Effect of Ammonium Halide Additives on the Performance of Methyl Amine Based Perovskite Solar Cells
Abstract
1. Introduction
2. Experimental Details
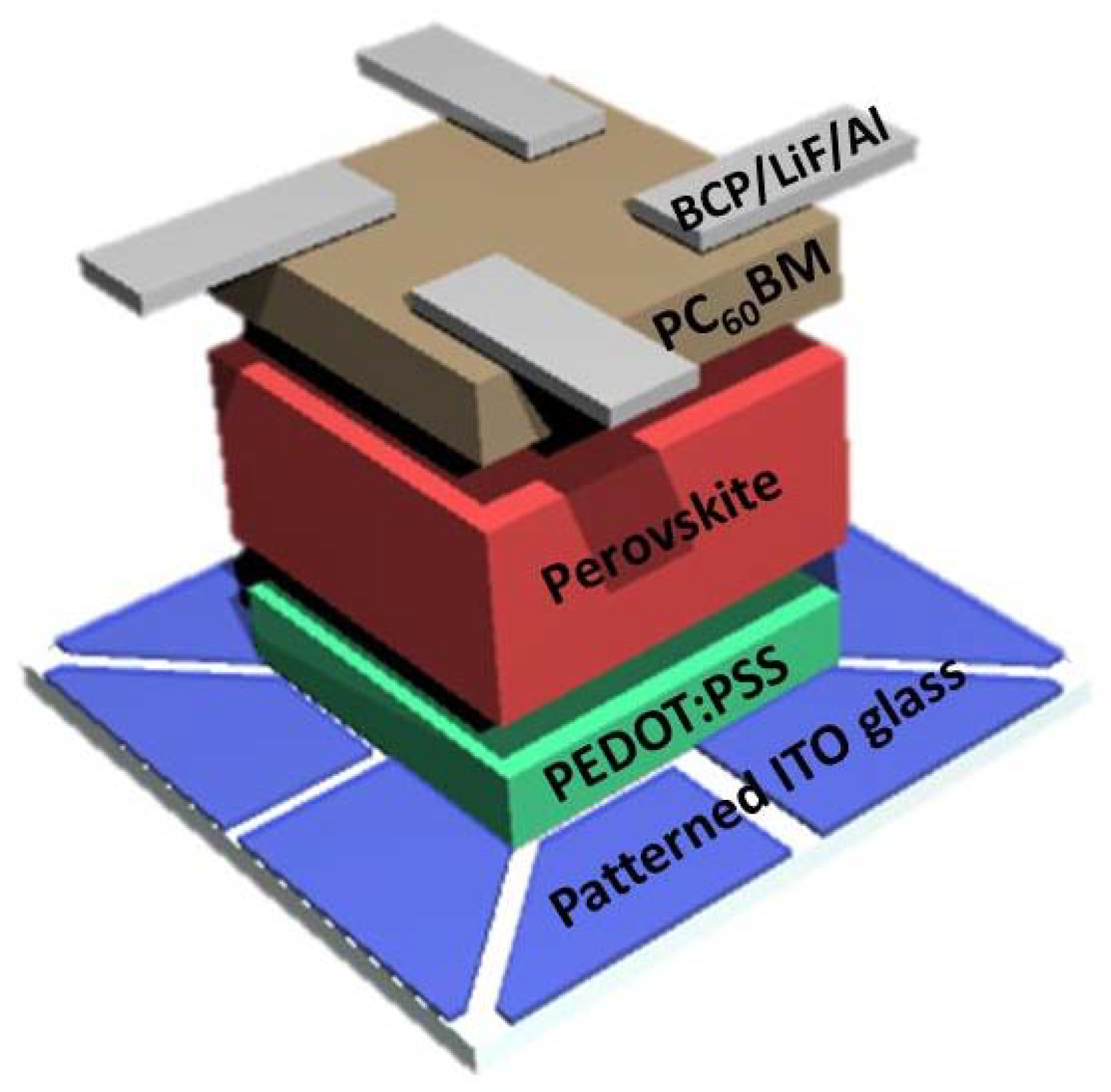
2.1. Fabrication of CH3NH3PbI3-xClx PSCs
2.2. Characterizations
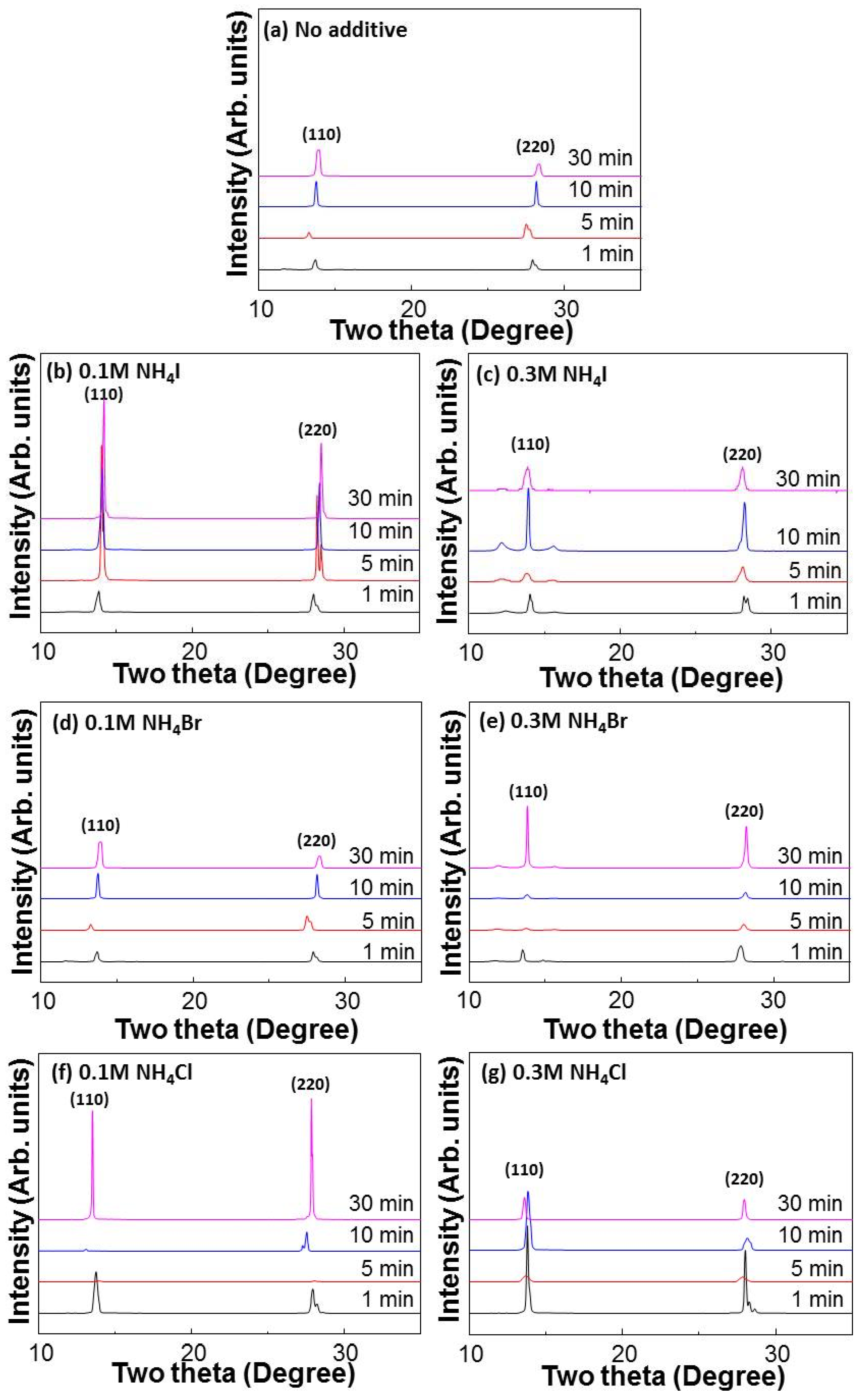
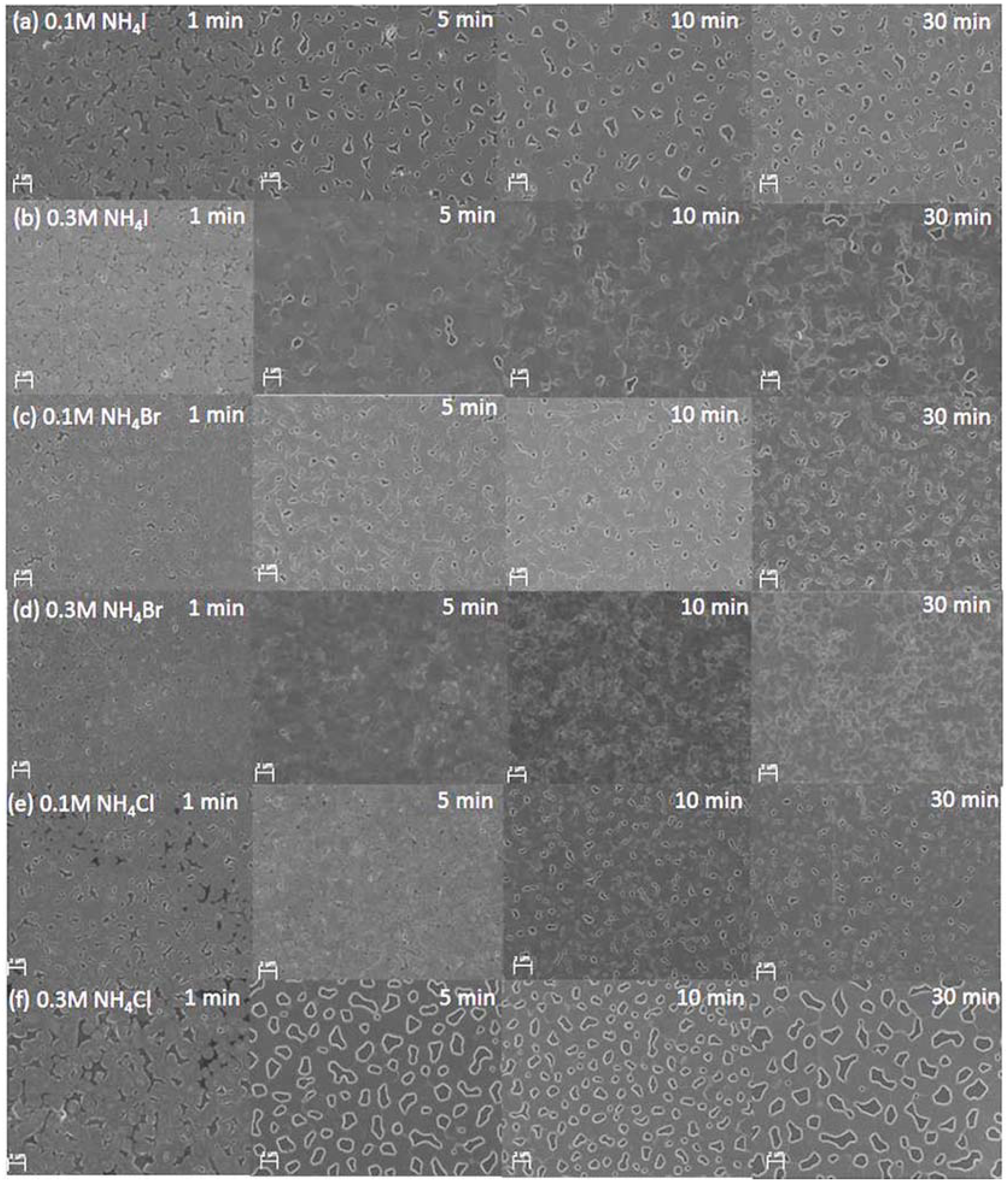
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, K.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Im, J.-H.; Lee, C.-R.; Lee, J.-W.; Park, S.-W.; Park, N.-G. 6.5% efficient perovskite quantum-dot-sensitized solar cell. Nanoscale 2011, 3, 4088–4093. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Lee, C.-R.; Im, J.-H.; Lee, K.-B.; Moehl, T.; Marchioro, A.; Moon, S.-J.; Humphry-Baker, R.; Yum, J.-H.; Moser, J.E.; et al. Lead Iodide Perovskite Sensitized All-Solid-State Submicron Thin Film Mesoscopic Solar Cell with Efficiency Exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient Hybrid Solar Cells Based on Meso-Superstructured Organometal Halide Perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.V.; Nguyen, T.P.; Choi, K.S.; Cho, Y.-H.; Hong, Y.J.; Kim, S.Y. Dual use of tantalum disulfides as hole and electron extraction layers in organic photovoltaic cells. Phys. Chem. Chem. Phys. 2014, 16, 25468–25472. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Le, Q.V.; Kim, C.; Kim, S.Y. Use of silane-functionalized graphene oxide on organic photovoltaic cells and organic light-emitting diodes. Phys. Chem. Chem. Phys. 2015, 17, 9369–9374. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.V.; Shin, J.W.; Jung, J.-H.; Park, J.; Ozturk, A.; Kim, S.Y. Control of the Crystal Growth Shape in CH3NH3PbBr3 Perovskite Materials. J. Nanosci. Nanotechnol. 2017, 17, 8169–8174. [Google Scholar] [CrossRef]
