Porous Titanium Scaffolds Fabricated by Metal Injection Moulding for Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Feedstock Preparation
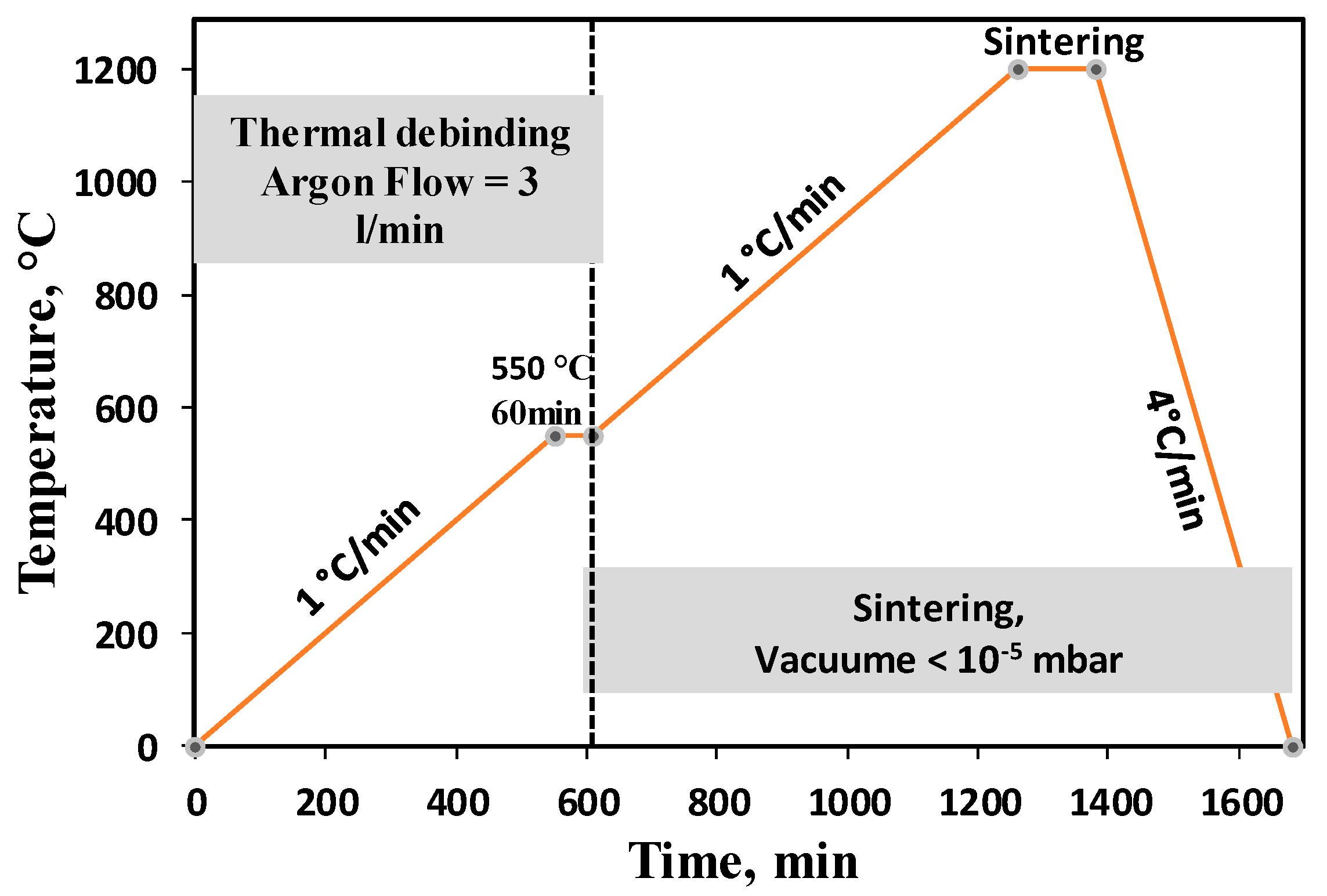
2.2. Injection Moulding, Debinding and Sintering
2.3. Materials Characterisation
2.4. Corrosion Testing
3. Results and Discussion
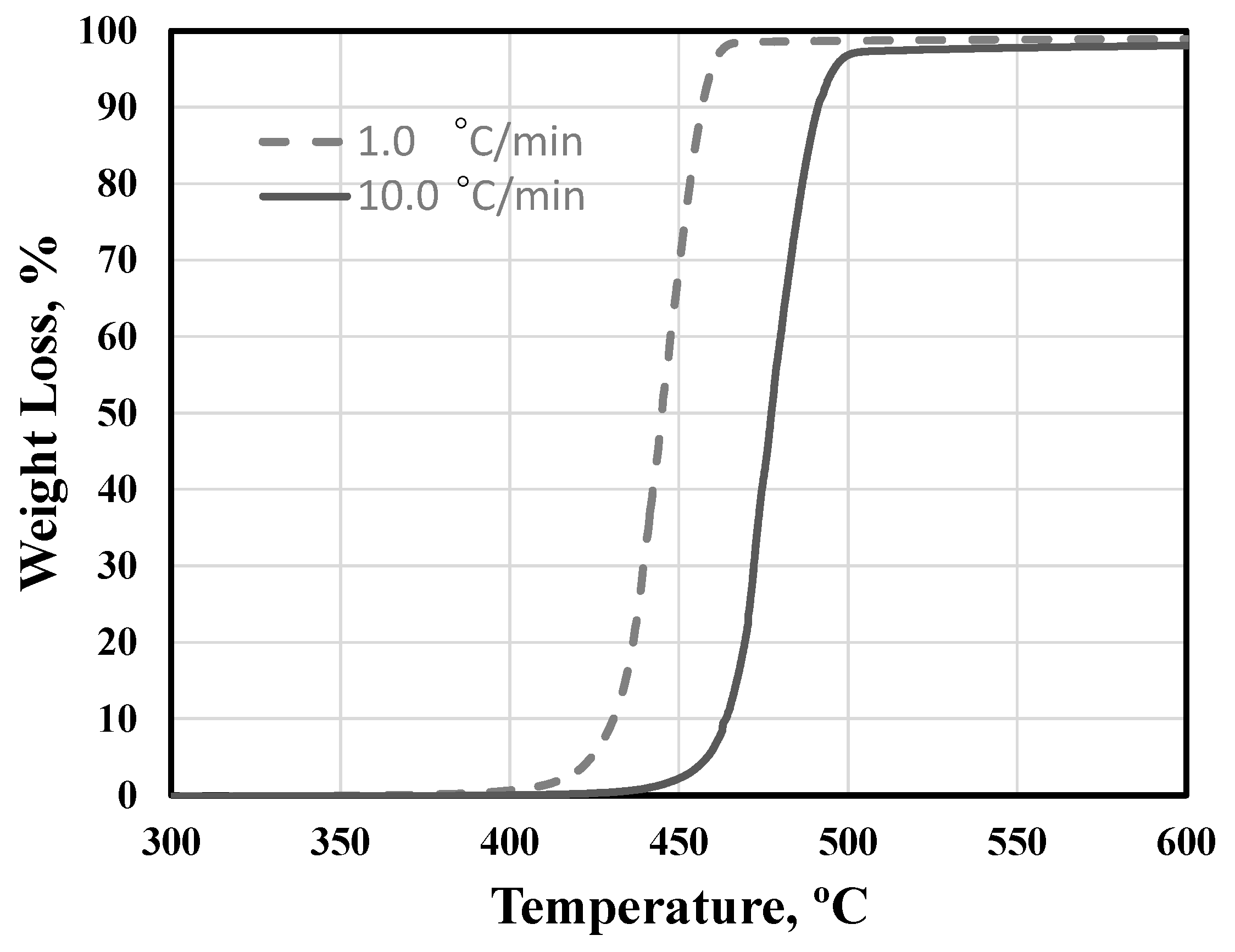
3.1. Binder Assessment and De-Binding
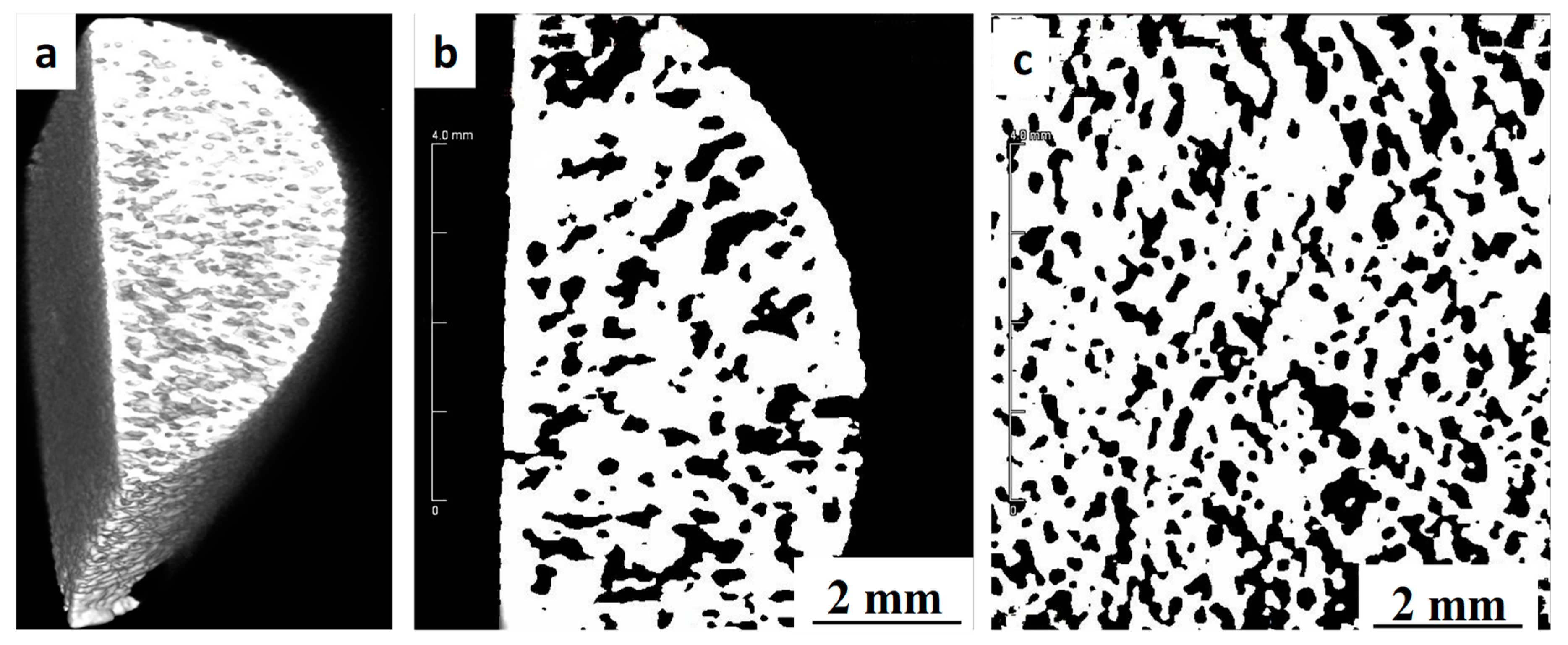
3.2. Shrinkage, Porosity and Pore Size Distribution
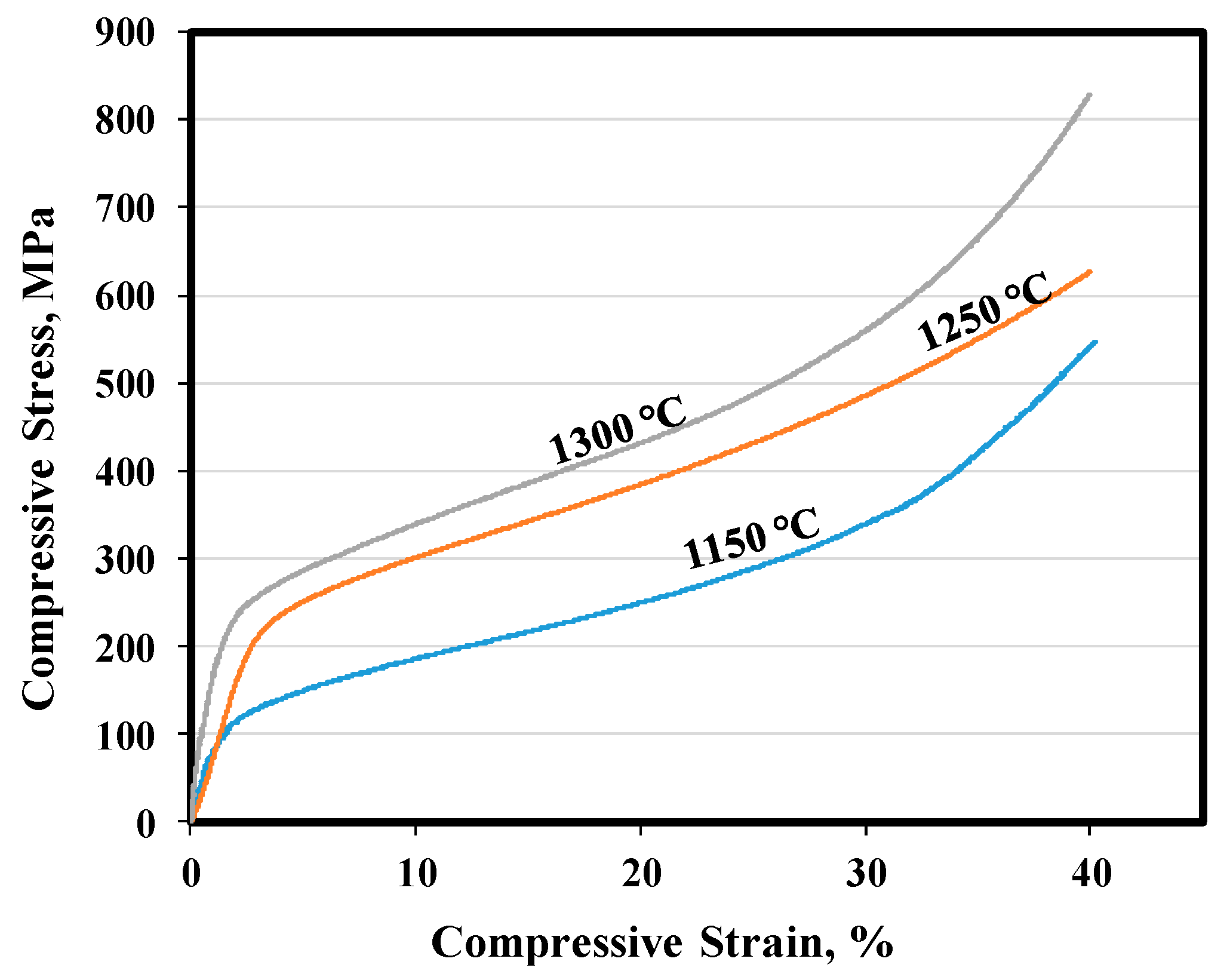
3.3. Mechanical Properties
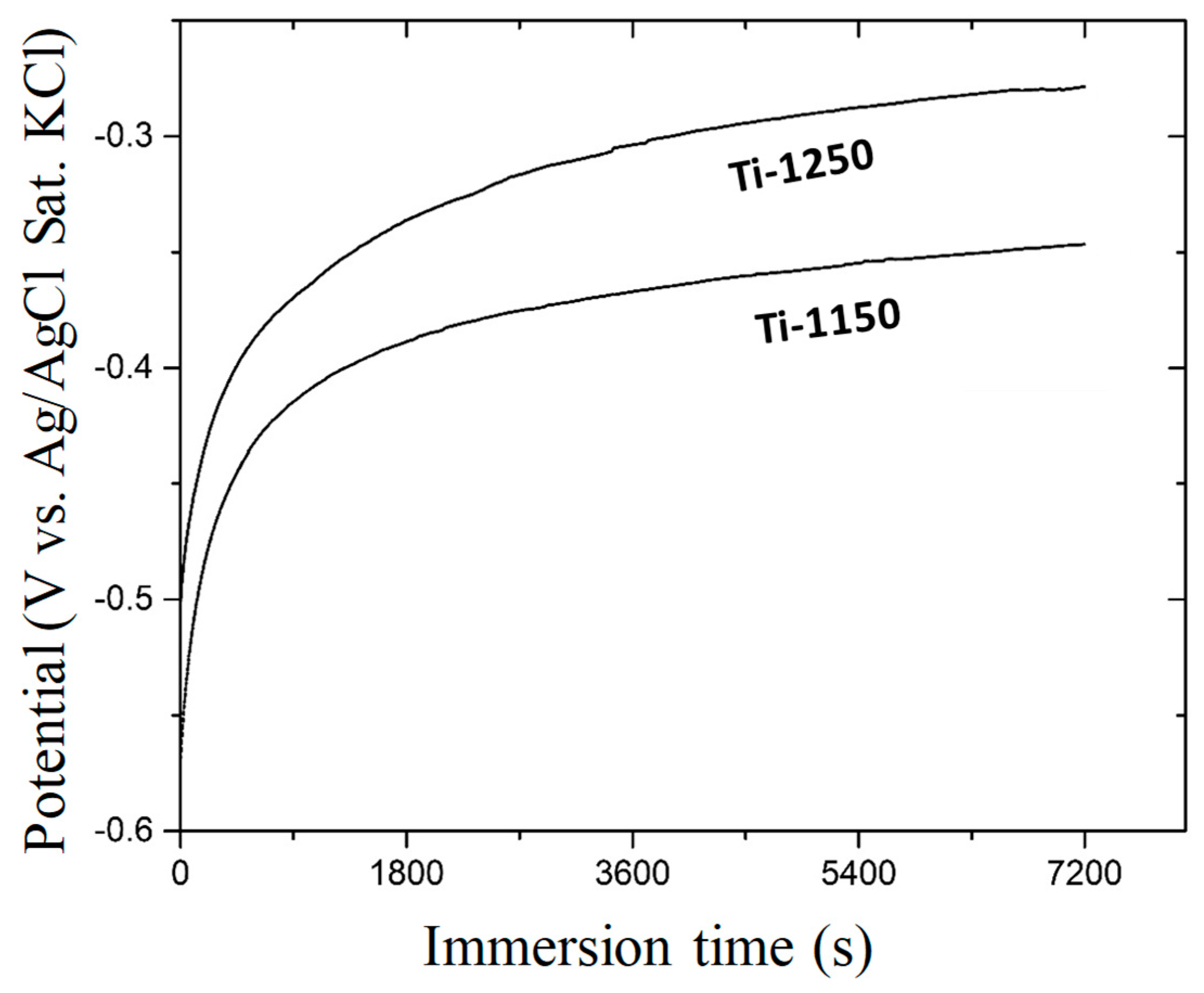
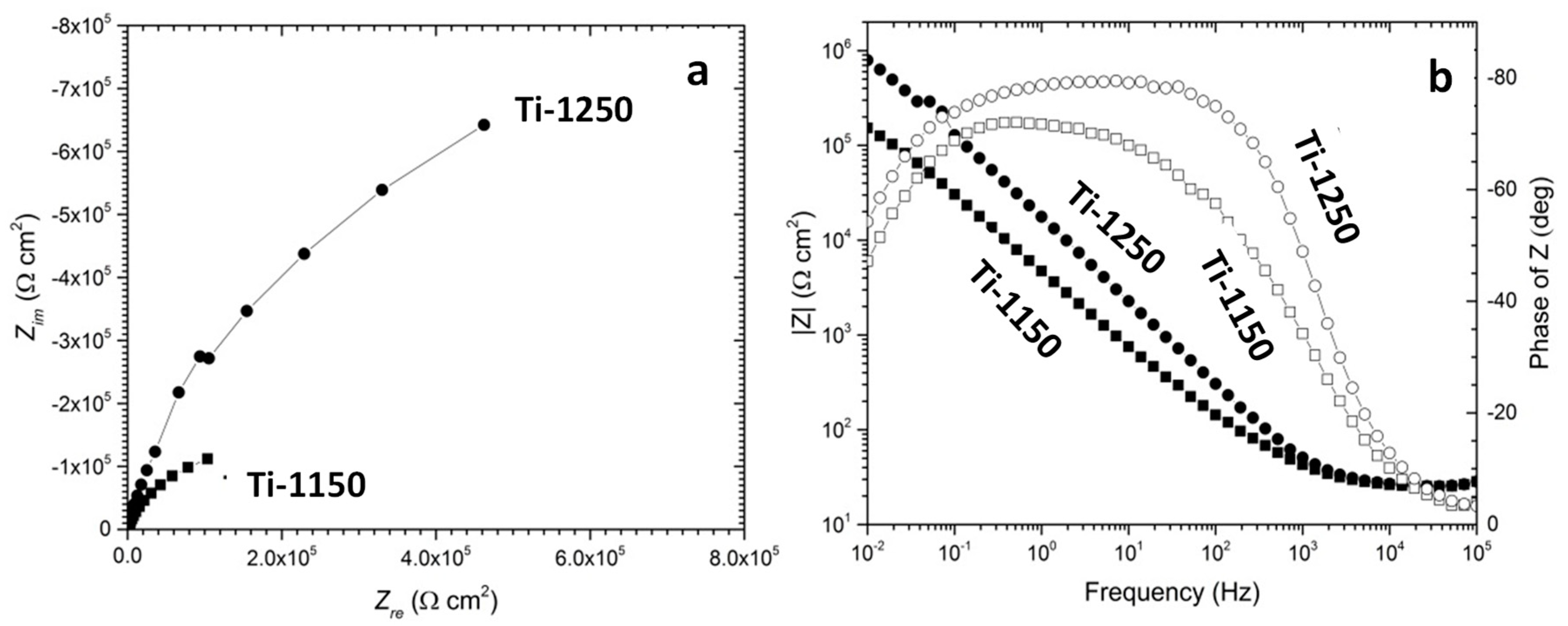
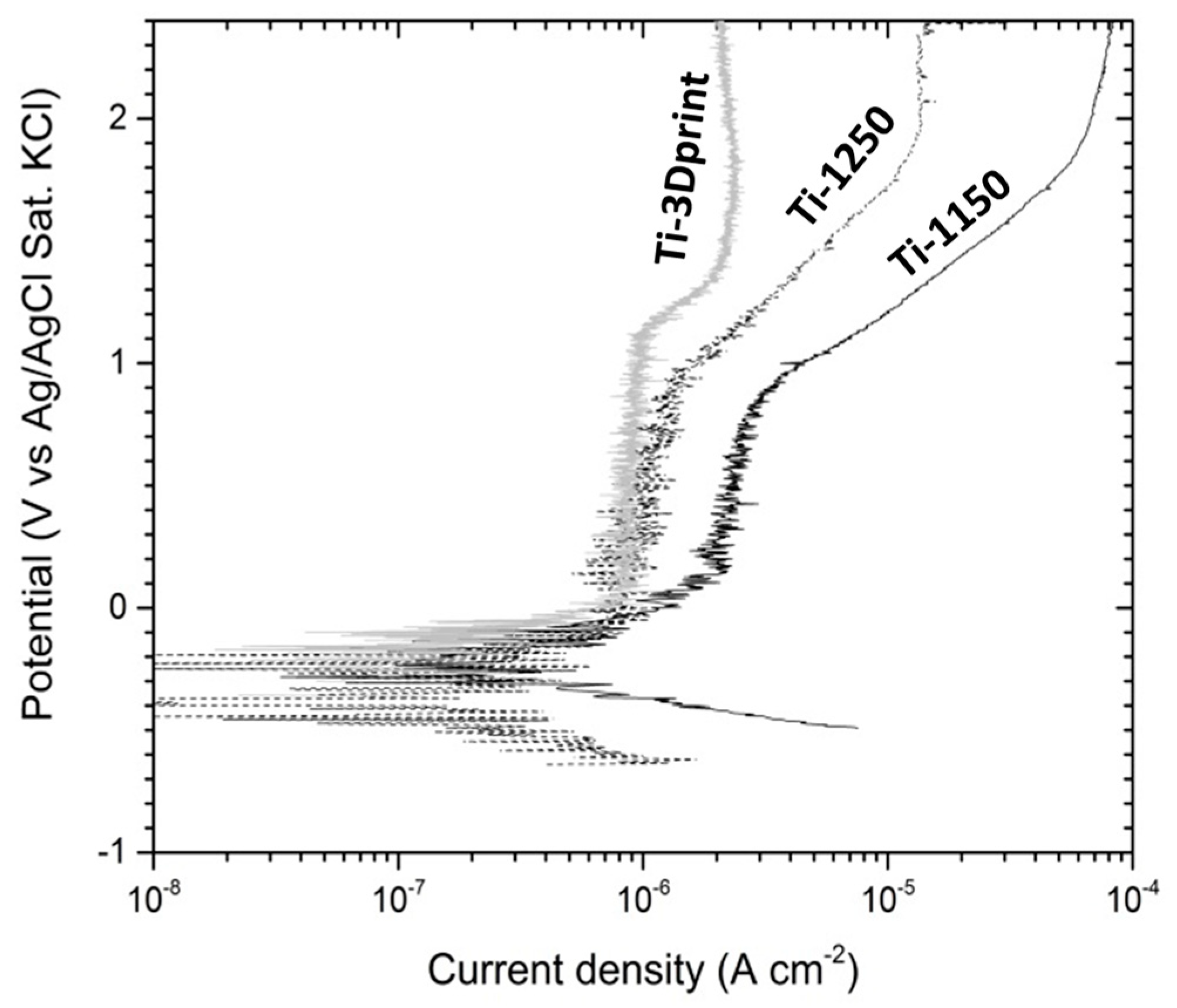
3.4. Evaluation of Corrosion Behaviours in Hank’s Solution
4. Conclusions
- (1)
- MIM is able to manufacture porous biomedical titanium scaffolds with controlled shrinkage, density, porosity and a highly interconnected pore structure;
- (2)
- Uniform shrinkage of around 12.0% was observed in all dimensions of the scaffold samples after sintering at 1250 °C or 1300 °C;
- (3)
- Samples sintered at 1250 °C for 120 min achieved mechanical properties that are very close to those of human cortical bone;
- (4)
- The corrosion resistance of scaffold titanium samples sintered at 1250 °C and 1150 °C in Hank’s solution changed with porosity. The higher the porosity, the lower the corrosion resistance;
- (5)
- Overall, sintering at 1250 °C for 120 min can be chosen as a desired sintering condition in terms of the resulting porosity level (40%), mechanical properties, dimensional control and corrosion resistance.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lütjering, G.; Williams, J.C. Titanium, Engineering Materials and Processes, 2nd ed.; Springer Science & Business Media: Berlin, Germany, 2007; p. 442. [Google Scholar]
