Polystyrene Opals Responsive to Methanol Vapors
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Polystyrene Photonic Crystals
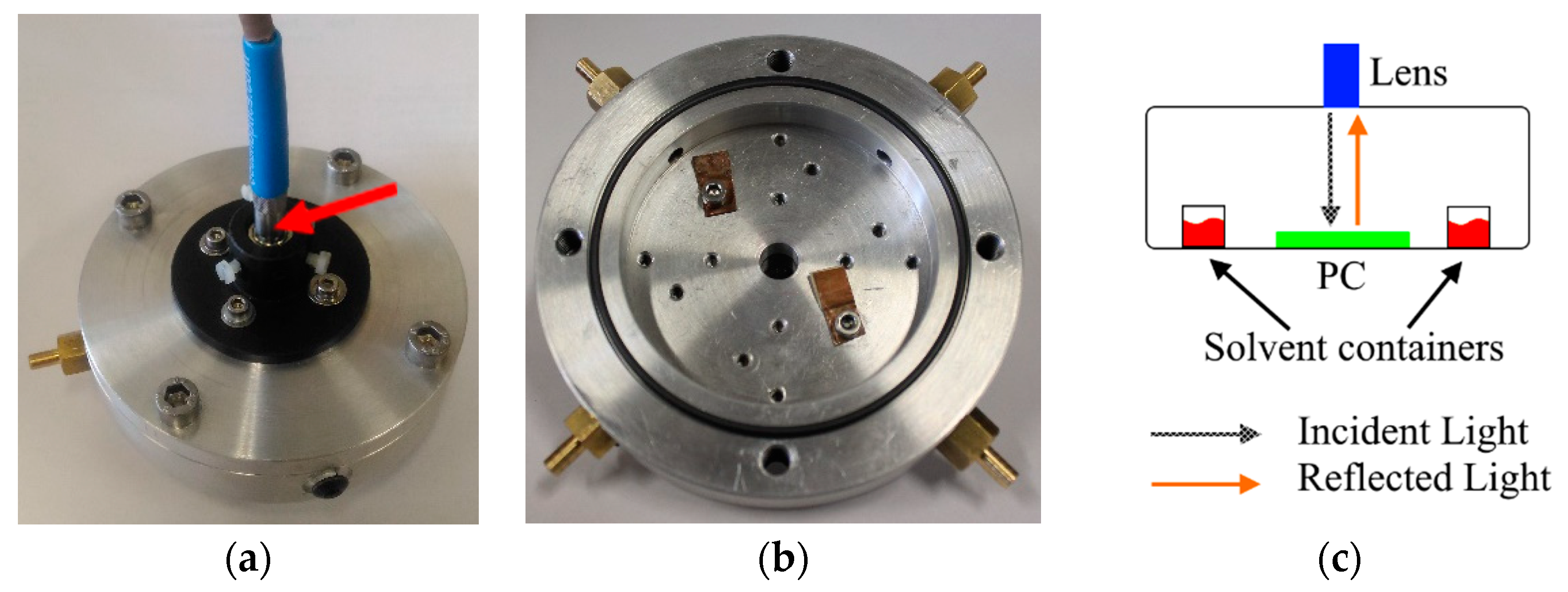
2.3. Apparatus
2.4. Detection of Methanol
3. Results and Discussion
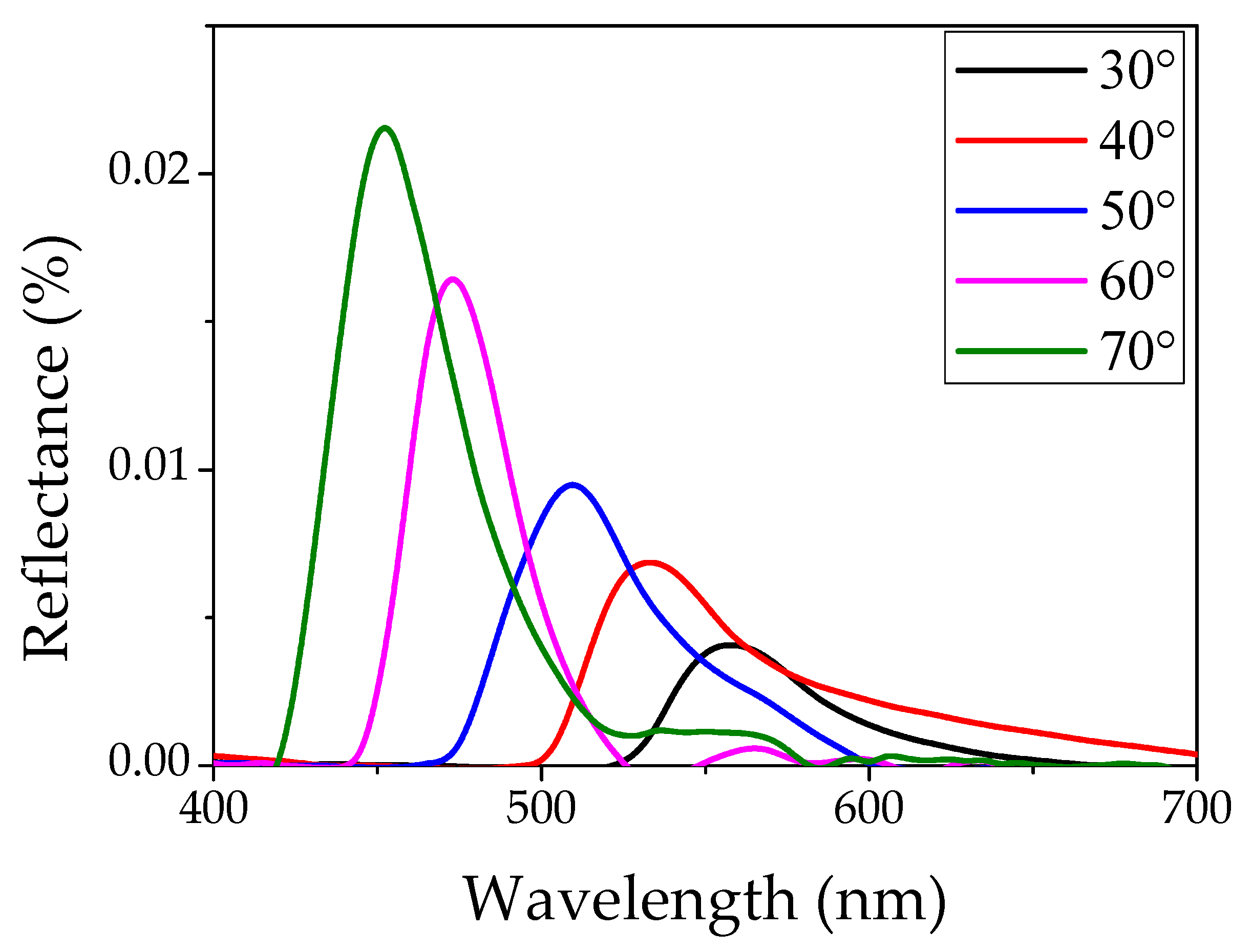
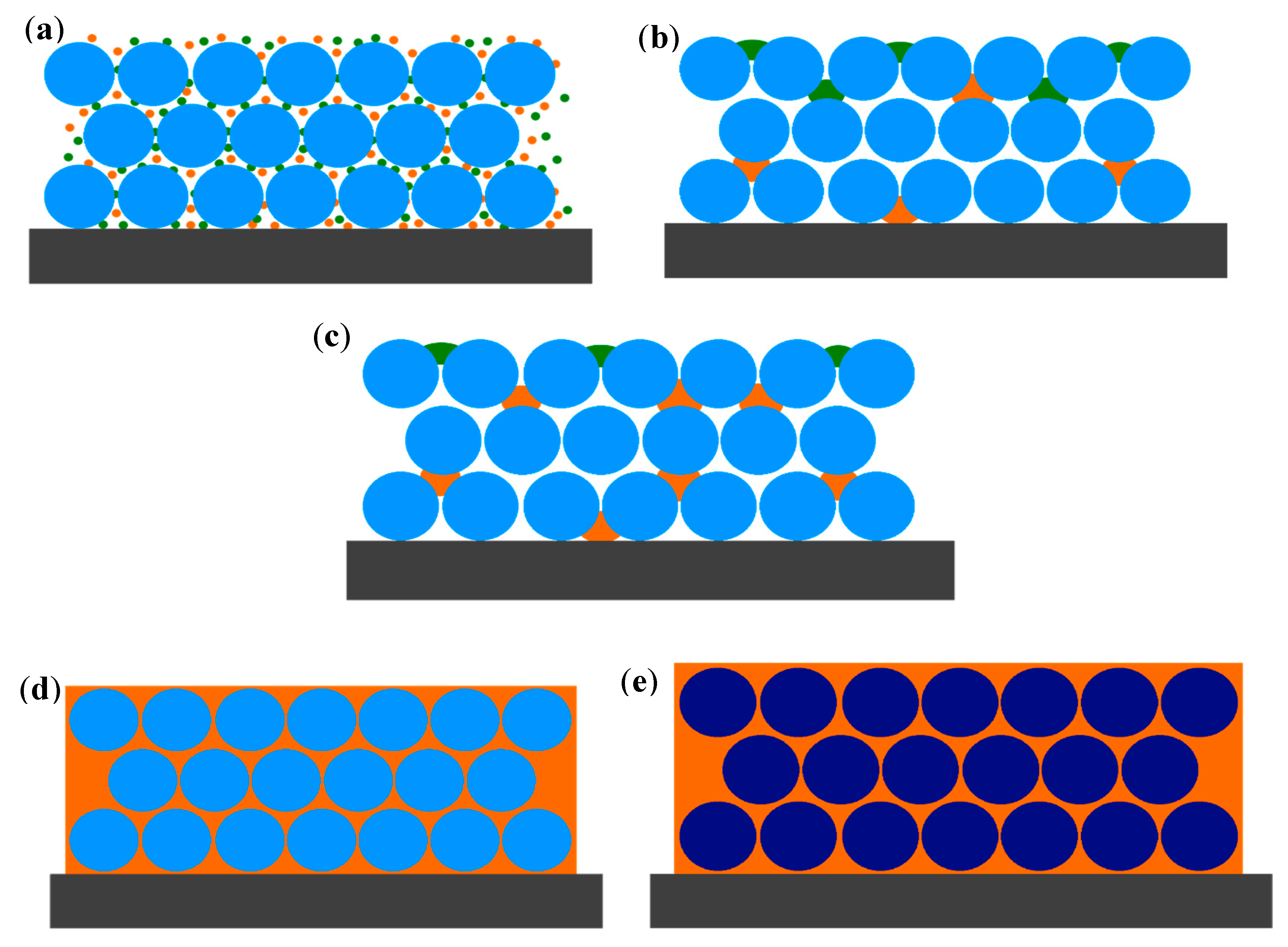
3.1. Optical and Structural Characterization of PS Opals
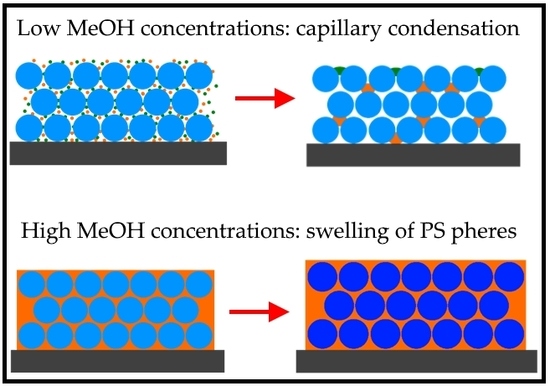
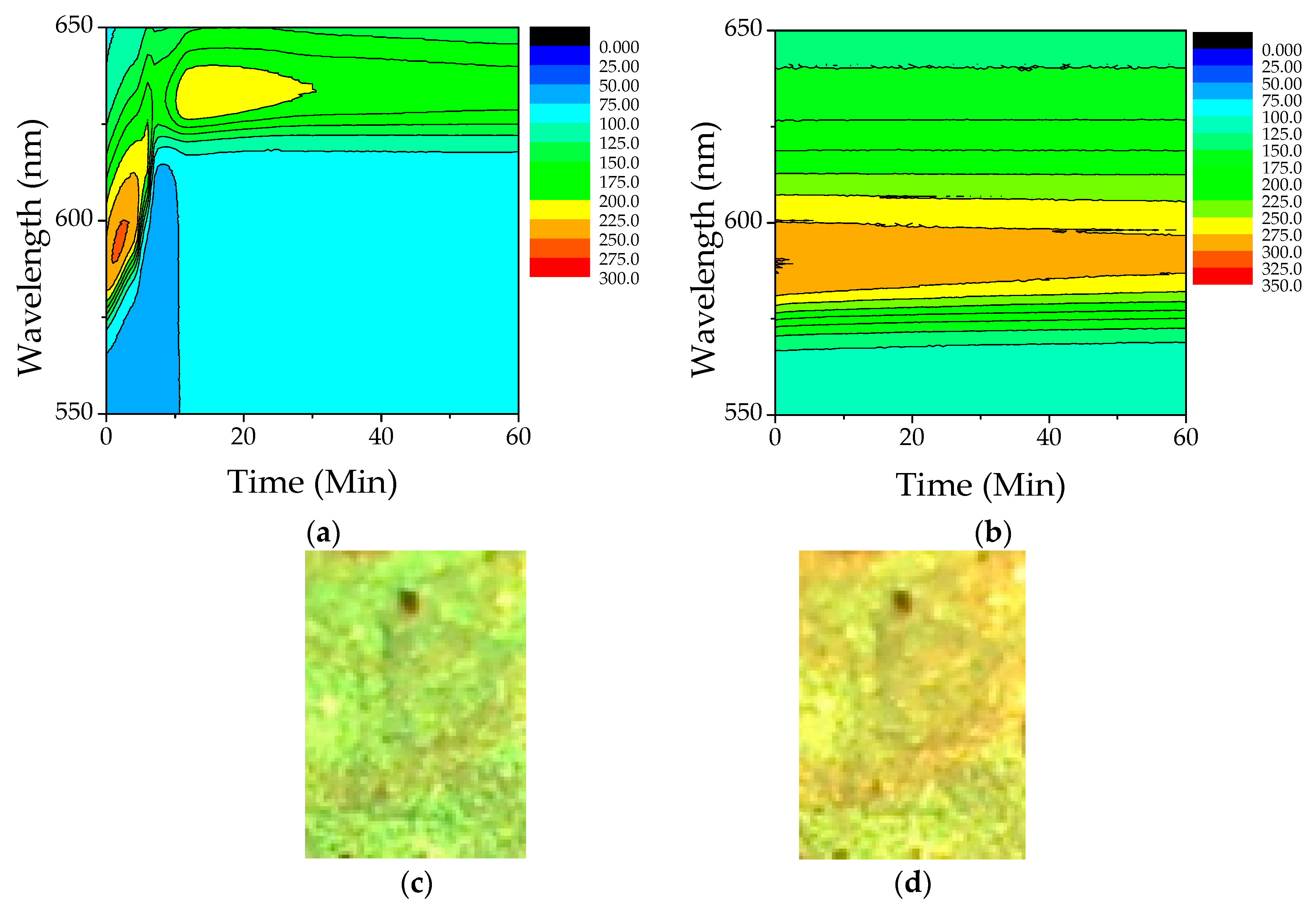
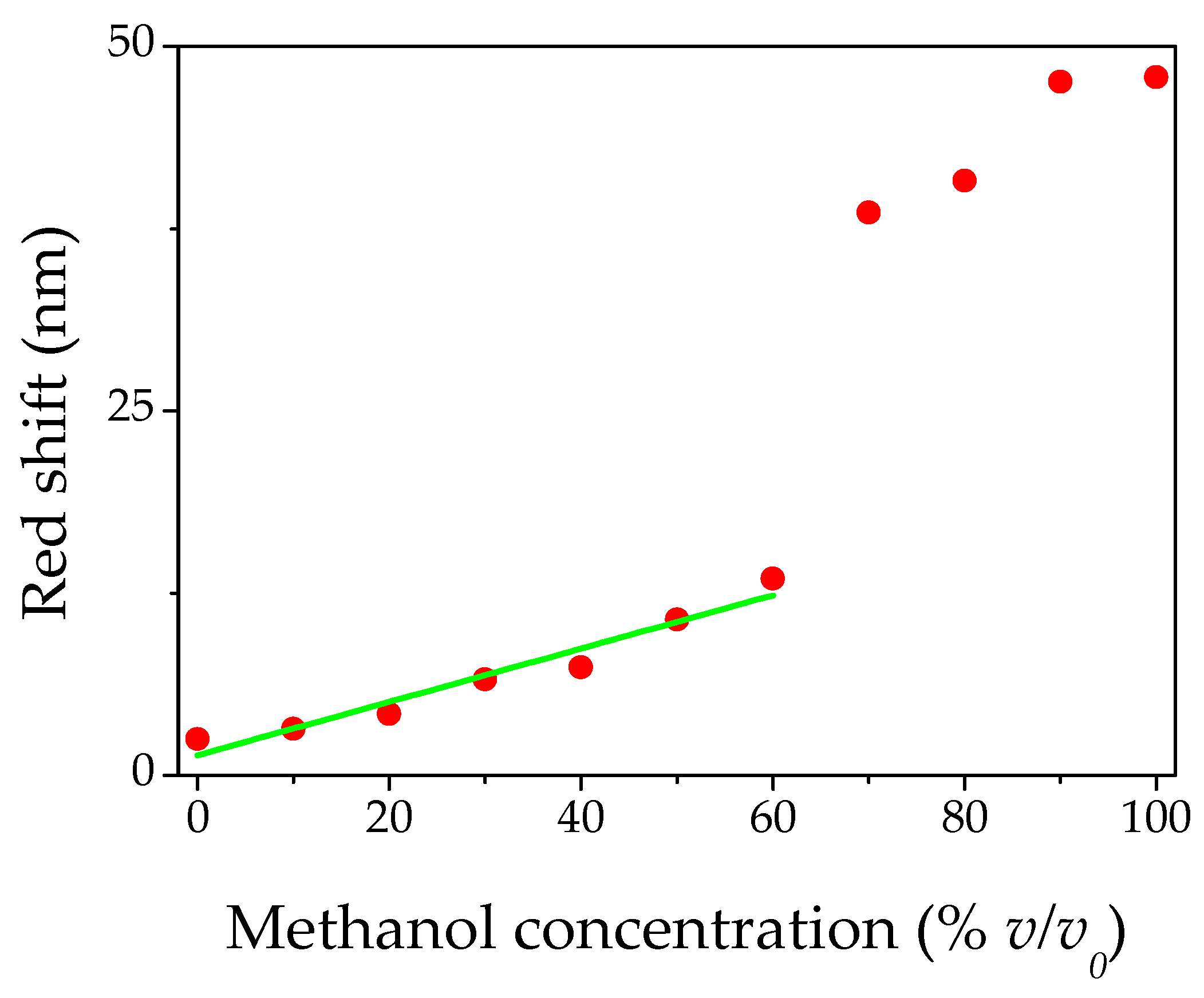
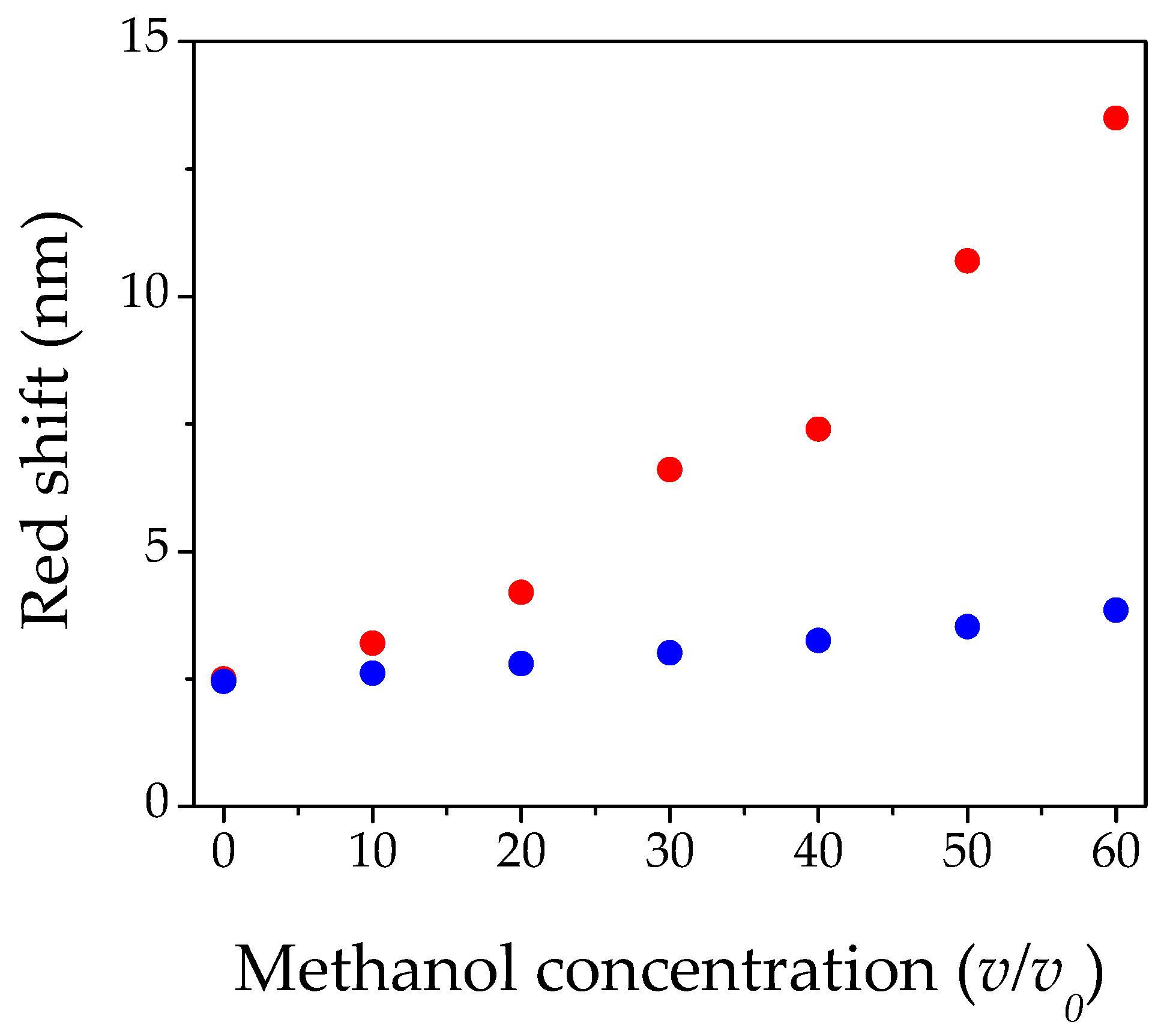
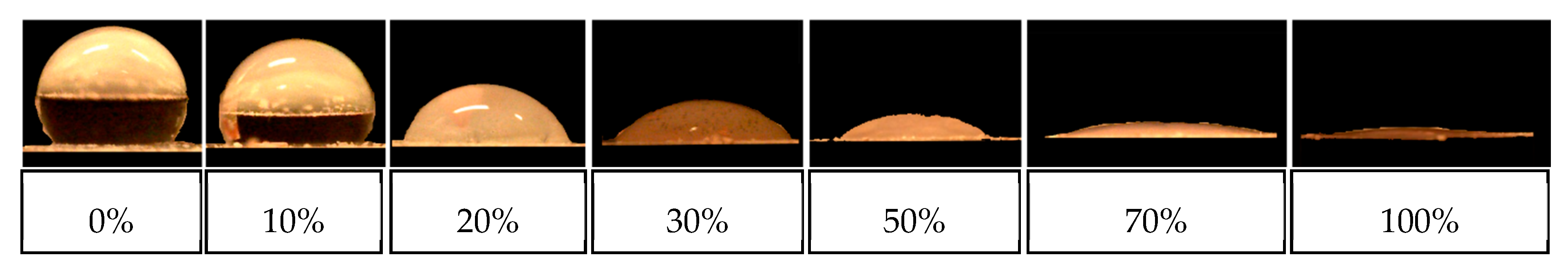
3.2. Optical Behavior of PS Opal in the Presence of Different Methanol Concentrations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ahmed, M.J.K.; Ahmaruzzaman, M. A review on potential usage of industrial waste materials for binding heavy metal ions from aqueous solutions. J. Water Process Eng. 2016, 10, 39–47. [Google Scholar] [CrossRef]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of heavy metal (loid)s contaminated soils—To mobilize or to immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef] [PubMed]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciotta, E.; Prosposito, P.; Tagliatesta, P.; Lorecchio, C.; Stella, L.; Kaciulis, S.; Soltani, P.; Placidi, E.; Pizzoferrato, R. Discriminating between different heavy metal ions with fullerene-derived nanoparticles. Sensors 2018, 18, 1496. [Google Scholar] [CrossRef] [PubMed]
- Ciotta, E.; Paoloni, S.; Richetta, M.; Prosposito, P.; Tagliatesta, P.; Lorecchio, C.; Venditti, I.; Fratoddi, I.; Casciardi, S.; Pizzoferrato, R. Sensitivity to heavy-metal ions of unfolded fullerene quantum dots. Sensors 2017, 17, 2614. [Google Scholar] [CrossRef] [PubMed]