- Huang, J.; Wang, M.; Ding, L.; Yang, Z.; Zhang, K. Hydrobromic acid assisted crystallization of MAPbI3-xClx for enhanced power conversion efficiency in perovskite solar cells. RSC Adv. 2016, 6, 55720–55725. [Google Scholar] [CrossRef]
- Hasani, A.; Gavgani, J.N.; Pashaki, R.M.; Baseghi, S.; Salehi, A.; Heo, D.; Kim, S.Y.; Mahyari, M. Poly(3,4 ethylenedioxythiophene):Poly(Styrenesulfonate)Iron(III) Porphyrin Supported on S and N Co-Doped Graphene Quantum Dots as a Hole Transport Layer in Polymer Solar Cells. Sci. Adv. Mater. 2017, 9, 1616–1625. [Google Scholar] [CrossRef]
- Le, Q.V.; Choi, J.-Y.; Kim, S.Y. Recent advances in the application of two-dimensional materials as charge transport layer in organic and perovskite solar cells. FlatChem 2017, 2, 54–66. [Google Scholar] [CrossRef]
- Kim, Y.G.; Kwon, K.C.; Le, Q.V.; Hong, K.; Jang, H.W.; Kim, S.Y. Atomically thin two-dimensional materials as hole extraction layers in organolead halide perovskite photovoltaic cells. J. Power Source 2016, 319, 1–8. [Google Scholar] [CrossRef]
- Kwon, K.C.; Hong, K.; Le, Q.V.; Lee, S.Y.; Choi, J.; Kim, K.-B.; Kim, S.Y.; Jang, H.W. Inhibition of Ion Migration for Reliable Operation of Organolead Halide Perovskite-Based Metal/Semiconductor/Metal Broadband Photodetectors. Adv. Fun. Mater. 2016, 26, 4213–4222. [Google Scholar] [CrossRef]