- Burg, K.J.; Porter, S.; Kellam, J.F. Biomaterial developments for bone tissue engineering. Biomaterials 2000, 21, 2347–2359. [Google Scholar] [CrossRef]
- Dehghan-Manshadi, A.; Dippenaar, R.J. Development of β-phase morphologies during low temperature isothermal heat treatment of a Ti-5Al-5Mo-5V-3Cr alloy. Mater. Sci. Eng. A 2011, 528, 1833–1839. [Google Scholar] [CrossRef]
- Elias, C.; Lima, J.H.; Valiev, R.; Meyers, M. Biomedical applications of titanium and its alloys. JOM 2008, 60, 46–49. [Google Scholar] [CrossRef]
- McCracken, M. Dental implant materials: Commercially pure titanium and titanium alloys. J. Prosthodont. 1999, 8, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Rack, H. Titanium alloys in total joint replacement—a materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.; Asokamani, R.; Gogia, A. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M. Titanium-based biomaterials for preventing stress shielding between implant devices and bone. Inter. J. Biomater. 2011, 2011, 836587. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Chang, K.; Ebel, T.; Qian, M.; Willumeit, R.; Yan, M.; Pyczak, F. Microstructure and mechanical behavior of metal injection molded Ti–Nb binary alloys as biomedical material. J. Mech. Prop. Biomater. 2013, 28, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Ryan, G.E.; Pandit, A.S.; Apatsidis, D.P. Porous titanium scaffolds fabricated using a rapid prototyping and powder metallurgy technique. Biomaterials 2008, 29, 3625–3635. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Xu, W.; Brandt, M.; Tang, H.P. Additive manufacturing and postprocessing of Ti-6Al-4V for superior mechanical properties. MRS Bull. 2016, 41, 775–783. [Google Scholar] [CrossRef]
