Microstructures, Mechanical Properties, and Corrosion Behavior of As-Cast Mg–2.0Zn–0.5Zr–xGd (wt %) Biodegradable Alloys
Abstract
:1. Introduction
2. Experimental Section
2.1. Sample Preparation
2.2. Microstructure Determination and Mechanical Properties Test
2.3. Electrochemical Corrosion Test
2.4. Immersion Corrosion Test
3. Results and Discussion
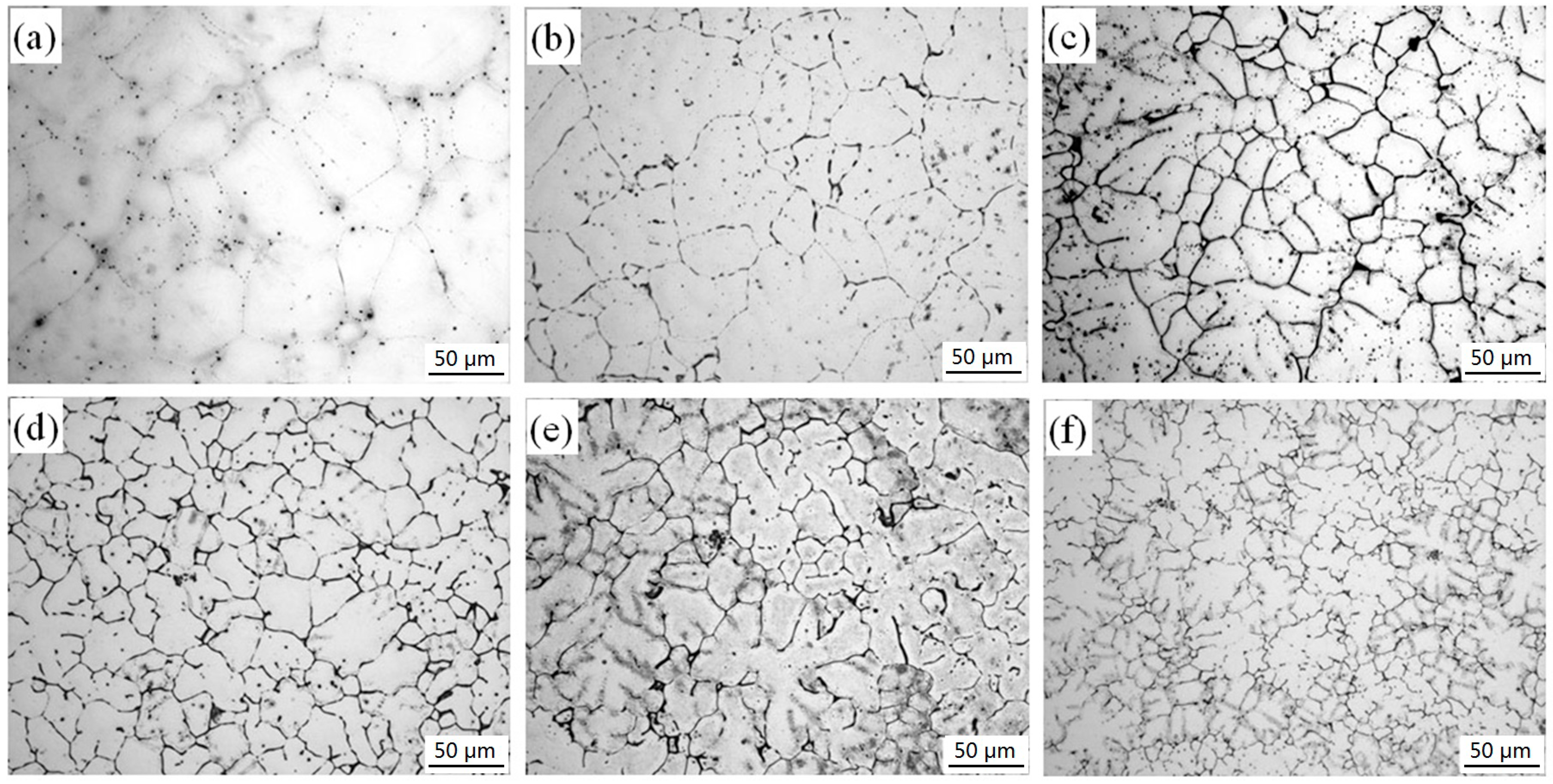
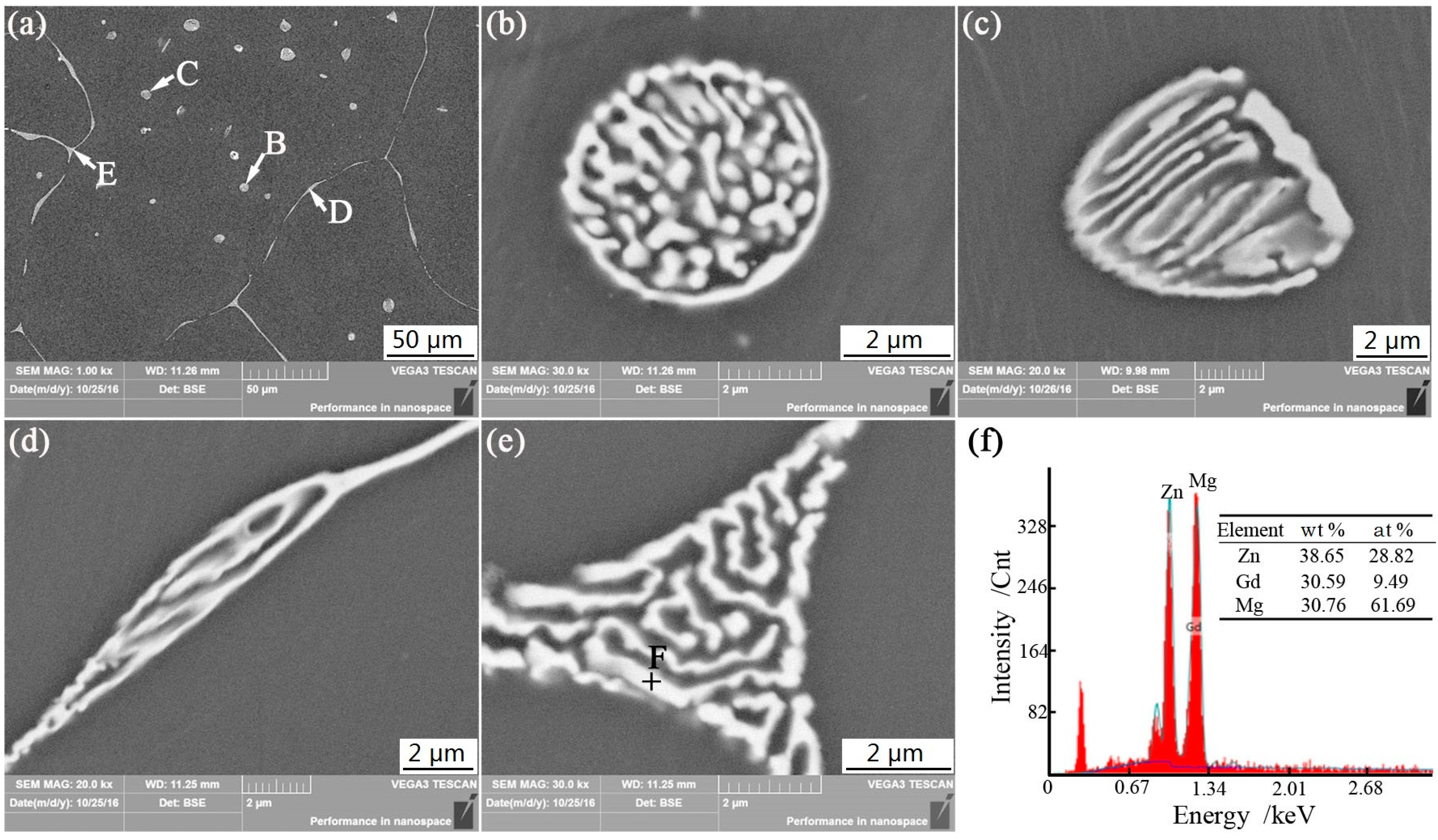
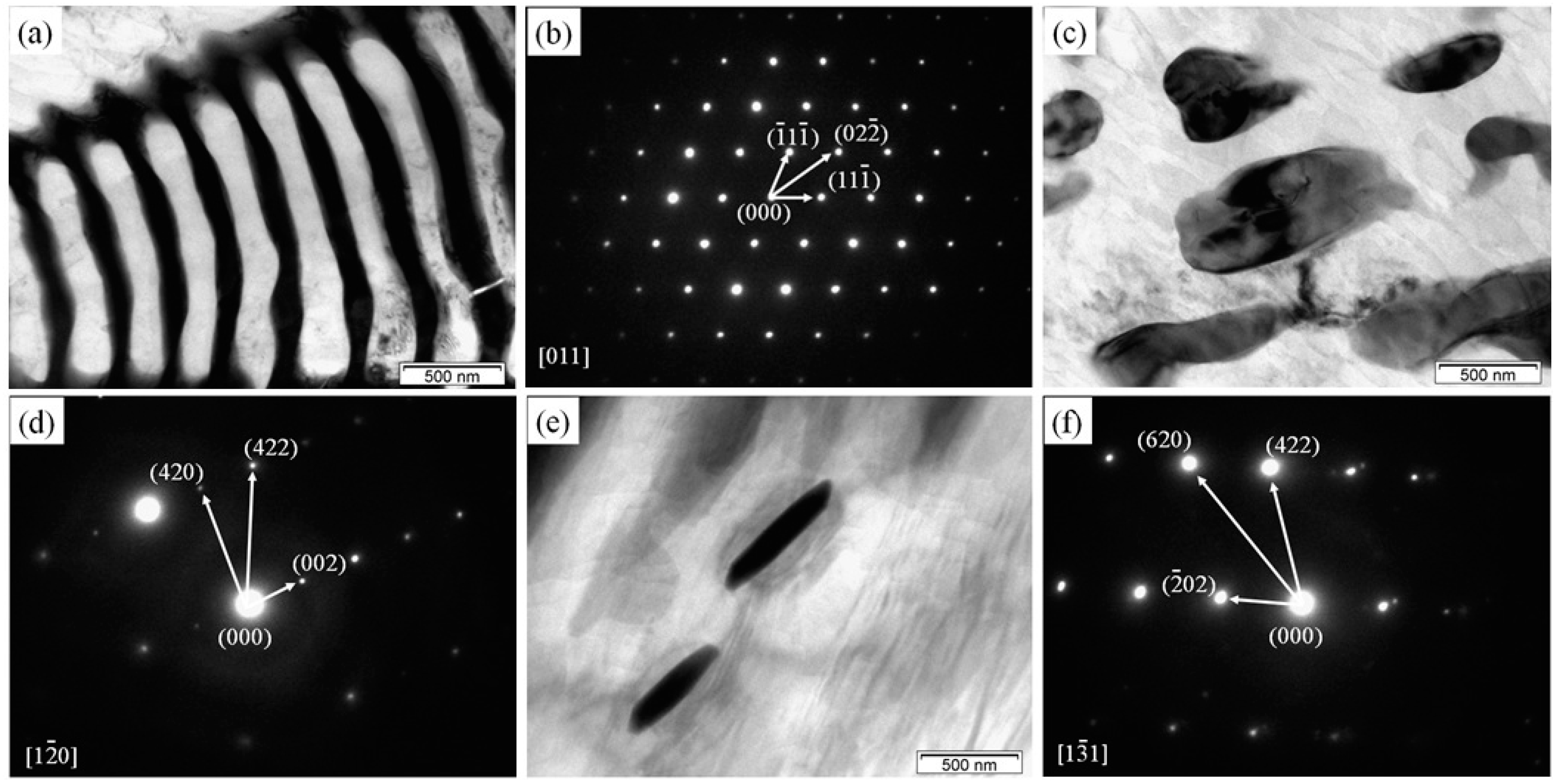
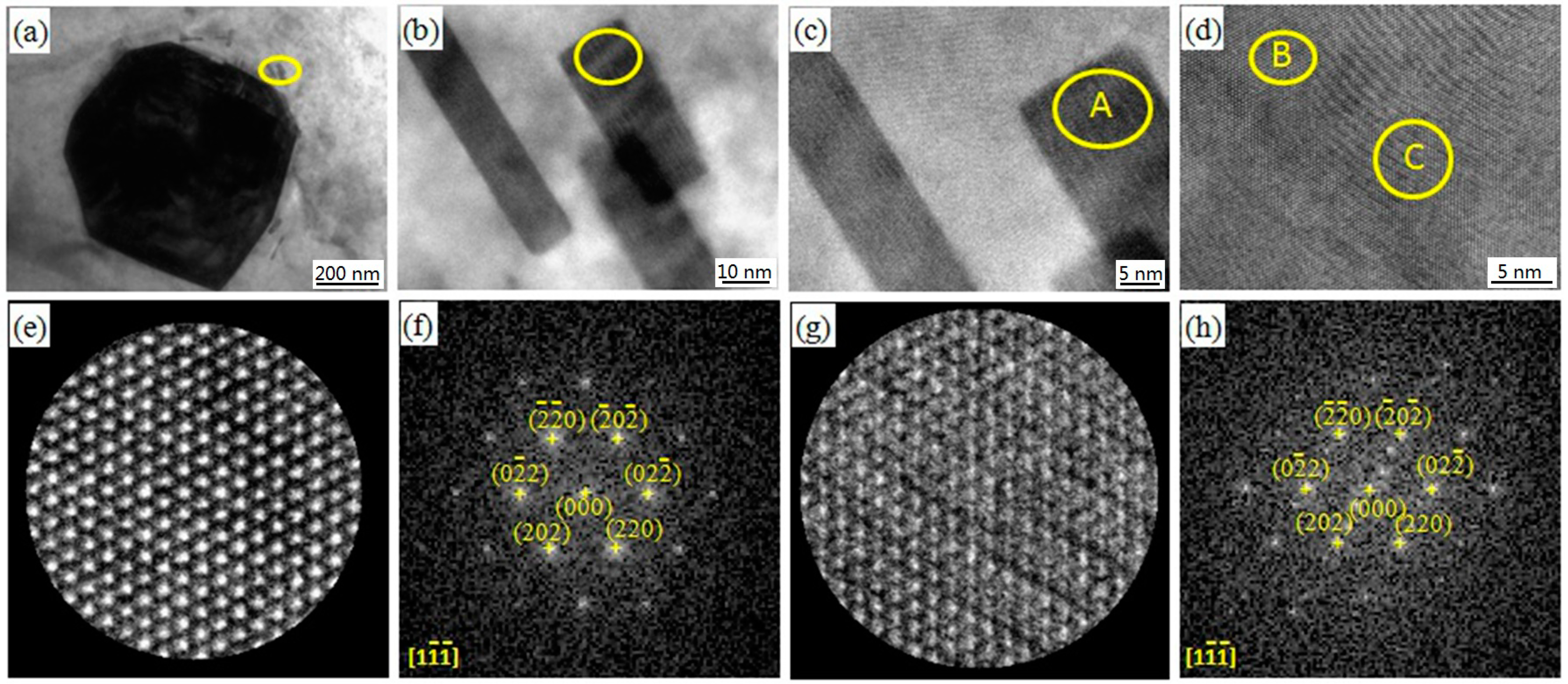
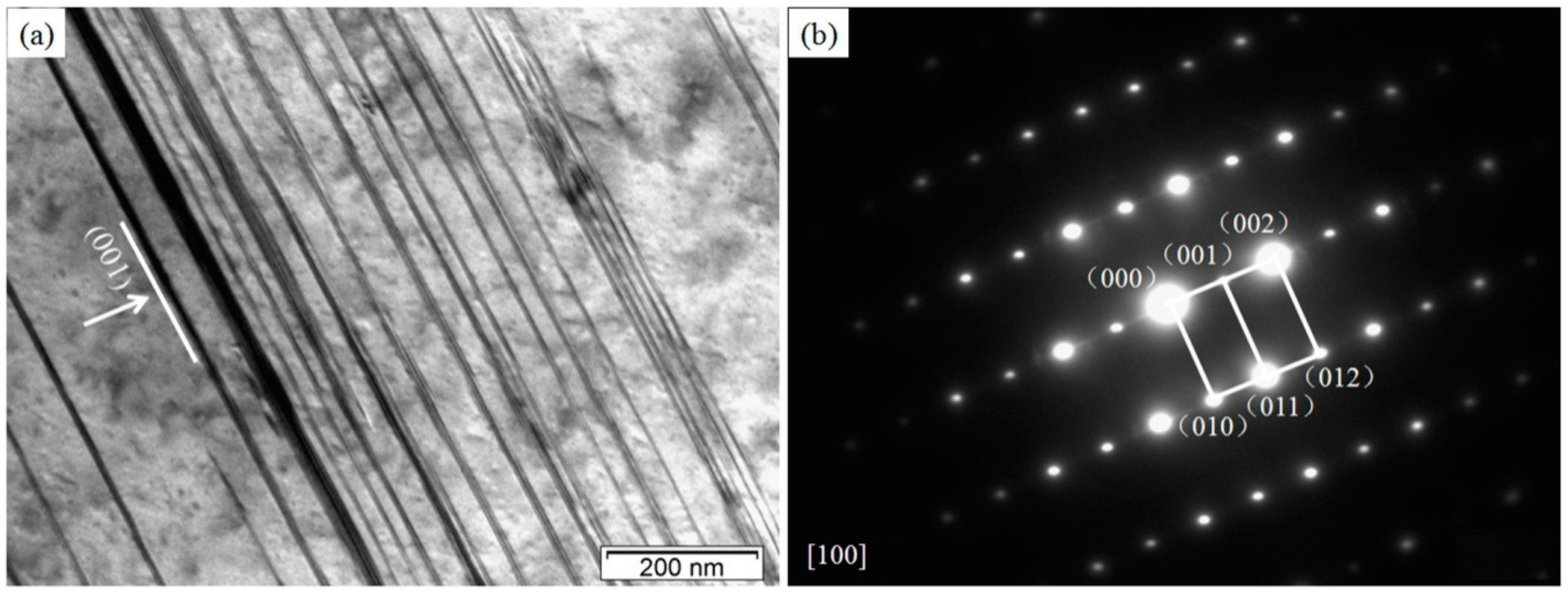
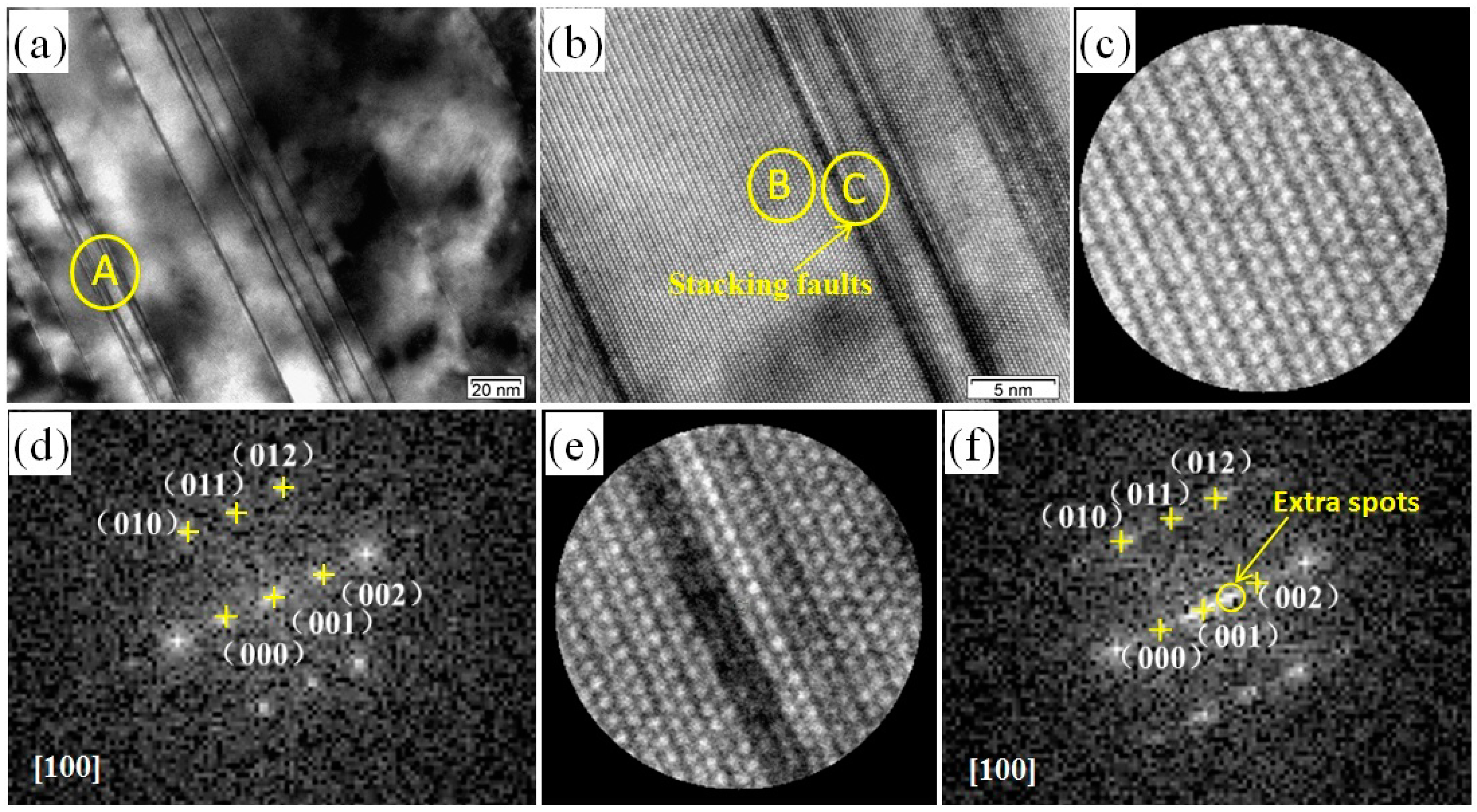
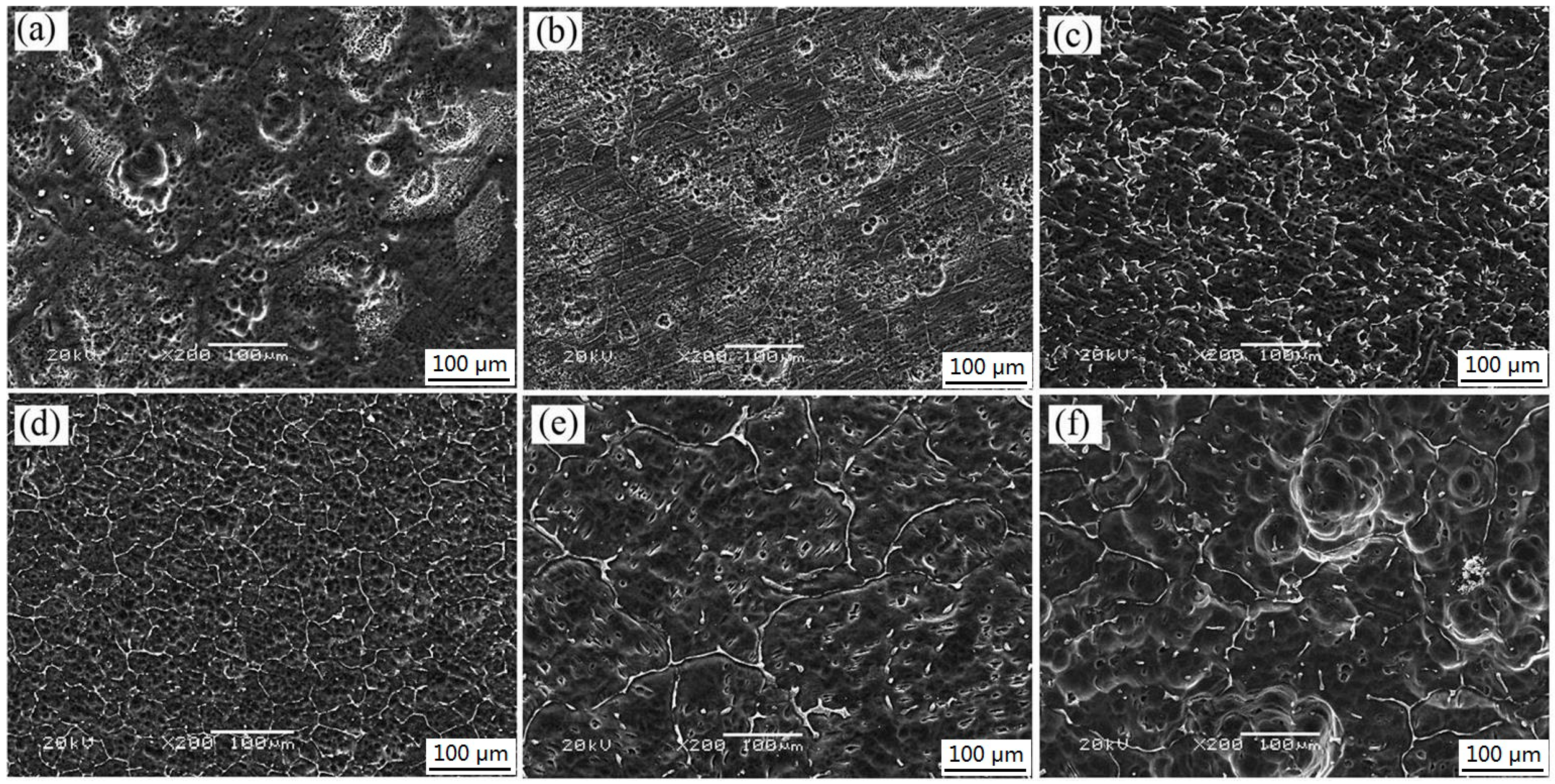
3.1. Microstructures
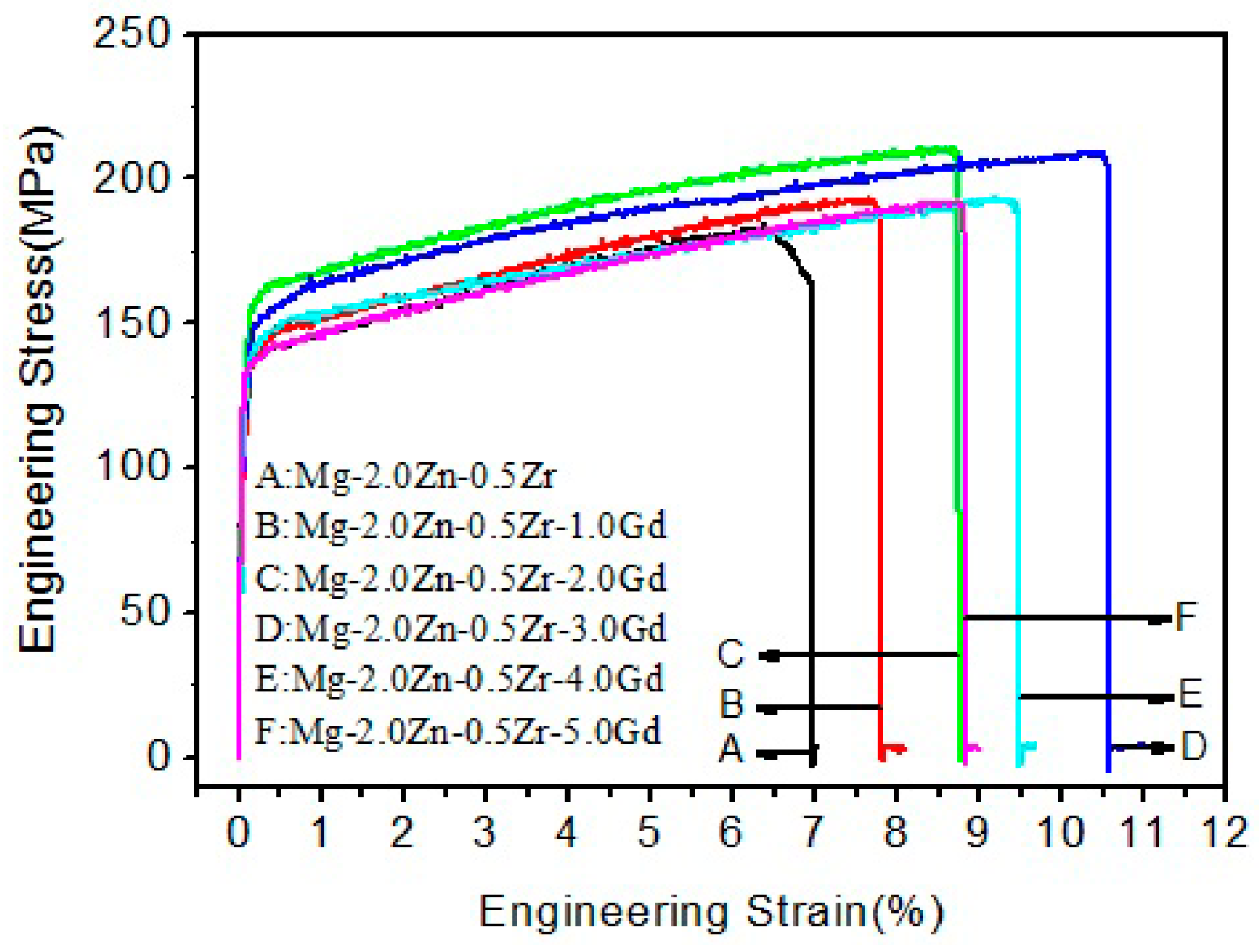
3.2. Mechanical Properties
3.3. Fracture Characteristics
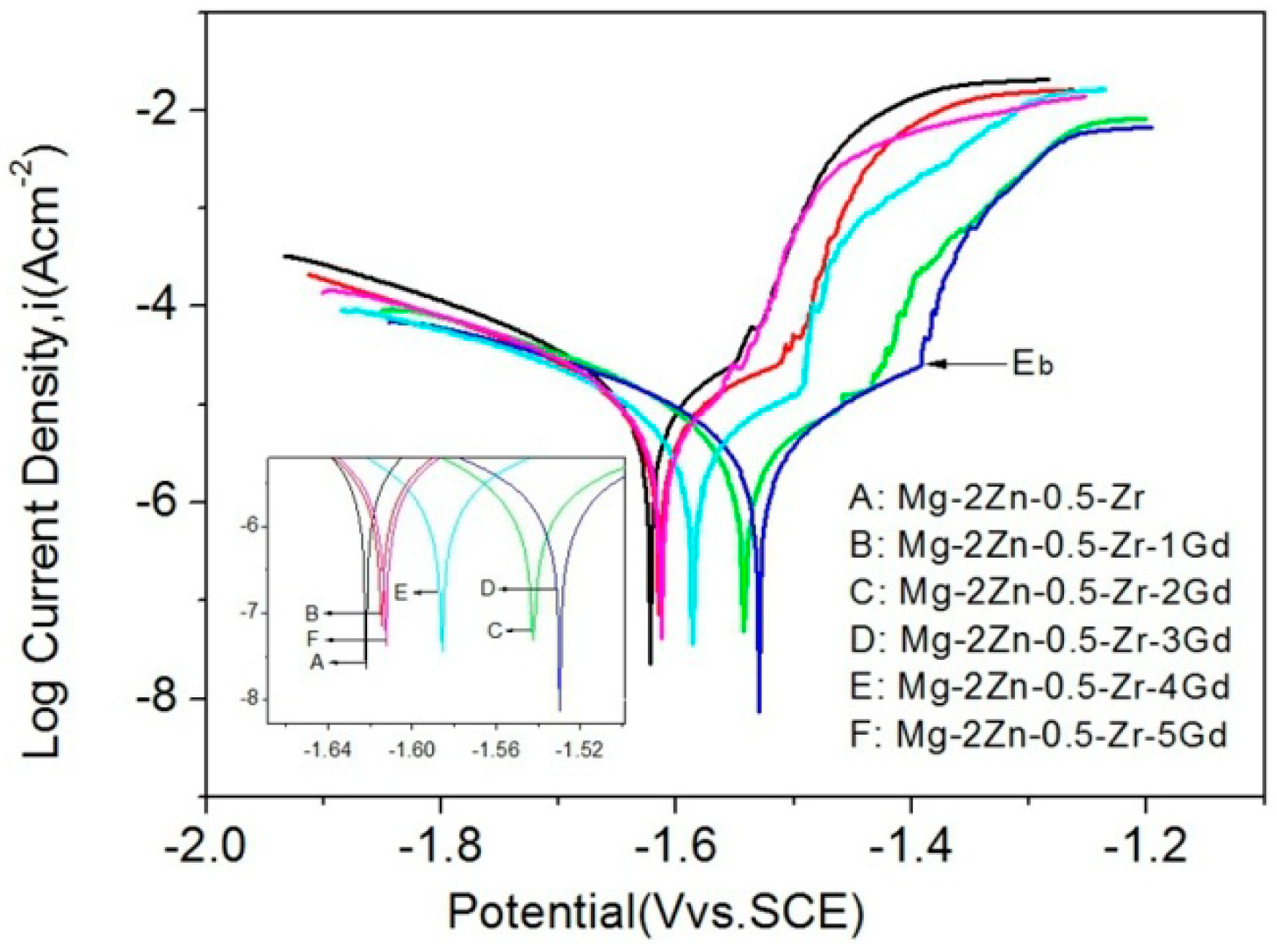
3.4. Corrosion Behavior
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Homma, T.; Mendis, C.L.; Hono, K.; Kamado, S. Effect of Zr addition on the mechanical properties of as-extruded Mg-Zn-Ca-Zr alloys. Mat. Sci. Eng. A 2010, 527, 2356–2362. [Google Scholar] [CrossRef]
- Homayun, B.; Afshar, A. Microstructure, mechanical properties, corrosion behavior and cytotoxicity of Mg-Zn-Al-Ca alloys as biodegradable materials. J. Alloys Compd. 2014, 607, 1–10. [Google Scholar] [CrossRef]