- Kim, Y.G.; Kim, T.-Y.; Oh, J.H.; Choi, K.S.; Kim, Y.-J.; Kim, S.Y. Cesium lead iodide solar cells controlled by annealing temperature. Phys. Chem. Chem. Phys. 2017, 19, 6257–6263. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.V.; Nguyen, T.P.; Jang, H.W.; Kim, S.Y. The use of UV/ozone-treated MoS2 nanosheets for extended air stability in organic photovoltaic cells. Phys. Chem. Chem. Phys. 2014, 16, 13123–13128. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Zhan, X.Y.; Wang, Y.J.; Muhammad, S.; Huang, Y.; He, J. A flexible UV nanosensor based on reduced graphene oxide decorated ZnO nanostructures. Nanoscale 2012, 4, 2678–2684. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhou, H.; Hong, Z.; Luo, S.; Duan, H.-S.; Wang, H.-H.; Liu, Y.; Li, G.; Yang, Y. Planar heterojunction perovskite solar cells via vapor-assisted solution process. J. Am. Chem. Soc. 2013, 136, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Huang, F.; Huang, W.; Dkhissi, Y.; Zhu, Y.; Etheridge, J.; Gray-Weale, A.; Bach, U.; Cheng, Y.-B.; Spiccia, L. A Fast Deposition-Crystallization Procedure for Highly Efficient Lead Iodide Perovskite Thin-Film Solar Cells. Angew. Chem. 2014, 126, 1–7. [Google Scholar] [CrossRef]
- Li, G.; Shrotriya, V.; Huang, J.; Yao, Y.; Moriarty, T.; Emery, K.; Yang, Y. High-efficiency solution processable polymer photovoltaic cells by self-organization of polymer blends. Nat. Mater. 2005, 4, 864–868. [Google Scholar] [CrossRef]
