chloride solution observed using a novel electrochemical measurement approach. Desalination 2013, 327, 39–45. [Google Scholar] [CrossRef]
- Gupta, R.K.; Parvathavarthini, N.; Vinod Kumar, A.; Dayal, R.K. Influence of inclusion and specimen orientations on intergranular corrosion testing of AISI 316LN stainless steel. Trans. Indian Inst. Met. 2011, 64, 365–375. [Google Scholar] [CrossRef]
- Farhadi, S.; Farzaneh, M.; Kulinich, S.A. Anti-icing performance of superhydrophobic surfaces. Appl. Surf. Sci. 2011, 257, 6264–6269. [Google Scholar] [CrossRef]
- Khorsand, S.; Raeissi, K.; Ashrafizadeh, F. Corrosion resistance and long-term durability of super-hydrophobic nickel film prepared by electrodeposition process. Appl. Surf. Sci. 2014, 305, 498–505. [Google Scholar] [CrossRef]
- Ahmad, Z.; Khan, A.U.; Farooq, R.; Mastoi, N.R.; Saif, T. Hydrophobicity A Green Technique for Enhancing Corrosion Resistance of Alloys. In New Trends in Alloy Development, Characterization and Application; Zaki, A., Ed.; IntechOpen: London, UK, 2015. [Google Scholar]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Hensel, R.; Helbig, R.; Aland, S.; Voigt, A.; Neinhuis, C.; Werner, C. Tunable nano-replication to explore the omniphobic characteristics of springtail skin. NPG Asia Mater. 2013, 5, e37. [Google Scholar] [CrossRef]
- Nishino, T.; Meguro, M.; Nakamae, K.; Matsushita, M.; Ueda, Y. The lowest surface free energy based on –CF3 alignment. Langmuir 1999, 15, 4321–4323. [Google Scholar] [CrossRef]
- Limongi, T.; Schipani, R.; Di Vito, A.; Giugni, A.; Francardi, M.; Torre, B.; Allione, M.; Miele, E.; Malara, N.; Alrasheed, S.; et al. Photolithography and micromolding techniques for the realization of 3D polycaprolactone scaffolds for tissue engineering applications. Microelectron. Eng. 2015, 141, 135–139. [Google Scholar] [CrossRef]
- Rodriguez, A.; Echeverría, M.; Ellman, M.; Perez, N.; Verevkin, Y.K.; Peng, C.S.; Berthou, T.; Wang, Z.; Ayerdi, I.; Savall, J.; et al. Laser interference lithography for nanoscale structuring of materials: From laboratory to industry. Microelectron. Eng. 2009, 86, 937–940. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, X.; Zhang, J.; Wang, Y.; Han, Z.; Ren, L. Biomimetic hydrophobic surface fabricated by chemical etching method from hierarchically structured magnesium alloy substrate. Appl. Surf. Sci. 2013, 280, 845–849. [Google Scholar] [CrossRef]
- Abson, D.J.; Pargeter, R.J. Factors influencing as-deposited strength, microstructure, and toughness of manual metal arc welds suitable for C-Mn steel fabrications. Int. Mater. Rev. 1986, 31, 141–196. [Google Scholar] [CrossRef]
- Yuan, S.; Pehkonen, S.O.; Liang, B.; Ting, Y.P.; Neoh, K.G.; Kang, E.T. Superhydrophobic fluoropolymer-modified copper surface via surface graft polymerisation for corrosion protection. Corros. Sci. 2011, 53, 2738–2747. [Google Scholar] [CrossRef]
- Geler, E.; Azambuja, D.S. Corrosion inhibition of copper in chloride solutions by pyrazole. Corros. Sci. 2000, 42, 631–643. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, A.M.; Modestov, A.D.; Domantovsky, A.G.; Emelyanenko, K.A. Synergistic effect of superhydrophobicity and oxidized layers on corrosion resistance of aluminum alloy surface textured by nanosecond laser treatment. ACS Appl. Mater. Interfaces 2015, 7, 19500–19508. [Google Scholar] [CrossRef] [PubMed]
- Latthe, S.S.; Sudhagar, P.; Devadoss, A.; Kumar, A.M.; Liu, S.; Terashima, C.; Nakata, K.; Fujishima, A. A mechanically bendable superhydrophobic steel surface with self-cleaning and corrosion-resistant properties. J. Mater. Chem. A 2015, 3, 14263–14271. [Google Scholar] [CrossRef]
- Park, B.; Hwang, W. A facile fabrication method for corrosion-resistant micro/nanostructures on stainless steel surfaces with tunable wettability. Scr. Mater. 2016, 113, 118–121. [Google Scholar] [CrossRef]
- Wang, N.; Xiong, D. Superhydrophobic membranes on metal substrate and their corrosion protection in different corrosive media. Appl. Surf. Sci. 2014, 305, 603–608. [Google Scholar] [CrossRef]
- Trdan, U.; Hocevar, M.; Gregorcic, P. Transition from superhydrophilic to superhydrophobic state of laser textured stainless steel surface and its effect on corrosion resistance. Corros. Sci. 2017, 123, 21–26. [Google Scholar] [CrossRef]
- Ogihara, H.; Xie, J.; Saji, T. Factors determining wettability of superhydrophobic paper prepared by spraying nanoparticle suspensions. Colloids Surf. A Physicochem. Eng. Asp. 2013, 434, 35–41. [Google Scholar] [CrossRef]
- Wu, B.; Zhou, M.; Li, J.; Ye, X.; Li, G.; Cai, L. Superhydrophobic surfaces fabricated by microstructuring of stainless steel using a femtosecond laser. Appl. Surf. Sci. 2009, 256, 61–66. [Google Scholar] [CrossRef]
- Aculon Aculon Performance Surface Solution. Available online: http://www.aculon.com/multisurfacehydrophobic.php (accessed on 20 April 2017).