- Prosposito, P.; Mochi, F.; Ciotta, E.; Casalboni, M.; De Matteis, F.; Venditti, I.; Fontana, L.; Testa, G.; Fratoddi, I. Hydrophilic silver nanoparticles with tunable optical properties: Application for the detection of heavy metals in water. Beilstein J. Nanotechnol. 2016, 7, 1654–1661. [Google Scholar] [CrossRef] [PubMed]
- Mochi, F.; Burratti, L.; Fratoddi, I.; Venditti, I.; Battocchio, C.; Carlini, L.; Iucci, G.; Casalboni, M.; Matteis, F.; Casciardi, S.; et al. Plasmonic Sensor Based on Interaction between Silver Nanoparticles and Ni2+ or Co2+ in Water. Nanomaterials 2018, 8, 488. [Google Scholar] [CrossRef] [PubMed]
- Wiedinmyer, C.; Yokelson, R.J.; Gullett, B.K. Global emissions of trace gases, particulate matter, and hazardous air pollutants from open burning of domestic waste. Environ. Sci. Technol. 2014, 48, 9523–9530. [Google Scholar] [CrossRef] [PubMed]
- Brunekreef, B.; Holgate, S.T. Air pollution and health. Lancet 2002, 360, 1233–1242. [Google Scholar] [CrossRef]
- Guerry, A.D.; Polasky, S.; Lubchenco, J.; Chaplin-Kramer, R.; Daily, G.C.; Griffin, R.; Ruckelshaus, M.; Bateman, I.J.; Duraiappah, A.; Elmqvist, T.; et al. Natural capital and ecosystem services informing decisions: From promise to practice. Proc. Natl. Acad. Sci. USA 2015, 112, 7348–7355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Ang, H.M.; Tade, M.O. Volatile organic compounds in indoor environment and photocatalytic oxidation: State of the art. Environ. Int. 2007, 33, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Field, M. Distribution of volatile organic chemicals i n outdoor and indoor air. Environ. Sci. Technol. 1988, 22, 1381–1388. [Google Scholar]