- Sista, S.; Park, M.-H.; Hong, Z.; Wu, Y.; Hou, J.; Kwan, W.L.; Li, G.; Yang, Y. Highly Efficient Tandem Polymer Photovoltaic Cells. Adv. Mater. 2010, 22, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Luo, J.; Son, M.-K.; Gao, P.; Cho, K.T.; Seo, J.; Zakeeruddin, S.M.; Grätzel, M.; Nazeeruddin, M.K. Enhanced Charge Collection with Passivation Layers in Perovskite Solar Cells. Adv. Mater. 2016, 28, 3966–3972. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, H.; Yang, T.; Zhang, W.; Gong, X. Solution-Processed Ultrasensitive Polymer Photodetectors with High External Quantum Efficiency and Detectivity. ACS Appl. Mater. Interfaces 2012, 4, 3701–3705. [Google Scholar] [CrossRef] [PubMed]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent engineering high-performance inorganic-organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.W.; Liao, Z.M.; Zhou, Y.B.; Wu, H.C.; Bie, Y.Q.; Xu, J.; Yu, D.-P. Graphene/ZnO nanowire/graphene vertical structure based fast-response ultraviolet photodetector. Appl. Phys. Lett. 2012, 100, 223114. [Google Scholar] [CrossRef]
- Kim, A.; Won, Y.; Woo, K.; Kim, C.H.; Jooho, M. Highly Transparent Low Resistance ZnO/Ag Nanowire/ZnO Composite Electrode for Thin Film Solar Cells. ACS Nano 2013, 7, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Mutiso, R.M.; Sherrott, M.C.; Rathmell, A.R.; Wiley, B.J.; Winey, K.I. Integrating Simulations and Experiments to Predict Sheet Resistance and Optical Transmittance in Nanowire Films for Transparent Conductors. ACS Nano 2013, 7, 7654–7663. [Google Scholar] [CrossRef] [PubMed]
- Dualeh, A.; Moehl, T.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Effect of Annealing Temperature on Film Morphology of Organic-Inorganic Hybrid Perovskite Solid-State Solar Cells. Funct. Mater. 2014, 24, 3250–3258. [Google Scholar] [CrossRef]