- Jamesh, M.I.; Wu, G.; Zhao, Y.; McKenzie, D.R.; Bilek, M.M.M.; Chu, P.K. Electrochemical corrosion behavior of biodegradable Mg-Y-RE and Mg-Zn-Zr alloys in Ringer’s solution and simulated body fluid. Corros. Sci. 2015, 91, 160–184. [Google Scholar] [CrossRef]
- Jamsh, M.; Kumar, S.; Sankara Narayanan, T.S.N. Corrosion behavior of commercially pure Mg and ZM21 Mg alloy in Ringer’s solution—Long term evaluation by EIS. Corros. Sci. 2011, 53, 645–654. [Google Scholar] [CrossRef]
- Hartwig, A. Role of magnesium in genomic stability. Mutat. Res. 2001, 475, 113–121. [Google Scholar] [CrossRef]
- Jafari, S.; Raman, R.K.S.; Davies, C.H.J. Corrosion fatigue of a magnesium alloy in modified simulated body fluid. Eng. Fract. Mech. 2015, 137, 2–11. [Google Scholar] [CrossRef]
- Witte, F. The history of biodegradable magnesium implants: A review. Acta Biomater. 2010, 23, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Yang, L. Microstructure, mechanical properties and bio-corrosion properties of Mg-Zn-Mn-Ca alloy for biomedical application. Mater. Sci. Eng. A 2008, 497, 111–118. [Google Scholar] [CrossRef]
- Ding, Y.; Wen, C.; Hodgson, P.; Li, Y. Effects of alloying elements on the corrosion behavior and biocompatibility of biodegradabl e magnesium alloys: A review. J. Mater. Chem. B 2014, 2, 1912–1933. [Google Scholar] [CrossRef]