- Hassanian-Moghaddam, H.; Pajoumand, A.; Dadgar, S.M.; Shadnia, S. Prognostic factors in methanol poisoning. Hum. Exp. Toxicol. 2007, 26, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.M.; Yates, C.; Megarbane, B.; Winchester, J.F.; Maclaren, R.; Gosselin, S.; Nolin, T.D.; Lavergne, V.; Hoffman, R.S.; Ghannoum, M. Recommendations for the role of extracorporeal treatments in the management of acute methanol poisoning: A systematic review and consensus statement. Crit. Care Med. 2015, 43, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Paasma, R.; Hovda, K.E.; Jacobsen, D. Methanol poisoning and long term sequelae—A six years follow-up after a large methanol outbreak. BMC Clin. Pharmacol. 2009, 9, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kruse, J.A. Intensive Care Medicine 9. Intensive Care Med. 1992, 18, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, R.J.; Mason, R.W.; Beasley, D.M.G.; Vale, J.A.; Schep, L.J. Isopropanol poisoning. Clin. Toxicol. 2014, 52, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Bunc, M.; Pezdir, T.; Moẑina, H.; Moẑina, M.; Brvar, M. Butanol ingestion in an airport hangar. Hum. Exp. Toxicol. 2006, 25, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Capone, S.; Forleo, A.; Francioso, L.; Rella, R.; Siciliano, P.; Spadavecchia, J.; Presicce, D.S.; Taurino, A.M. Solid state gas sensors: State of the art and future activities. J. Optoelectron. Adv. Mater. 2003, 5, 1335–1348. [Google Scholar] [CrossRef]
- Badhulika, S.; Myung, N.V.; Mulchandani, A. Conducting polymer coated single-walled carbon nanotube gas sensors for the detection of volatile organic compounds. Talanta 2014, 123, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Plecenik, T.; Moško, M.; Haidry, A.A.; Ďurina, P.; Truchlý, M.; Grančič, B.; Gregor, M.; Roch, T.; Satrapinskyy, L.; Mošková, A.; et al. Fast highly-sensitive room-temperature semiconductor gas sensor based on the nanoscale Pt–TiO2–Pt sandwich. Sens. Actuators B Chem. 2015, 207, 351–361. [Google Scholar] [CrossRef]