- Carnie, M.J.; Charbonneau, C.; Davies, M.L.; Troughton, J.; Watson, T.M.; Wojciechowski, K.; Snaith, H.; Worsley, D.A. A one-step low temperature processing route for organolead halide perovskite solar cells. Chem. Commun. 2013, 49, 7893–7895. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Park, B.-K.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H.; et al. Iodide management in formamidinium-lead-halide–based perovskite layers for efficient solar cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef] [PubMed]
- McMeekin, D.P.; Sadoughi, G.; Rehman, W.; Eperon, G.E.; Saliba, M.; HöNrantner, M.T.; Haghighirad, A.; Sakai, N.; Korte, L.; Rech, B.; et al. A mixed-cation lead mixed-halide perovskite absorber for tandem solar cells. Science 2016, 351, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ibrahim Dar, M.; Yi, C.; Luo, J.; Tschumi, M.; Zakeeruddin, S.M.; Nazeeruddin, M.K.; Han, H.; Grätzel, M. Improved performance and stability of perovskite solar cells by crystal crosslinking with alkylphosphonic acid ω-ammonium chlorides. Nat. Chem. 2015, 7, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.H.; Song, D.H.; Im, S.H. Planar CH3NH3PbBr3 Hybrid Solar Cells with 10.4% Power Conversion Efficiency, Fabricated by Controlled Crystallization in the Spin-Coating Process. Adv. Mater. 2014, 26, 8179–8183. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, K. Three-step sequential solution deposition of PbI2-free CH3NH3PbI3 perovskite. J. Mater. Chem. A 2015, 3, 9086–9091. [Google Scholar] [CrossRef]