- Chen, Y.; Kent, D.; Bermingham, M.; Dehghan-Manshadi, A.; Dargusch, M. Manufacturing of biocompatible porous titanium scaffolds using a novel spherical sugar pellet space holder. Mater. Lett. 2017, 195, 92–95. [Google Scholar] [CrossRef]
- Torres-Sanchez, C.; Al Mushref, F.; Norrito, M.; Yendall, K.; Liu, Y.; Conway, P.P. The effect of pore size and porosity on mechanical properties and biological response of porous titanium scaffolds. Mater. Sci. Eng. C 2017, 77, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chang, L.; Liu, H.; Li, Y.; Yang, H.; Ruan, J. Microstructure, mechanical behavior and biocompatibility of powder metallurgy Nb-Ti-Ta alloys as biomedical material. Mater. Sci. Eng. C 2017, 71, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Frith, J.E.; Dehghan-Manshadi, A.; Attar, H.; Kent, D.; Soro, N.D.M.; Bermingham, M.J.; Dargusch, M.S. Mechanical Properties and Biocompatibility of Porous Titanium Scaffolds for Bone Tissue Engineering. J. Mech. Prop. Biomater. 2017, 75, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.M.; Tseng, L.-F.; Iannolo, M.T.; Oest, M.E.; Henderson, J.H. Self-deploying shape memory polymer scaffolds for grafting and stabilizing complex bone defects: A mouse femoral segmental defect study. Biomaterials 2016, 76, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Ebel, T. Advances in the Metal Injection Moulding of Titanium at Euro PM 2014. PIM Int. 2015, 9, 51–61. [Google Scholar]