- Lee, D.S.; Jung, J.K.; Lim, J.W.; Huh, J.S.; Lee, D.D. Recognition of volatile organic compounds using SnO2 sensor array and pattern recognition analysis. Sens. Actuators B Chem. 2001, 77, 228–236. [Google Scholar] [CrossRef]
- Barettin, D.; De Angelis, R.; Prosposito, P.; Auf Der Maur, M.; Casalboni, M.; Pecchia, A. Model of a realistic InP surface quantum dot extrapolated from atomic force microscopy results. Nanotechnology 2014, 25, 195201. [Google Scholar] [CrossRef] [PubMed]
- Akrajas; Mat Salleh, M.; Yahaya, M. Enriching the selectivity of metalloporphyrins chemical sensors by means of optical technique. Sens. Actuators B Chem. 2002, 85, 191–196. [Google Scholar] [CrossRef]
- De Angelis, R.; D’Amico, L.; Casalboni, M.; Hatami, F.; Masselink, W.T.; Prosposito, P. Photoluminescence sensitivity to methanol vapours of surface InP quantum dot: Effect of dot size and coverage. Sens. Actuators, B Chem. 2013, 189, 113–117. [Google Scholar] [CrossRef]
- Elosua, C.; Matias, I.R.; Bariain, C.; Arregui, F.J. Volatile organic compound optical fiber sensors: A review. Sensors 2006, 6, 1440–1465. [Google Scholar] [CrossRef]
- Consales, M.; Crescitelli, A.; Penza, M.; Aversa, P.; Veneri, P.D.; Giordano, M.; Cusano, A. SWCNT nano-composite optical sensors for VOC and gas trace detection. Sens. Actuators B Chem. 2009, 138, 351–361. [Google Scholar] [CrossRef]
- Kuo, W.-K.; Weng, H.-P.; Hsu, J.-J.; Yu, H. Photonic Crystal-Based Sensors for Detecting Alcohol Concentration. Appl. Sci. 2016, 6, 67. [Google Scholar] [CrossRef]
- Burratti, L.; De Matteis, F.; Casalboni, M.; Francini, R.; Pizzoferrato, R.; Prosposito, P. Polystyrene photonic crystals as optical sensors for volatile organic compounds. Mater. Chem. Phys. 2018, 212, 274–281. [Google Scholar] [CrossRef]
- Fenzl, C.; Hirsch, T.; Wolfbeis, O.S. Photonic crystals for chemical sensing and biosensing. Angew. Chem. Int. Ed. 2014, 53, 3318–3335. [Google Scholar] [CrossRef] [PubMed]