- Gui, Z.Z.; Kang, Z.X.; Li, Y.Y. Mechanical and corrosion properties of Mg-Gd-Zn-Zr-Mn biodegradable alloy by hot extrusion. J. Alloys Compd. 2016, 685, 222–230. [Google Scholar] [CrossRef]
- Sun, M.; Wu, G.H.; Wang, W.; Ding, W.J. Effect of Zr on the microstructure, mechanical properties and corrosion resistance of Mg-10Gd-3Y magnesium alloy. Mater. Sci. Eng. A 2009, 523, 145–151. [Google Scholar] [CrossRef]
- Feyerabend, F.; Fischer, J.; Holtz, J.; Witte, F.; Willumeit, R.; Drücker, H.; Vogt, C.; Hort, N. Evaluation of short-term effects of rare earth and other elements used in magnesium alloys on primary cells and cell lines. Acta Biomater. 2010, 6, 1834–1842. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.B.; Ba, Z.X.; Wang, Z.Z.; Wu, Y.J.; Xue, Y.J. Effect of LPSO structure on mechanical properties and corrosion behavior of as-extruded GZ51K magnesium alloy. Mater. Lett. 2016, 163, 250–253. [Google Scholar] [CrossRef]
- Gao, Y.C.; Li, J.P.; Li, J.S.; Yang, Z.; Zhao, J.; Xia, F.; Liang, M.X. Mg-Gd-Y system phase diagram calculation and experimental clarification. J. Alloys Compd. 2008, 450, 446–451. [Google Scholar] [CrossRef]
- Jeong, J.S.; Kim, W.J. Enhancement of mechanical properties and corrosion resistance of Mg-Ca alloys through microstructural refinement by indirect extrusion. Corros. Sci. 2014, 82, 392–403. [Google Scholar] [CrossRef]
