Structure-Function Correlative Microscopy of Peritubular and Intertubular Dentine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
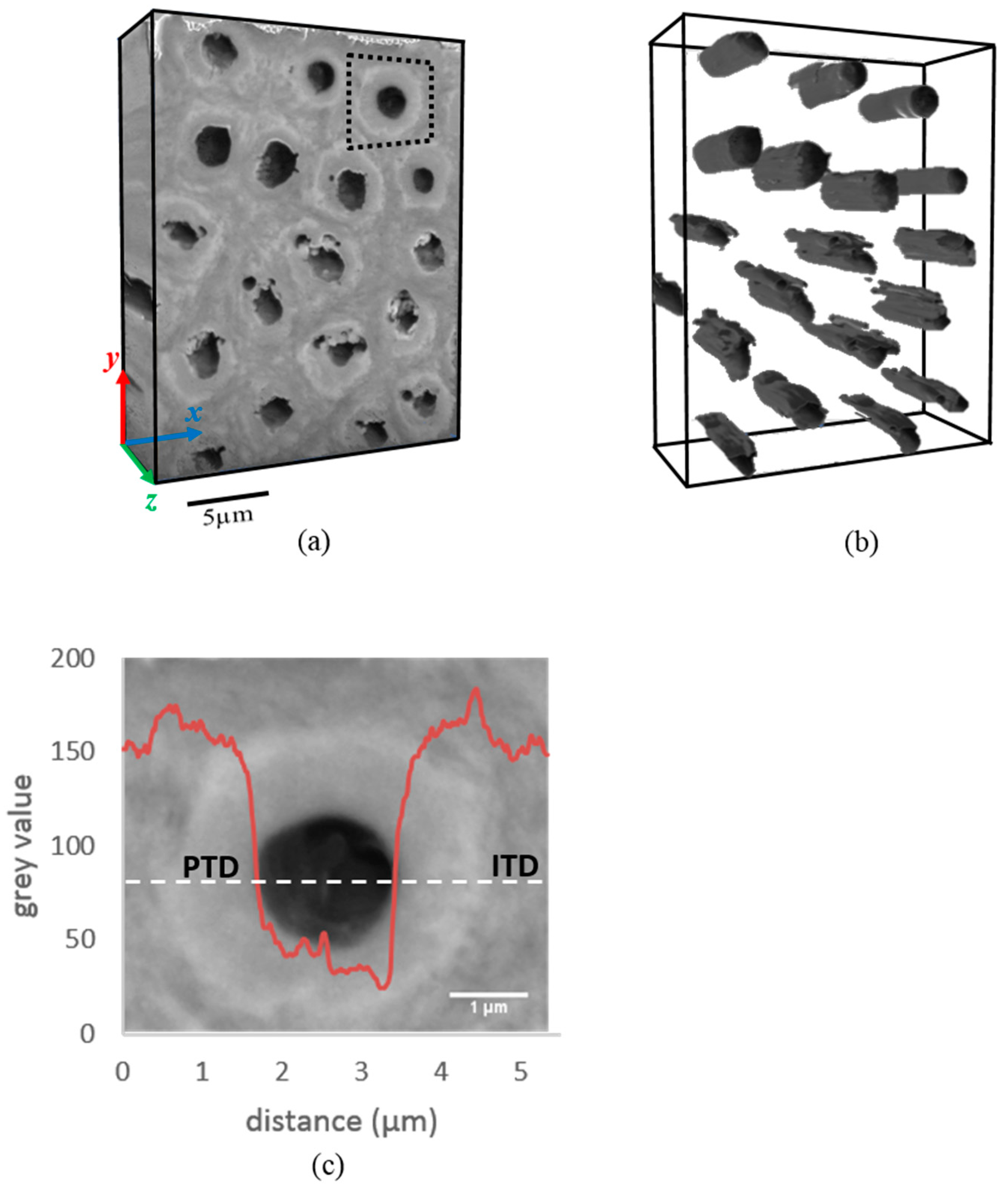
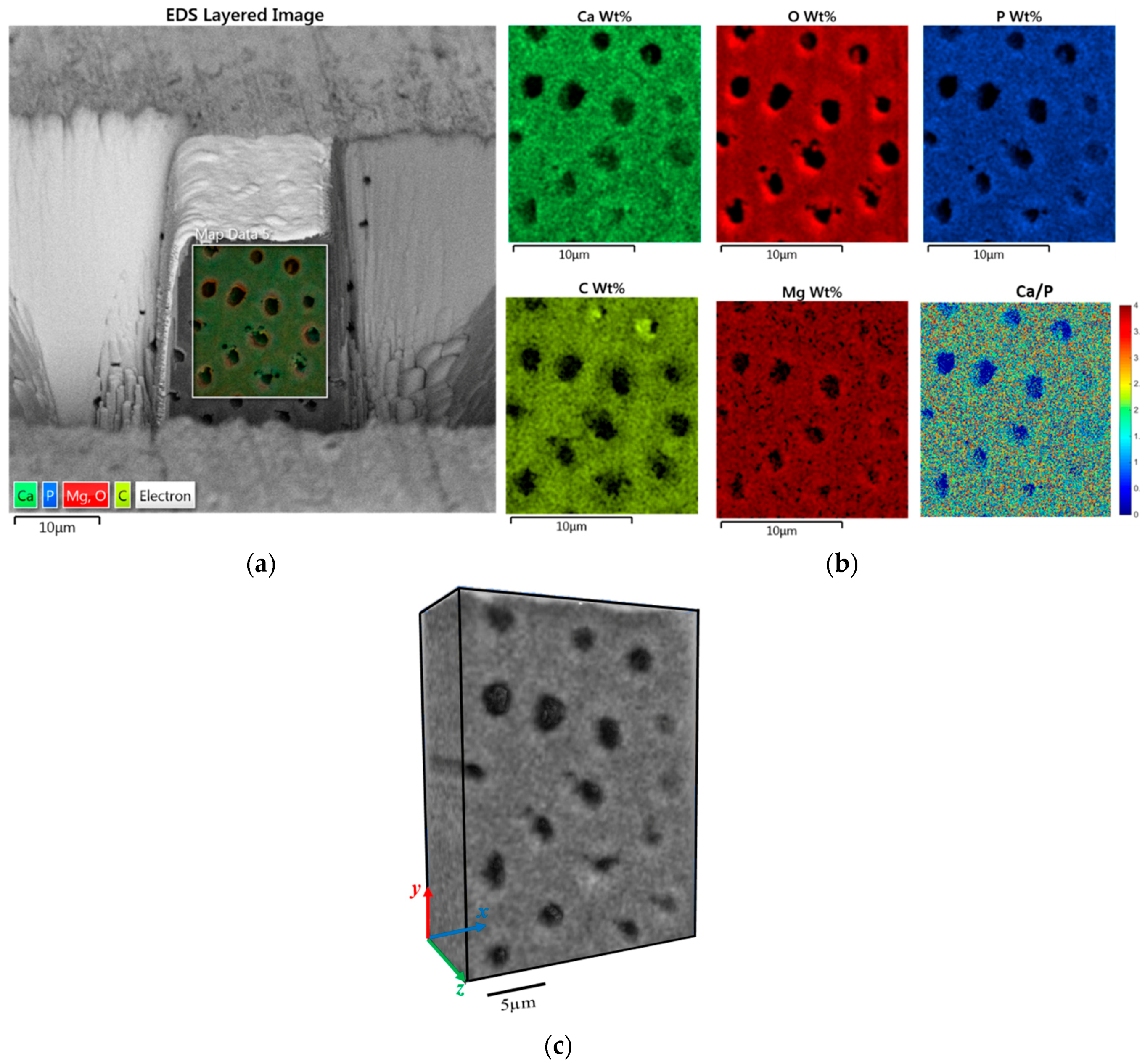
2.2. 3D FIB-SEM-EDS Tomography
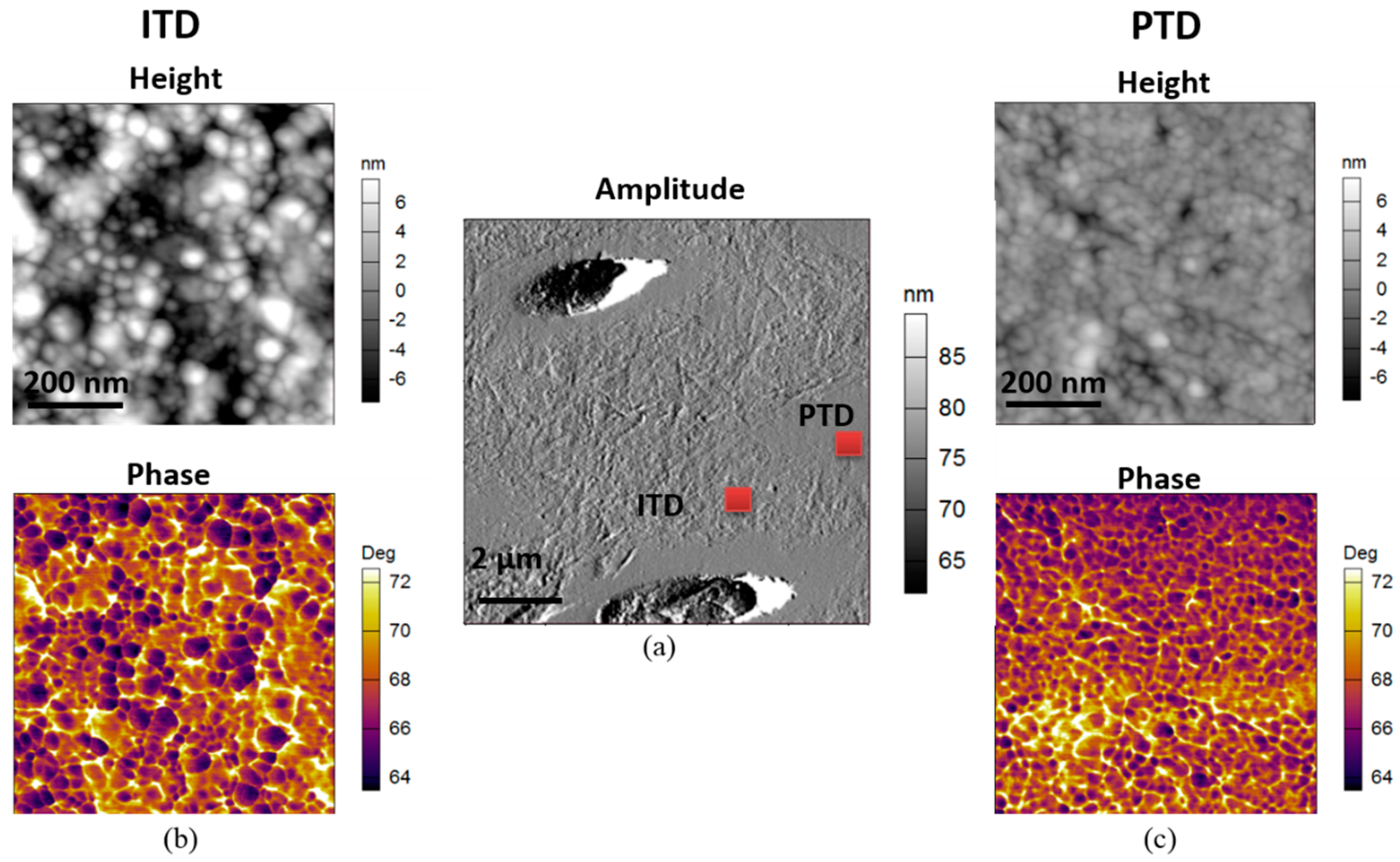
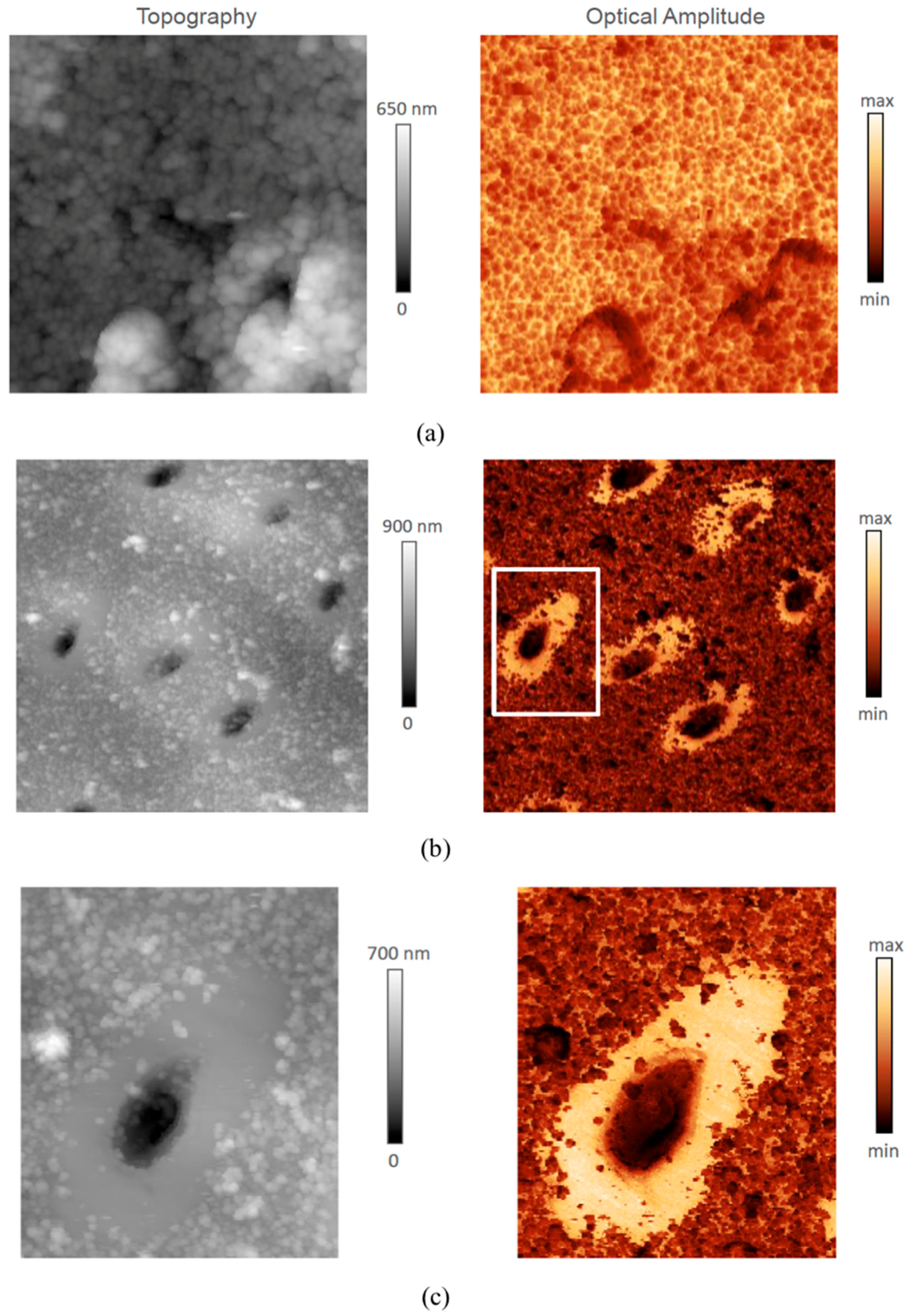
2.3. 2D Tapping Mode AFM
2.4. s-SNOM Imaging
3. Results
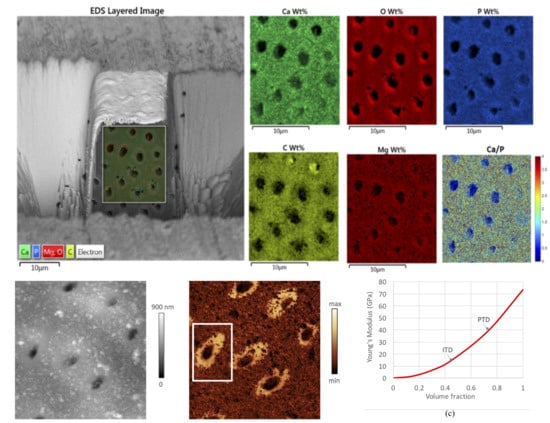
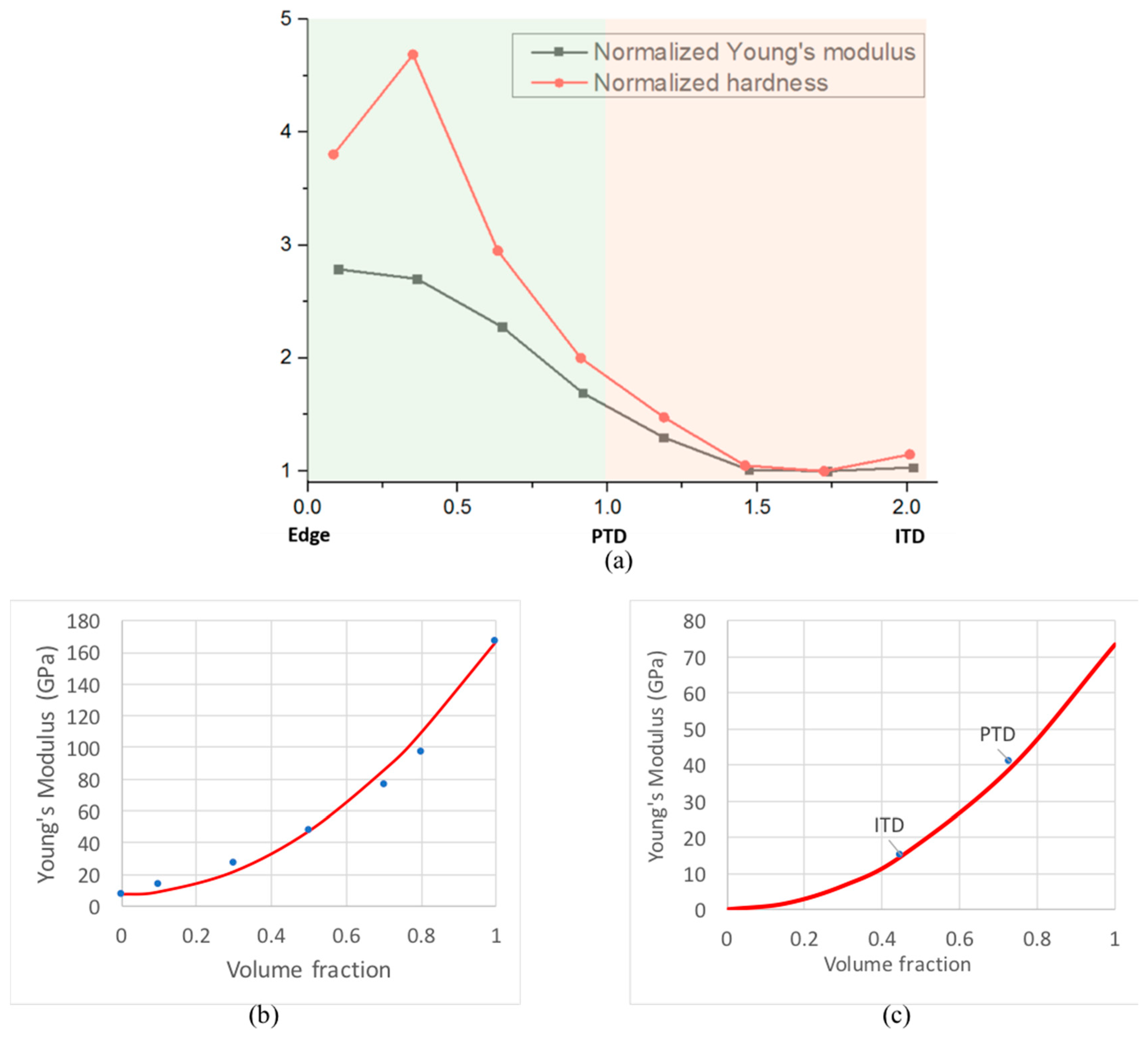
3.1. Structural Difference between PTD and ITD
3.2. Chemical Composition Difference between PTD and ITD
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ten Cate, A.R.; Dale, A.C. Oral Histology: Development, Structure, and Function; Mosby: Maryland Heights, MO, USA, 1980. [Google Scholar]
- Petrovic, L.M.; Spasic, D.T.; Atanackovic, T.M. On a mathematical model of a human root dentin. Dent. Mater. 2005, 21, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Pashley, D.H.; Ciucchi, B.; Sano, H.; Carvalho, R.M.; Russell, C.M. Bond strength versus dentine structure: A modelling approach. Arch. Oral Biol. 1995, 40, 1109–1118. [Google Scholar] [CrossRef]