- Fenzl, C.; Hirsch, T.; Wolfbeis, O. Photonic Crystal Based Sensor for Organic Solvents and for Solvent-Water Mixtures. Sensors 2012, 12, 16954–16963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.Y.; Mamak, M.; Von Freymann, G.; Chopra, N.; Ozin, G.A. Mesoporous Bragg Stack Color Tunable Sensors. Nano Lett. 2006, 6, 2456–2461. [Google Scholar] [CrossRef] [PubMed]
- Lova, P.; Bastianini, C.; Giusto, P.; Patrini, M.; Rizzo, P.; Guerra, G.; Iodice, M.; Soci, C.; Comoretto, D. Label-Free Vapor Selectivity in Poly( p -Phenylene Oxide) Photonic Crystal Sensors. ACS Appl. Mater. Interfaces 2016, 8, 31941–31950. [Google Scholar] [CrossRef] [PubMed]
- Mönch, W.; Dehnert, J.; Prucker, O.; Rühe, J.; Zappe, H. Tunable Bragg filters based on polymer swelling. Appl. Opt. 2006, 45, 4284. [Google Scholar] [CrossRef] [PubMed]
- Lazarova, K.; Georgiev, R.; Vasileva, M.; Georgieva, B.; Spassova, M.; Malinowski, N.; Babeva, T. One-dimensional PMMA–V2O5 photonic crystals used as color indicators of chloroform vapors. Opt. Quantum Electron. 2016, 48, 310. [Google Scholar] [CrossRef]
- Schutzmann, S.; Venditti, I.; Prosposito, P.; Casalboni, M.; Russo, M.V. High-energy angle resolved reflection spectroscopy on three-dimensional photonic crystals of self-organized polymeric nanospheres. Opt. Express 2008, 16, 897. [Google Scholar] [CrossRef] [PubMed]
- Schutzmann, S.; Prosposito, P.; Casalboni, M.; Venditti, I.; Russo, M.V. Spectroscopic ellipsometry on photonic crystals made by self-assembled dye-doped P(S/HEMA) nanospheres. Phys. Status Solidi Curr. Top. Solid State Phys. 2008, 5, 1403–1406. [Google Scholar] [CrossRef]