- Virtanen, S. Biodegradable Mg and Mg alloys: Corrosion and biocompatibility. Mater. Sci. Eng. B 2011, 176, 1600–1608. [Google Scholar] [CrossRef]
- Shi, Z.M.; Liu, M.; Atrens, A. Measurement of the corrosion rate of magnesium alloys using Tafel extrapolation. Corros. Sci. 2010, 52, 579–588. [Google Scholar] [CrossRef]
- Zhao, M.C.; Liu, M.; Song, G.; Atrens, A. Influence of pH and chloride ion concentration on the corrosion of Mg alloy ZE41. Corros. Sci. 2015, 50, 3168–3178. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A. Understanding magnesium corrosion-A framework for improved alloy performance. Adv. Eng. Mater. 2003, 5, 837–858. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Idris, M.H.; Abdul-Kadir, M.R.; Ourdjini, A.; Medraj, M.; Daroonparvar, M. Mechanical and bio-corrosion properties of quaternary Mg-Ca-Mn-Zn alloys compared with binary Mg-Ca alloys. Mater. Des. 2014, 53, 283–292. [Google Scholar] [CrossRef]
- Zhang, J.S.; Zhang, W.B.; Bian, L.P.; Cheng, W.L.; Niu, X.F.; Xu, C.X.; Wu, S.J. Study of Mg-Gd-Zn-Zr alloys with long period stacking ordered structures. Mater. Sci. Eng. A 2013, 585, 268–276. [Google Scholar] [CrossRef]
- Xu, C.; Zheng, M.Y.; Wu, K.; Wang, E.D.; Fan, G.H.; Xu, S.W.; Kamado, S.; Liu, X.D.; Wang, G.J.; Lv, X.Y.; et al. Effect of final rolling reduction on the microstructure and mechanical properties of Mg-Gd-Y-Zn-Zr alloy sheets. Mater. Sci. Eng. A 2013, 559, 232–240. [Google Scholar] [CrossRef]