- Habelitz, S.; Rodriguez, B.J.; Marshall, S.J.; Marshall, G.W.; Kalinin, S.V.; Gruverman, A. Peritubular dentin lacks piezoelectricity. J. Dent. Res. 2007, 86, 908–911. [Google Scholar] [CrossRef] [PubMed]
- Bertassoni, L.E.; Stankoska, K.; Swain, M.V. Insights into the structure and composition of the peritubular dentin organic matrix and the lamina limitans. Micron 2012, 43, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Weiner, S.; Veis, A.; Beniash, E.; Arad, T.; Dillon, J.W.; Sabsay, B.; Siddiqui, F. Peritubular dentin formation: Crystal organization and the macromolecular constituents in human teeth. J. Struct. Biol. 1999, 126, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Stock, S.R.; Deymier-Black, A.C.; Veis, A.; Telser, A.; Lux, E.; Cai, Z. Bovine and equine peritubular and intertubular dentin. Acta Biomater. 2014, 10, 3969–3977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stock, S.R.; Veis, A.; Telser, A.; Cai, Z. Near tubule and intertubular bovine dentin mapped at the 250 nm level. J. Struct. Biol. 2011, 176, 203–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forien, J.B.; Fleck, C.; Cloetens, P.; Duda, G.; Fratzl, P.; Zolotoyabko, E.; Zaslansky, P. Compressive residual strains in mineral nanoparticles as a possible origin of enhanced crack resistance in human tooth dentin. Nano Lett. 2015, 15, 3729–3734. [Google Scholar] [CrossRef] [PubMed]