- Pizzoferrato, R.; Ziller, T.; Micozzi, A.; Ricci, A.; Lo Sterzo, C.; Ustione, A.; Oliva, C.; Cricenti, A. Suppression of the excimer photoluminescence in a poly(arylene-ethynylene) co-polymer. Chem. Phys. Lett. 2005, 414, 234–238. [Google Scholar] [CrossRef]
- Barettin, D.; Di Carlo, A.; De Angelis, R.; Casalboni, M.; Prosposito, P. Effect of dielectric Bragg grating nanostructuring on dye sensitized solar cells. Opt. Express 2012, 20, A888. [Google Scholar] [CrossRef] [PubMed]
- Le Guével, X.; Palazzesi, C.; Prosposito, P.; Della Giustina, G.; Brusatin, G. Influence of chelating agents on the photopolymerization of hybrid Ti-based waveguides. J. Mater. Chem. 2008, 18, 3556–3562. [Google Scholar] [CrossRef]
- Kuo, C.Y.; Lu, S.Y.; Chen, S.; Bernards, M.; Jiang, S. Stop band shift based chemical sensing with three-dimensional opal and inverse opal structures. Sens. Actuators B Chem. 2007, 124, 452–458. [Google Scholar] [CrossRef]
- De Angelis, R.; Venditti, I.; Fratoddi, I.; De Matteis, F.; Prosposito, P.; Cacciotti, I.; D’Amico, L.; Nanni, F.; Yadav, A.; Casalboni, M.; et al. From nanospheres to microribbons: Self-assembled Eosin Y doped PMMA nanoparticles as photonic crystals. J. Colloid Interface Sci. 2014, 414, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Joannopoulos, J.J.D.; Johnson, S.; Winn, J.N.J.; Meade, R.R.D. Photonic Crystals: Molding the Flow of Light, 2nd ed.; Princeton University Press: Princeton, NJ, USA, 2008; ISBN 9780691124568. [Google Scholar]
- Kuo, W.K.; Weng, H.P.; Hsu, J.J.; Yu, H.H. A bioinspired color-changing polystyrene microarray as a rapid qualitative sensor for methanol and ethanol. Mater. Chem. Phys. 2016, 173, 285–290. [Google Scholar] [CrossRef]
- Prosposito, P.; Casalboni, M.; Orsini, E.; Palazzesi, C.; Stella, F. UV-nanoimprinting lithography of Bragg Gratings on hybrid sol-gel based channel waveguides. Solid State Sci. 2010, 12, 1886–1889. [Google Scholar] [CrossRef]