- Lu, F.M.; Ma, A.B.; Jiang, J.H.; Yang, D.H.; Yuan, Y.C.; Zhang, L.Y. Formation of profuse long period stacking ordered microcells in Mg-Gd-Zn-Zr alloy during multipass ECAP process. J. Alloys Compd. 2014, 601, 140–145. [Google Scholar] [CrossRef]
- Tang, Y.X.; Li, B.; Tang, H.X.; Xu, Y.C.; Gao, Y.P.; Wang, L.H.; Guan, J.Y. Effect of long period stacking ordered structure on mechanical and damping properties of as-cast Mg-Zn-Y-Zr alloy. Mater. Sci. Eng. A 2015, 640, 287–294. [Google Scholar] [CrossRef]
- Zhang, X.B.; Ba, Z.X.; Wang, Z.Z.; Xue, Y.J. Microstructures and corrosion behavior of biodegradable Mg-6Gd-xZn-0.4Zr alloys with and without long period stacking ordered structure. Corros. Sci. 2016, 105, 68–77. [Google Scholar] [CrossRef]
- Xu, D.K.; Han, E.H.; Xu, Y.B. Effect of long-period stacking ordered phase on microstructure, mechanical property and corrosion resistance of Mg alloys: A review. Prog. Nat. Sci. Mater. 2016, 26, 117–128. [Google Scholar] [CrossRef]
- Zhang, S.; Yuan, G.Y.; Lu, C.; Ding, W.J. The relationship between (Mg, Zn)3RE phase and 14H-LPSO phase in Mg-Gd-Y-Zn-Zr alloys solidified at different cooling rates. J. Alloys Compd. 2011, 509, 3515–3521. [Google Scholar] [CrossRef]