- German, R.M. Progress in Titanium Metal Powder Injection Molding. Materials 2013, 6, 3641–3662. [Google Scholar] [CrossRef] [PubMed]
- Qian, M. Metal Injection Moulding (MIM) of titanium and titanium hydride reviewed at PM Titanium 2013. PIM Int. 2013, 8, 67–74. [Google Scholar]
- Dehghan-Manshadi, A.; Bermingham, M.; Dargusch, M.; StJohn, D.; Qian, M. Metal Injection Moulding of Titanium and Titanium Alloys: Challenges and Recent Development. Powder Technol. 2017, 319, 289–301. [Google Scholar] [CrossRef]
- Ergul, E.; Gulsoy, H.O.; Gunay, V. Effect of sintering parameters on mechanical properties of injection moulded Ti-6Al-4V alloys. Powder Metall. 2009, 52, 65–71. [Google Scholar] [CrossRef]
- Hamidi, M.; Harun, W.; Samykano, M.; Ghani, S.; Ghazalli, Z.; Ahmad, F.; Sulong, A. A review of biocompatible metal injection moulding process parameters for biomedical applications. Mater. Sci. Eng. C 2017, 78, 1263–1276. [Google Scholar] [CrossRef] [PubMed]
- Bidaux, J.; Closuit, C.; Rodriguez-Arbaizar, M.; Zufferey, D.; Carreño-Morelli, E. Processing of a low modulus Ti-Nb biomaterial by Metal Injection Molding (MIM). PIM Int. 2012, 6, 72–75. [Google Scholar]
- Nelles, H.; Bram, M.; Buchkremer, H.P.; Stöver, D. Method for the Production of Near Net-Shaped Metallic and/or Ceramic Parts. U.S. Patent 7351371, 1 April 2008. [Google Scholar]
- Carreño-Morelli, E.; Rodríguez-Arbaizar, M.; Amherd, A.; Bidaux, J.E. Porous titanium processed by powder injection moulding of titanium hydride and space holders. Powder Metall. 2014, 57, 93–97. [Google Scholar] [CrossRef]