- Wang, Z.; Li, H.; Shen, Q.; Liu, W.; Wang, Z. Nano-precipitates evolution and their effects on mechanical properties of 17–4 precipitation-hardening stainless steel. Acta Mater. 2018, 156, 158–171. [Google Scholar] [CrossRef]
- Murayama, M.; Hono, K.; Katayama, Y. Microstructural evolution in a 17-4 PH stainless steel after aging at 400C. Metall. Mater. Trans. A 1999, 30, 345–353. [Google Scholar] [CrossRef]
- Youn, K.T.; Rhyim, Y.M.; Yoo, W.D.; Lee, J.H. Study on the Microstructure and Mechanical Properties of 17-4 PH Stainless Steel Depending on Heat Treatment and Aging Time. In Solid State Phenomena; Trans Tech Publications: Stafa-Zurich, Switzerland, 2006; Volume 118, pp. 15–20. [Google Scholar]
- Park, S.H.C.; Sato, Y.S.; Kokawa, H.; Okamoto, K.; Hirano, S.; Inagaki, M. Corrosion resistance of friction stir welded 304 stainless steel. Scr. Mater. 2004, 51, 101–105. [Google Scholar] [CrossRef]
- Wenzel, R.N. Surface roughness and contact angle. J. Phys. Chem. 1949, 53, 1466–1467. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Jung, Y.C.; Bhushan, B. Contact angle, adhesion and friction properties of micro-and nanopatterned polymers for superhydrophobicity. Nanotechnology 2006, 17, 4970. [Google Scholar] [CrossRef]
- Ogawa, T.; Koseki, T. Effect of composition profiles on metallurgy and corrosion behavior of duplex stainless steel weld metals. Weld. J. 1989, 68, 181. [Google Scholar]
- Gao, Q.; Zhang, Y.; Zhang, H.; Li, H.; Qu, F.; Han, J.; Lu, C.; Wu, B.; Lu, Y.; Ma, Y. Precipitates and Particles Coarsening of 9Cr–1.7W–0.4Mo–Co Ferritic Heat-Resistant Steel after Isothermal Aging. Sci. Rep. 2017, 7, 5859. [Google Scholar] [CrossRef] [PubMed]
- Chun, D.M.; Ngo, C.V.; Lee, K.M. Fast fabrication of superhydrophobic metallic surface using nanosecond laser texturing and low-temperature annealing. CIRP Ann. Manuf. Technol. 2016, 65, 519–522. [Google Scholar] [CrossRef]
- Leon, A.; Aghion, E. Effect of surface roughness on corrosion fatigue performance of AlSi10Mg alloy produced by Selective Laser Melting (SLM). Mater. Charact. 2017, 131, 188–194. [Google Scholar] [CrossRef]



| Elements | Cr | Ni | Cu | Si | Mn | Fe |
|---|---|---|---|---|---|---|
| 17-4 PH | 16.70 ± 0.05 | 3.70 ± 0.06 | 2.91 ± 0.07 | 0.26 ± 0.08 | 0.46 ± 0.01 | Bal. |
| Phase | Cr | Ni | Cu | Si | Mn | Fe |
|---|---|---|---|---|---|---|
| δ-Ferrite | 22.3 ± 0.2 | 1.6 ± 0.1 | 1.2 ± 0.2 | 0.4 ± 0.1 | 0.1 ± 0.1 | Bal. |
| Martesite | 16.5 ± 0.1 | 3.7 ± 0.1 | 2.9 ± 0.1 | 0.3 ± 0.1 | 0.1 ± 0.9 | Bal. |
| Surface Type | Corrosion Potential (VAg/AgCl) | Corrosion Current (A) | Corrosion Current Density (A·cm−2) | Corrosion Rate (mm/y) |
|---|---|---|---|---|
| Base metal | −0.136 | 2.070 × 10−6 | 1.035 × 10−6 | 0.012 |
| Coated base metal | −0.110 | 8.036 × 10−7 | 4.018 × 10−7 | 0.005 |
| Coated channeled | −0.209 | 1.657 × 10−5 | 2.776 × 10−6 | 0.032 |
| Coated varied channeled | −0.205 | 1.878 × 10−5 | 3.799 × 10−6 | 0.043 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafieazad, M.; Jaffer, J.A.; Cui, C.; Duan, X.; Nasiri, A. Nanosecond Laser Fabrication of Hydrophobic Stainless Steel Surfaces: The Impact on Microstructure and Corrosion Resistance. Materials 2018, 11, 1577. https://doi.org/10.3390/ma11091577
Rafieazad M, Jaffer JA, Cui C, Duan X, Nasiri A. Nanosecond Laser Fabrication of Hydrophobic Stainless Steel Surfaces: The Impact on Microstructure and Corrosion Resistance. Materials. 2018; 11(9):1577. https://doi.org/10.3390/ma11091577
Chicago/Turabian StyleRafieazad, Mehran, Jaffer Alkarim Jaffer, Cong Cui, Xili Duan, and Ali Nasiri. 2018. "Nanosecond Laser Fabrication of Hydrophobic Stainless Steel Surfaces: The Impact on Microstructure and Corrosion Resistance" Materials 11, no. 9: 1577. https://doi.org/10.3390/ma11091577
APA StyleRafieazad, M., Jaffer, J. A., Cui, C., Duan, X., & Nasiri, A. (2018). Nanosecond Laser Fabrication of Hydrophobic Stainless Steel Surfaces: The Impact on Microstructure and Corrosion Resistance. Materials, 11(9), 1577. https://doi.org/10.3390/ma11091577