- Liu, L.Z.; Chen, X.H.; Pan, F.S.; Wang, Z.W.; Liu, W.; Cao, P.; Yan, T.; Xu, X.Y. Effect of Y and Ce additions on microstructure and mechanical properties of Mg-Zn-Zr alloys. Mater. Sci. Eng. A 2015, 644, 247–253. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, J.H.; Rokhlin, L.L.; Elkin, F.M.; Tang, D.X.; Meng, J. Microstructures and mechanical properties of extruded Mg-8Gd-0.4Zr alloys containing Zn. Mater. Sci. Eng. A 2009, 505, 13–19. [Google Scholar] [CrossRef]
- Bae, D.H.; Kim, S.H.; Kim, D.H.; Kim, W.T. Deformation behavior of Mg-Zn-Y alloys reinforced by icosahedral quasicrystalline particles. Acta Mater. 2002, 50, 2343–2356. [Google Scholar] [CrossRef]
- Feng, H.; Liu, H.P.; Cao, H.; Yang, Y.; Xu, Y.C.; Guan, J.Y. Effect of precipitates on mechanical and damping properties of Mg-Zn-Y-Nd alloys. Mater. Sci. Eng. A 2015, 639, 1–7. [Google Scholar] [CrossRef]
- Xu, D.K.; Liu, L.; Xu, Y.B.; Han, E.H. The influence of element Y on the mechanical properties of the as-extruded Mg-Zn-Y-Zr alloys. J. Alloys Compd. 2006, 426, 155–161. [Google Scholar] [CrossRef]
- Liu, K.; Wang, X.D.; Wen, K. Effect of isothermal homogenization on microstructure and mechanical properties of the Mg-5Y-4Gd-0.5Zn-0.4Zr alloy. Mater. Des. 2013, 52, 1035–1042. [Google Scholar] [CrossRef]