- Docampo, P.; Hanusch, F.-C.; Stranks, S.-D.; Döblinger, M.; Feckl, J.-M.; Ehrensperger, M.; Minar, N.-K.; Johnston, M.-B.; Snaith, H.-J.; Bein, T. Solution Depositon-Conversion for Planar Heterojunction Mixed Halide Perovskite Solar Cell. Adv. Energy Mater. 2014, 4, 1400355. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Y.; Liang, Z. Nonvolatile chlorinated additives adversely influence CH3NH3PbI3 based planar solar cells. J. Mater. Chem. A 2015, 3, 9137–9140. [Google Scholar] [CrossRef]
- Wang, Z.-K.; Li, M.; Yang, Y.-G.; Hu, Y.; Ma, H.; Gao, X.-Y.; Liao, L.-S. High Efficiency Pb-In Binary Metal Perovskite Solar Cells. Adv. Mater. 2016, 28, 6695–6703. [Google Scholar] [CrossRef] [PubMed]
- Zuo, C.; Ding, L. An 80.11% FF record achieved for perovskite solar cells by using the NH4Cl additive. Nanoscale 2014, 6, 9935–9938. [Google Scholar] [CrossRef] [PubMed]
- Oku, T.; Ohishi, Y.; Ueoka, N. Highly (100)-oriented CH3CH3PbI3(Cl) perovskite solar cells prepared with NH4Cl using an air blow method. RSC Adv. 2018, 8, 10389–10395. [Google Scholar] [CrossRef]
- Abdi-Jalebi, M.; Dar, M.-I.; Sadhanala, A.; Senanayak, S.-P.; Franckevičius, M.; Arora, N.; Hu, Y.; Nazeeruddin, M.-K.; Zakeeruddin, S.-M.; Grätzel, M.; Friend, R.-H. Impact of Monovalent Cation Halide Additives on the Structural and Optoelectronic Properties of CH3NH3PbI3 perovskite. Adv. Energy Mater. 2016, 6, 1502472. [Google Scholar] [CrossRef]