- Carreño-Morelli, E.; Amherd, A.; Rodriguez-Arbaizar, M.; Zufferey, D.; Várez, A.; Bidaux, J.-E. Porous titanium by powder injection moulding of titanium hydride and PMMA space holders. Eur. Cells Mater. 2013, 26, 16. [Google Scholar]
- Chen, L.-J.; Li, T.; Li, Y.-M.; He, H.; Hu, Y.-H. Porous titanium implants fabricated by metal injection molding. Trans. Nonferr. Met. Soc. China 2009, 19, 1174–1179. [Google Scholar] [CrossRef]
- Daudt, N.D.F.; Bram, M.; Barbosa, A.P.C.; Laptev, A.M.; Alves, C., Jr. Manufacturing of highly porous titanium by metal injection molding in combination with plasma treatment. J. Mater. Proc. Technol. 2017, 239, 202–209. [Google Scholar] [CrossRef]
- Hu, G.; Zhang, L.; Fan, Y.; Li, Y. Fabrication of high porous NiTi shape memory alloy by metal injection molding. J. Mater. Proc. Technol. 2008, 206, 395–399. [Google Scholar]
- Laptev, A.M.; Daudt, N.A.F.; Guillon, O.; Bram, M. Increased Shape Stability and Porosity of Highly Porous Injection-Molded Titanium Parts. Adv. Eng. Mater. 2015, 17, 1579–1587. [Google Scholar] [CrossRef]
- Tuncer, N.; Bram, M.; Laptev, A.; Beck, T.; Moser, A.; Buchkremer, H.P. Study of metal injection molding of highly porous titanium by physical modeling and direct experiments. J. Mater. Proc. Technol. 2014, 214, 1352–1360. [Google Scholar] [CrossRef]
- Demangel, C.; Auzène, D.; Vayssade, M.; Duval, J.-L.; Vigneron, P.; Nagel, M.-D.; Puippe, J.-C. Cytocompatibility of titanium metal injection molding with various anodic oxidation post-treatments. Mater. Sci. Eng. C 2012, 32, 1919–1925. [Google Scholar] [CrossRef]