- Ardelean, H.; Frateur, I.; Zanna, S.; Atrens, A.; Marcus, P. Corrosion protection of AZ91 magnesium alloy by anodizing in niobium and zirconium-containing electrolytes. Corros. Sci. 2009, 51, 3030–3038. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, B.P.; Wang, Y.; Geng, L.; Jiao, X.H. Preparation and characterization of a new biomedical Mg-Zn-Ca alloy. Mater. Des. 2012, 34, 58–64. [Google Scholar] [CrossRef]
- Abidin, N.I.Z.; Atrens, A.D.; Martin, D.; Atrens, A. Corrosion of high purity Mg, Mg2Zn0.2Mn, ZE41 and AZ91 in Hank’s solution at 37 °C. Corros. Sci. 2011, 53, 3542–3556. [Google Scholar] [CrossRef]
- Srinivasan, A.; Huang, Y.; Mendis, C.L.; Blawert, C.; Kainer, K.U.; Hort, N. Investigations on microstructures, mechanical and corrosion properties of Mg-Gd-Zn alloys. Mater. Sci. Eng. A 2014, 595, 224–234. [Google Scholar] [CrossRef]

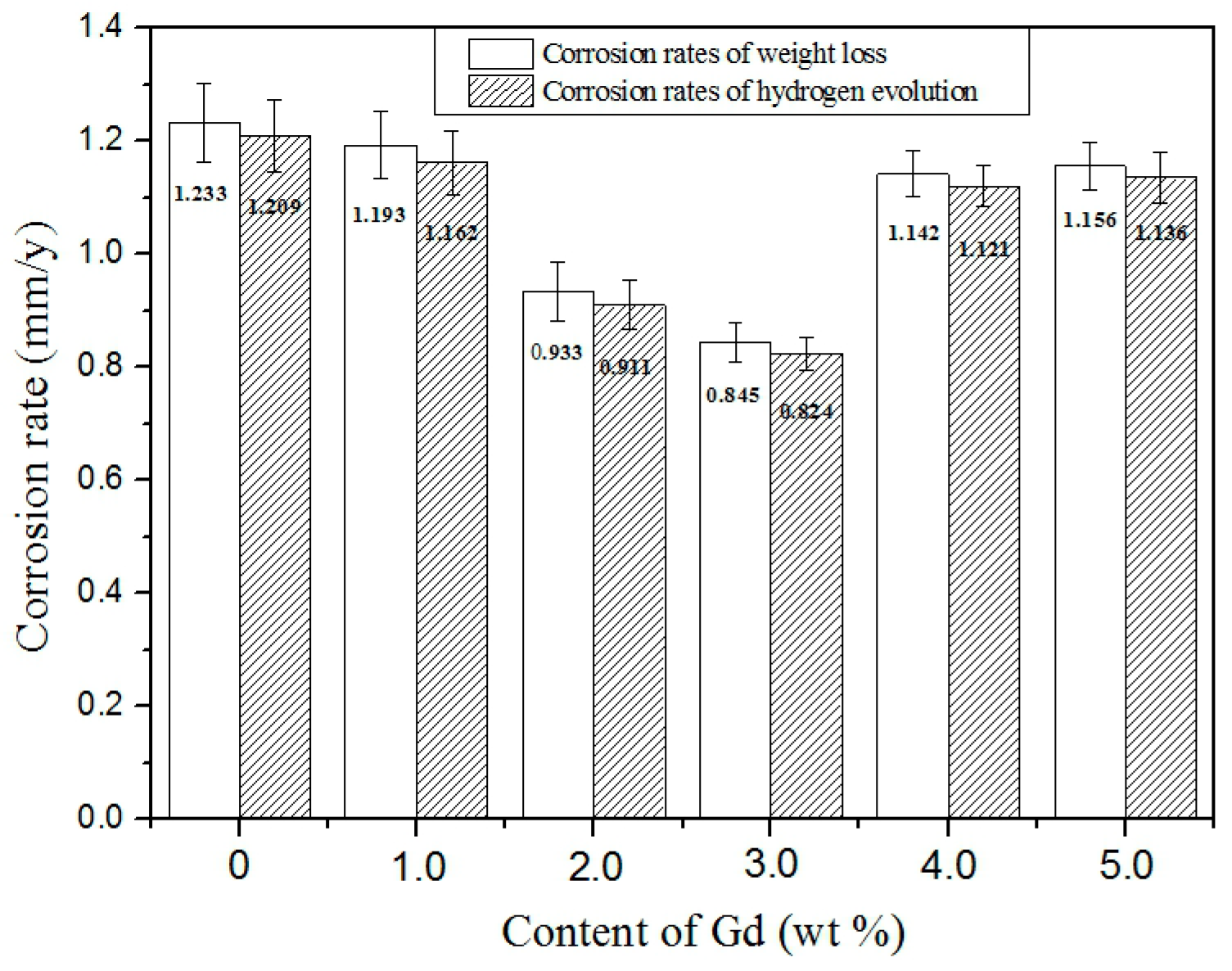
| Material | 0Gd | 1Gd | 2Gd | 3Gd | 4Gd | 5Gd |
|---|---|---|---|---|---|---|
| UTS (MPa) | 183 ± 3 | 193 ± 4 | 210 ± 3 | 204 ± 3 | 196 ± 4 | 192 ± 3 |
| YS (MPa) | 137 ± 3 | 143 ± 4 | 158 ± 3 | 155 ± 3 | 146 ± 4 | 141 ± 3 |
| EL (%) | 7 ± 0.6 | 7.8 ± 0.5 | 8.7 ± 0.6 | 10.6 ± 0.6 | 9.5 ± 0.5 | 8.9 ± 0.5 |
| Material | 0Gd | 1Gd | 2Gd | 3Gd | 4Gd | 5Gd |
|---|---|---|---|---|---|---|