- Zanette, I.; Enders, B.; Dierolf, M.; Thibault, P.; Gradl, R.; Diaz, A.; Guizar-Sicairos, M.; Menzel, A.; Pfeiffer, F.; Zaslansky, P. Ptychographic X-ray nanotomography quantifies mineral distributions in human dentine. Sci. Rep. 2015, 5, 9210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sezen, M.; Sadighikia, S. 3D electron microscopy investigations of human dentin at the micro/nano-scale using focused ion beam based nanostructuring. RSC Adv. 2015, 5, 7196–7199. [Google Scholar] [CrossRef]
- Charadram, N.; Austin, C.; Trimby, P.; Simonian, M.; Swain, M.V.; Hunter, N. Structural analysis of reactionary dentin formed in response to polymicrobial invasion. J. Struct. Biol. 2013, 181, 207–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gotliv, B.A.; Robach, J.S.; Veis, A. The composition and structure of bovine peritubular dentin: Mapping by time of flight secondary ion mass spectroscopy. J. Struct. Biol. 2006, 156, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, R.; Frank, R.M.; Voegel, J.C. The study of human dentine with secondary ion microscopy and electron diffraction. Calcif. Tissue Res. 1976, 19, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zeng, K.Y. Nanoscale elasticity mappings of micro-constituents of abalone shell by band excitation-contact resonance force microscopy. Nanoscale 2014, 6, 2177–2185. [Google Scholar] [CrossRef] [PubMed]
- Balooch, G.; Marshall, G.W.; Marshall, S.J.; Warren, O.L.; Asif, S.A.; Balooch, M. Evaluation of a new modulus mapping technique to investigate microstructural features of human teeth. J. Biomech. 2004, 37, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Marshall, G.W., Jr.; Marshall, S.J.; Kinney, J.H.; Balooch, M. The dentin substrate: Structure and properties related to bonding. J. Dent. 1997, 25, 441–458. [Google Scholar] [CrossRef]
- Habelitz, S.; Marshall, G.W., Jr.; Balooch, M.; Marshall, S.J. Nanoindentation and storage of teeth. J. Biomech. 2002, 35, 995–998. [Google Scholar] [CrossRef]
- Bertassoni, L.E.; Marshall, G.W.; Swain, M.V. Mechanical heterogeneity of dentin at different length scales as determined by AFM phase contrast. Micron 2012, 43, 1364–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landis, W.J. Mineral characterization in calcifying tissues: Atomic, molecular and macromolecular perspectives. Connect. Tissue Res. 1996, 34, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Kinney, J.H.; Balooch, M.; Haupt, D.L.; Marshall, S.J.; Marshall, G.W. Mineral distribution and dimensional changes in human dentin during demineralization. J. Dent. Res. 1995, 74, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Ziskind, D.; Hasday, M.; Cohen, S.R.; Wagner, H.D. Young’s modulus of peritubular and intertubular human dentin by nano-indentation tests. J. Struct. Biol. 2011, 174, 23–30. [Google Scholar] [CrossRef] [PubMed]
- DeLaurier, A.; Boyde, A.; Horton, M.A.; Price, J.S. Analysis of the surface characteristics and mineralization status of feline teeth using scanning electron microscopy. J. Anat. 2006, 209, 655–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voegel, J.C.; Frank, R.M. Ultrastructural-study of apatite crystal dissolution in human dentin and bone. J. Biol. Buccale 1977, 5, 181–194. [Google Scholar] [PubMed]
- Schroeder, L.; Frank, R.M. High-resolution transmission electron-microscopy of adult human peritubular dentin. Cell Tissue Res. 1985, 242, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Pugach, M.K.; Strother, J.; Darling, C.L.; Fried, D.; Gansky, S.A.; Marshall, S.J.; Marshall, G.W. Dentin caries zones: Mineral, structure, and properties. J. Dent. Res. 2009, 88, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Darda, S.; Madria, K.; Jamenis, R.; Heda, A.; Khanna, A.; Sardar, L. An in-vitro evaluation of effect of EDTAC on root dentin with respect to time. J. Int. Oral. Health 2014, 6, 22. [Google Scholar] [PubMed]
- Fritzen, F.; Bohlke, T. Periodic three-dimensional mesh generation for particle reinforced composites with application to metal matrix composites. Int. J. Solids Struct. 2011, 48, 706–718. [Google Scholar] [CrossRef]
- Dutov, P.; Antipova, O.; Varma, S.; Orgel, J.P.R.O.; Schieber, J.D. Measurement of elastic modulus of collagen type I single fiber. PLoS ONE 2016, 11, e0145711. [Google Scholar] [CrossRef] [PubMed]
- Rees, J.S.; Jacobsen, P.H. The elastic moduli of enamel and dentine. Clin. Mater. 1993, 14, 35–39. [Google Scholar] [CrossRef]
- Sui, T.; Sandholzer, M.A.; Baimpas, N.; Dolbnya, I.P.; Walmsley, A.D.; Lumley, P.J.; Landini, G.; Korsunsky, A.M. Multiscale modelling and diffraction-based characterization of elastic behaviour of human dentine. Acta Biomater. 