- Heo, J.-H.; Song, D.-H.; Han, H.-J.; Kim, S.-Y.; Kim, J.-H.; Kim, D.; Shin, H.-W.; Ahn, T.-K.; Wolf, C.; Lee, T.; et al. Planar CH3NH3PbI3 Perovskite Solar Cells with Constant 17.2% Average Power Conversion Efficiency Irrespective of the Scan Rate. Adv. Mater. 2015, 27, 3424–3430. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Liu, G.; Zhu, L.; Ye, J.; Zhang, X.; Alsaedi, A.; Hayat, T.; Pan, X.; Dai, S. Enhanced Performance and Stability of Perovskite Solar Cells Using NH4I Interfacial Modifier. ACS Appl. Mater. Interfaces 2017, 9, 41006–41013. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Chen, Q.; Li, G.; Luo, S.; Song, T.-B.; Duan, H.-S.; Hong, Z.; You, J.; Liu, Y.; Yang, Y. Interface engineering of highly efficient perovskite solar cells. Science 2014, 345, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, M.; Ding, L.; Igbari, F.; Yao, X. Efficiency enhancement of MAPbIxCl3-x based perovskite solar cell by modifying the TiO2 interface with Silver Nanowires. Sol. Energy 2016, 130, 273–280. [Google Scholar] [CrossRef]

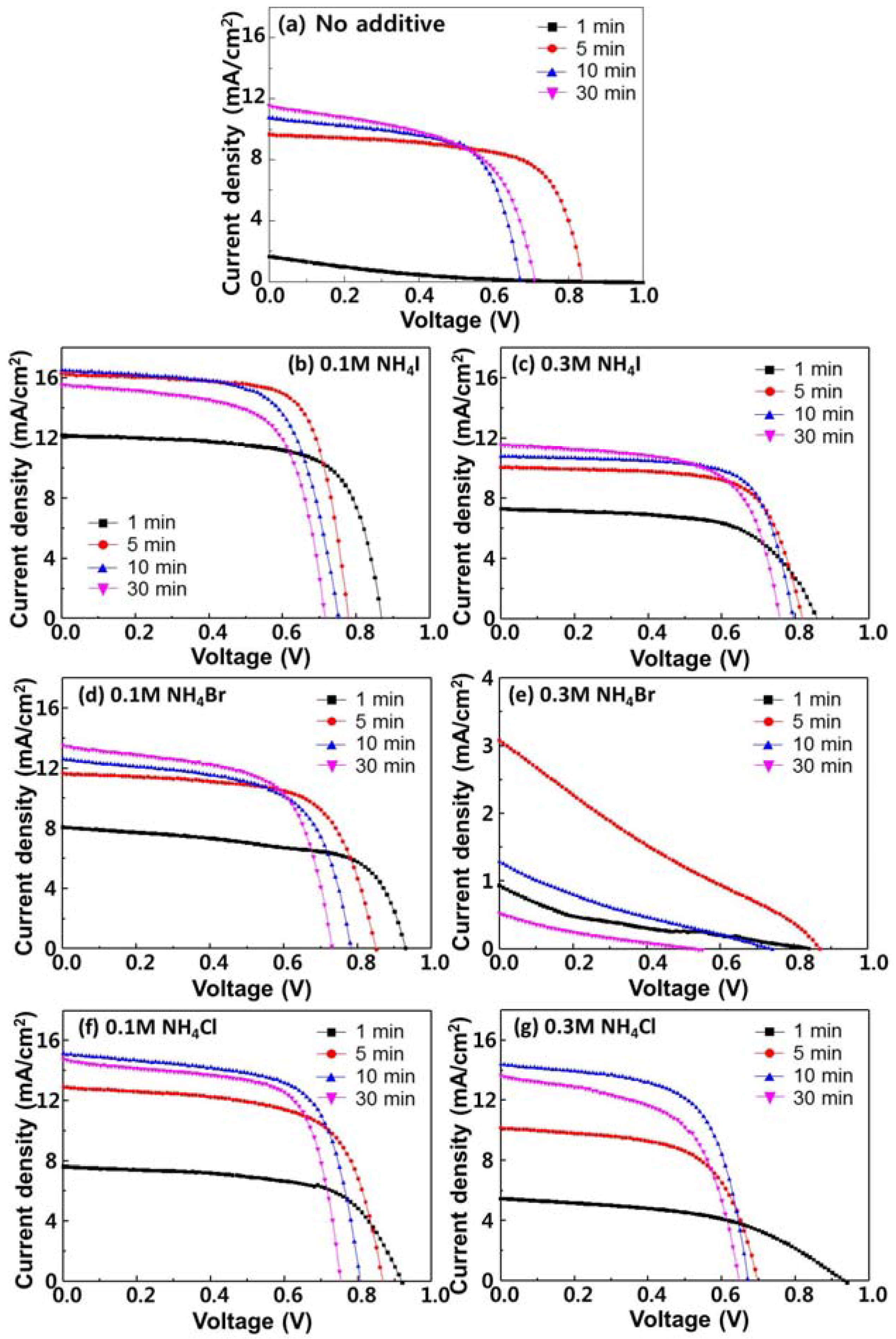
| Additives | Annealing Time (min) | VOC (V) | JSC (mA/cm2) | FF | PCE (%) |
|---|---|---|---|---|---|
| NH4I, 0.1 M | 1 | 0.86 | 12.2 | 0.67 | 7.31 |
| 5 | 0.78 | 16.3 | 0.72 | 9.13 | |
| 10 | 0.75 | 16.5 | 0.66 | 8.20 | |
| 30 | 0.71 | 15.6 | 0.66 | 7.31 | |
| NH4I, 0.3 M | 1 | 0.86 | 7.31 | 0.62 | 3.87 |
| 5 | 0.82 | 10.1 | 0.69 | 5.72 | |
| 10 | 0.79 | 10.8 | 0.70 | 6.06 | |
| 30 | 0.76 | 11.6 | 0.65 | 5.67 | |
| NH4Br, 0.1 M | 1 | 0.93 | 8.08 | 0.62 | 4.69 |
| 5 | 0.85 | 11.7 | 0.66 | 6.57 | |
| 10 | 0.78 | 12.6 | 0.62 | 4.69 | |