- Santos, P.F.; Niinomi, M.; Liu, H.; Cho, K.; Nakai, M.; Itoh, Y.; Narushima, T.; Ikeda, M. Fabrication of low-cost beta-type Ti-Mn alloys for biomedical applications by metal injection molding process and their mechanical properties. J. Mech. Prop. Biomater. 2016, 59, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.P.C.; Bram, M.; Stöver, D.; Buchkremer, H.P. Realization of a Titanium Spinal Implant with a Gradient in Porosity by 2-Component-Metal Injection Moulding. Adv. Eng. Mater. 2013, 15, 510–521. [Google Scholar] [CrossRef]
- Deing, A.; Luthringer, B.; Laipple, D.; Ebel, T.; Willumeit, R. A porous TiAl6V4 implant material for medical application. Int. J. Biomater. 2014, 2014, 904230. [Google Scholar] [CrossRef] [PubMed]
- Dehghan-Manshadi, A.; Qian, M.; Dargusch, M.; Chen, Y.; StJohn, D. Optimisation of Processing Parameters for Metal Injection Moulding of Titanium Using Non-Spherical Titanium Powders. J. Manuf. Process 2018, 31, 416–423. [Google Scholar] [CrossRef]
- Wiria, F.E.; Shyan, J.Y.M.; Lim, P.N.; Wen, F.G.C.; Yeo, J.F.; Cao, T. Printing of titanium implant prototype. Mater. Des. 2010, 31, S101–S105. [Google Scholar] [CrossRef]
- Molly, L. Bone density and primary stability in implant therapy. Clin. Oral Implants Res. 2006, 17, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, B.; Takemoto, M.; Fujibayashi, S.; Neo, M.; Kokubo, T.; Nakamura, T. Pore throat size and connectivity determine bone and tissue ingrowth into porous implants: Three-dimensional micro-CT based structural analyses of porous bioactive titanium implants. Biomaterials 2006, 27, 5892–5900. [Google Scholar] [CrossRef] [PubMed]
- Itälä, A.I.; Ylänen, H.O.; Ekholm, C.; Karlsson, K.H.; Aro, H.T. Pore diameter of more than 100 μm is not requisite for bone ingrowth in rabbits. J. Biomed. Mater. Res. A 2001, 58, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Reilly, D.T.; Burstein, A.H. The mechanical properties of cortical bone. JBJS 1974, 56, 1001–1022. [Google Scholar] [CrossRef]