- Yadav, A.; De Angelis, R.; Casalboni, M.; De Matteis, F.; Prosposito, P.; Nanni, F.; Cacciotti, I. Spectral properties of self-assembled polystyrene nanospheres photonic crystals doped with luminescent dyes. Opt. Mater. 2013, 35, 1538–1543. [Google Scholar] [CrossRef]
- Serpe, M.J.; Kang, Y.; Zhang, Q.M. Photonic Materials for Sensing, Biosensing and Display Devices; Serpe, M.J., Kang, Y., Zhang, Q.M., Eds.; Springer Series in Materials Science; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-24988-9. [Google Scholar]
- Wang, F.; Zhu, Z.; Xue, M.; Xue, F.; Wang, Q.; Meng, Z.; Lu, W.; Chen, W.; Qi, F.; Yan, Z. Cellulose photonic crystal film sensor for alcohols. Sens. Actuators B Chem. 2015, 220, 222–226. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, Q.; Ge, J. Photonic sensing of organic solvents through geometric study of dynamic reflection spectrum. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, T.; Do, D.D.; Nicholson, D. Capillary condensation of adsorbates in porous materials. Adv. Colloid Interface Sci. 2011, 169, 40–58. [Google Scholar] [CrossRef] [PubMed]
- Rojas, F.; Kornhauser, I.; Felipe, C.; Esparza, J.M.; Cordero, S.; Domínguez, A.; Riccardo, J.L. Capillary condensation in heterogeneous mesoporous networks consisting of variable connectivity and pore-size correlation. Phys. Chem. Chem. Phys. 2002, 4, 2346–2355. [Google Scholar] [CrossRef]
- Hidalgo, N.; Calvo, M.E.; Colodrero, S.; Miguez, H. Porous One-Dimensional Photonic Crystal Coatings for Gas Detection. IEEE Sens. J. 2010, 10, 1206–1212. [Google Scholar] [CrossRef] [Green Version]
- Mönch, W.; Dehnert, J.; Jaufmann, E.; Zappe, H. Flory-Huggins swelling of polymer Bragg mirrors. Appl. Phys. Lett. 2006, 89, 164104. [Google Scholar] [CrossRef]