2013, 9, 7937–7947. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.O. The deformation and ageing of mild steel: III discussion of results. Proc. Phys. Soc. Sect. B 1951, 64, 747–753. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sui, T.; Dluhoš, J.; Li, T.; Zeng, K.; Cernescu, A.; Landini, G.; Korsunsky, A.M. Structure-Function Correlative Microscopy of Peritubular and Intertubular Dentine. Materials 2018, 11, 1493. https://doi.org/10.3390/ma11091493
Sui T, Dluhoš J, Li T, Zeng K, Cernescu A, Landini G, Korsunsky AM. Structure-Function Correlative Microscopy of Peritubular and Intertubular Dentine. Materials. 2018; 11(9):1493. https://doi.org/10.3390/ma11091493
Chicago/Turabian StyleSui, Tan, Jiří Dluhoš, Tao Li, Kaiyang Zeng, Adrian Cernescu, Gabriel Landini, and Alexander M. Korsunsky. 2018. "Structure-Function Correlative Microscopy of Peritubular and Intertubular Dentine" Materials 11, no. 9: 1493. https://doi.org/10.3390/ma11091493
APA StyleSui, T., Dluhoš, J., Li, T., Zeng, K., Cernescu, A., Landini, G., & Korsunsky, A. M. (2018). Structure-Function Correlative Microscopy of Peritubular and Intertubular Dentine. Materials, 11(9), 1493. https://doi.org/10.3390/ma11091493