- Sidambe, A.T.; Figueroa, I.A.; Hamilton, H.G.; Todd, I. Taguchi optimization of MIM titanium sintering. Int. J. Powder Metall. 2011, 47, 21–28. [Google Scholar]
- Takekawa, J.; Sakurai, N. Effect of the Processing Conditions on Density, Strength and Microstructure of Ti-12Mo Alloy Fabricated by PIM Process. J. Jpn. Soc. Powder Metall. 1999, 46, 877–881. [Google Scholar] [CrossRef]
- Niinomi, M. Recent research and development in titanium alloys for biomedical applications and healthcare goods. Sci. Technol. Adv. Mater. 2003, 4, 445. [Google Scholar] [CrossRef]
- Fojt, J.; Joska, L.; Málek, J. Corrosion behaviour of porous Ti–39Nb alloy for biomedical applications. Corros. Sci. 2013, 71, 78–83. [Google Scholar] [CrossRef]
- Alves, A.C.; Sendao, I.; Ariza, E.; Toptan, F.; Ponthiaux, P.; Pinto, A.M.P. Corrosion behaviour of porous Ti intended for biomedical applications. J. Porous Mater. 2016, 23, 1261–1268. [Google Scholar] [CrossRef]


| Components | g/L |
|---|---|
| CaCl2 | 0.1396 |
| MgSO4 (anhydrous) | 0.09767 |
| KCl | 0.4 |
| KH2PO4 (anhydrous) | 0.06 |
| NaCl | 8.0 |
| Na2HPO4 (anhydrous) | 0.04788 |
| D-Glucose | 1.0 |
| Sintering Temperature (°C) | Radial Shrinkage (%) | Longitudinal Shrinkage (%) | Density (g/cm3) | Overall Porosity (%) | Open Porosity (%) | Pore Interconnectivity (%) |
|---|---|---|---|---|---|---|
| 1150 | 9.54 ± 0.65 | 9.81 ± 0.47 | 2.60 ± 0.05 | 42.5 | 40.6 | 95.5 |
| 1250 | 12.38 ± 0.77 | 12.62 ± 0.71 | 2.86 ± 0.05 | 36.5 | 33.4 | 91.5 |