- Lazarova, K.; Awala, H.; Thomas, S.; Vasileva, M.; Mintova, S.; Babeva, T. Vapor Responsive One-Dimensional Photonic Crystals from Zeolite Nanoparticles and Metal Oxide Films for Optical Sensing. Sensors 2014, 14, 12207–12218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Zheng, T. A comparison of chemical sensors based on the different ordered inverse opal films. Sens. Actuators B Chem. 2008, 131, 190–195. [Google Scholar] [CrossRef]

| Liquid Phase MeOH:H2O (vol:vol) | Percentage of MeOH in Liquid Phase | MeOH in Liquid Phase (Molar Fraction) | MeOH in Vapor Phase (Molar Fraction) | MeOH in Vapor Phase (ppm) |
|---|---|---|---|---|
| 1:9 | 10% | 0.047 | 0.070 | 7843 |
| 1:4 | 20% | 0.100 | 0.015 | 16,669 |
| 1:2.3 | 30% | 0.160 | 0.024 | 26,627 |
| 1:1.5 | 40% | 0.229 | 0.034 | 37,966 |
| 1:1 | 50% | 0.308 | 0.046 | 50,998 |
| 1.5:1 | 60% | 0.400 | 0.060 | 66,130 |
| 2.3:1 | 70% | 0.509 | 0.076 | 83,915 |
| 4:1 | 80% | 0.640 | 0.096 | 105,117 |
| 9:1 | 90% | 0.800 | 0.119 | 130,828 |
| 10:0 | 100% | 1.000 | 0.149 | 162,655 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burratti, L.; Casalboni, M.; De Matteis, F.; Pizzoferrato, R.; Prosposito, P. Polystyrene Opals Responsive to Methanol Vapors. Materials 2018, 11, 1547. https://doi.org/10.3390/ma11091547
Burratti L, Casalboni M, De Matteis F, Pizzoferrato R, Prosposito P. Polystyrene Opals Responsive to Methanol Vapors. Materials. 2018; 11(9):1547. https://doi.org/10.3390/ma11091547
Chicago/Turabian StyleBurratti, Luca, Mauro Casalboni, Fabio De Matteis, Roberto Pizzoferrato, and Paolo Prosposito. 2018. "Polystyrene Opals Responsive to Methanol Vapors" Materials 11, no. 9: 1547. https://doi.org/10.3390/ma11091547
APA StyleBurratti, L., Casalboni, M., De Matteis, F., Pizzoferrato, R., & Prosposito, P. (2018). Polystyrene Opals Responsive to Methanol Vapors. Materials, 11(9), 1547. https://doi.org/10.3390/ma11091547