| Ecorr (Vvs.SCE) | −1.621 ± 0.004 | −1.615 ± 0.003 | −1.542 ± 0.004 | −1.531 ± 0.003 | −1.586 ± 0.003 | −1.613 ± 0.004 |
| Icorr (μA cm−2) | 9.278 ± 0.005 | 8.947 ± 0.004 | 8.136 ± 0.005 | 7.686 ± 0.004 | 8.746 ± 0.004 | 8.863 ± 0.005 |
| Pi (mm/y) | 0.416 ± 0.003 | 0.401 ± 0.002 | 0.367 ± 0.003 | 0.343 ± 0.002 | 0.389 ± 0.002 | 0.398 ± 0.003 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, H.; Wen, J.; Xiong, Y.; Liu, Y.; Lu, Y.; Cao, W. Microstructures, Mechanical Properties, and Corrosion Behavior of As-Cast Mg–2.0Zn–0.5Zr–xGd (wt %) Biodegradable Alloys. Materials 2018, 11, 1564. https://doi.org/10.3390/ma11091564
Yao H, Wen J, Xiong Y, Liu Y, Lu Y, Cao W. Microstructures, Mechanical Properties, and Corrosion Behavior of As-Cast Mg–2.0Zn–0.5Zr–xGd (wt %) Biodegradable Alloys. Materials. 2018; 11(9):1564. https://doi.org/10.3390/ma11091564
Chicago/Turabian StyleYao, Huai, Jiuba Wen, Yi Xiong, Ya Liu, Yan Lu, and Wei Cao. 2018. "Microstructures, Mechanical Properties, and Corrosion Behavior of As-Cast Mg–2.0Zn–0.5Zr–xGd (wt %) Biodegradable Alloys" Materials 11, no. 9: 1564. https://doi.org/10.3390/ma11091564
APA StyleYao, H., Wen, J., Xiong, Y., Liu, Y., Lu, Y., & Cao, W. (2018). Microstructures, Mechanical Properties, and Corrosion Behavior of As-Cast Mg–2.0Zn–0.5Zr–xGd (wt %) Biodegradable Alloys. Materials, 11(9), 1564. https://doi.org/10.3390/ma11091564