| 1300 | 13.04 ± 0.69 | 13.21 ± 0.65 | 2.96 ± 0.03 | 34.4 | 33.8 | 98.2 |
| Sintering Temperature (°C) | σ0.2 (MPa) | σ40 (MPa) | Young’s Modulus (GPa) |
|---|---|---|---|
| 1150 | 123 | 553 | 8.40 |
| 1250 | 220 | 630 | 7.82 |
| 1300 | 230 | 831 | 21.69 |
| Human cortical bone [38] | 104–121 | - | 4–30 |
| Sample | Ecorr (V) | Icorr (µA cm−2) | Bc (mV) | Eb (V) | Etp (V) |
|---|---|---|---|---|---|
| Ti-1150 | −0.297 ± 0.009 | 0.32 ± 0.06 | −164 ± 22 | 0.806 ± 0.032 | 1.545 ± 0.338 |
| Ti-1250 | −0.510 ± 0.021 | 0.19 ± 0.02 | −109 ± 1 | 0.874 ± 0.071 | 1.893 ± 0.014 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dehghan-Manshadi, A.; Chen, Y.; Shi, Z.; Bermingham, M.; StJohn, D.; Dargusch, M.; Qian, M. Porous Titanium Scaffolds Fabricated by Metal Injection Moulding for Biomedical Applications. Materials 2018, 11, 1573. https://doi.org/10.3390/ma11091573
Dehghan-Manshadi A, Chen Y, Shi Z, Bermingham M, StJohn D, Dargusch M, Qian M. Porous Titanium Scaffolds Fabricated by Metal Injection Moulding for Biomedical Applications. Materials. 2018; 11(9):1573. https://doi.org/10.3390/ma11091573
Chicago/Turabian StyleDehghan-Manshadi, Ali, Yunhui Chen, Zhiming Shi, Michael Bermingham, David StJohn, Matthew Dargusch, and Ma Qian. 2018. "Porous Titanium Scaffolds Fabricated by Metal Injection Moulding for Biomedical Applications" Materials 11, no. 9: 1573. https://doi.org/10.3390/ma11091573
APA StyleDehghan-Manshadi, A., Chen, Y., Shi, Z., Bermingham, M., StJohn, D., Dargusch, M., & Qian, M. (2018). Porous Titanium Scaffolds Fabricated by Metal Injection Moulding for Biomedical Applications. Materials, 11(9), 1573. https://doi.org/10.3390/ma11091573