| 30 | 0.73 | 13.6 | 0.63 | 6.23 | |
| NH4Br, 0.3 M | 1 | 0.91 | 0.91 | 0.19 | 0.15 |
| 5 | 0.87 | 3.09 | 0.23 | 0.61 | |
| 10 | 0.73 | 1.29 | 0.20 | 0.19 | |
| 30 | 0.53 | 0.53 | 0.18 | 0.051 | |
| NH4Cl, 0.1 M | 1 | 0.92 | 7.63 | 0.63 | 4.42 |
| 5 | 0.87 | 12.9 | 0.65 | 7.30 | |
| 10 | 0.80 | 15.1 | 0.67 | 8.13 | |
| 30 | 0.75 | 14.8 | 0.68 | 7.56 | |
| NH4Cl, 0.3 M | 1 | 0.93 | 5.25 | 0.51 | 2.49 |
| 5 | 0.70 | 10.2 | 0.61 | 4.36 | |
| 10 | 0.67 | 14.4 | 0.63 | 6.13 | |
| 30 | 0.64 | 13.1 | 0.60 | 5.11 | |
| No additive | 1 | 0.81 | 1.64 | 0.15 | 0.20 |
| 5 | 0.84 | 9.45 | 0.68 | 5.40 | |
| 10 | 0.67 | 10.8 | 0.64 | 4.64 | |
| 30 | 0.71 | 11.6 | 0.56 | 4.64 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heo, D.Y.; Luo, Z.; Kim, S.Y. Effect of Ammonium Halide Additives on the Performance of Methyl Amine Based Perovskite Solar Cells. Materials 2018, 11, 1417. https://doi.org/10.3390/ma11081417
Heo DY, Luo Z, Kim SY. Effect of Ammonium Halide Additives on the Performance of Methyl Amine Based Perovskite Solar Cells. Materials. 2018; 11(8):1417. https://doi.org/10.3390/ma11081417
Chicago/Turabian StyleHeo, Do Yeon, Zhengtang Luo, and Soo Young Kim. 2018. "Effect of Ammonium Halide Additives on the Performance of Methyl Amine Based Perovskite Solar Cells" Materials 11, no. 8: 1417. https://doi.org/10.3390/ma11081417
APA StyleHeo, D. Y., Luo, Z., & Kim, S. Y. (2018). Effect of Ammonium Halide Additives on the Performance of Methyl Amine Based Perovskite Solar Cells. Materials, 11(8), 1417. https://doi.org/10.3390/ma11081417







