Structural Relaxation of Lix(Ni0.874Co0.090Al0.036)O2 after Lithium Extraction down to x = 0.12
Abstract
:1. Introduction
2. Materials and Methods
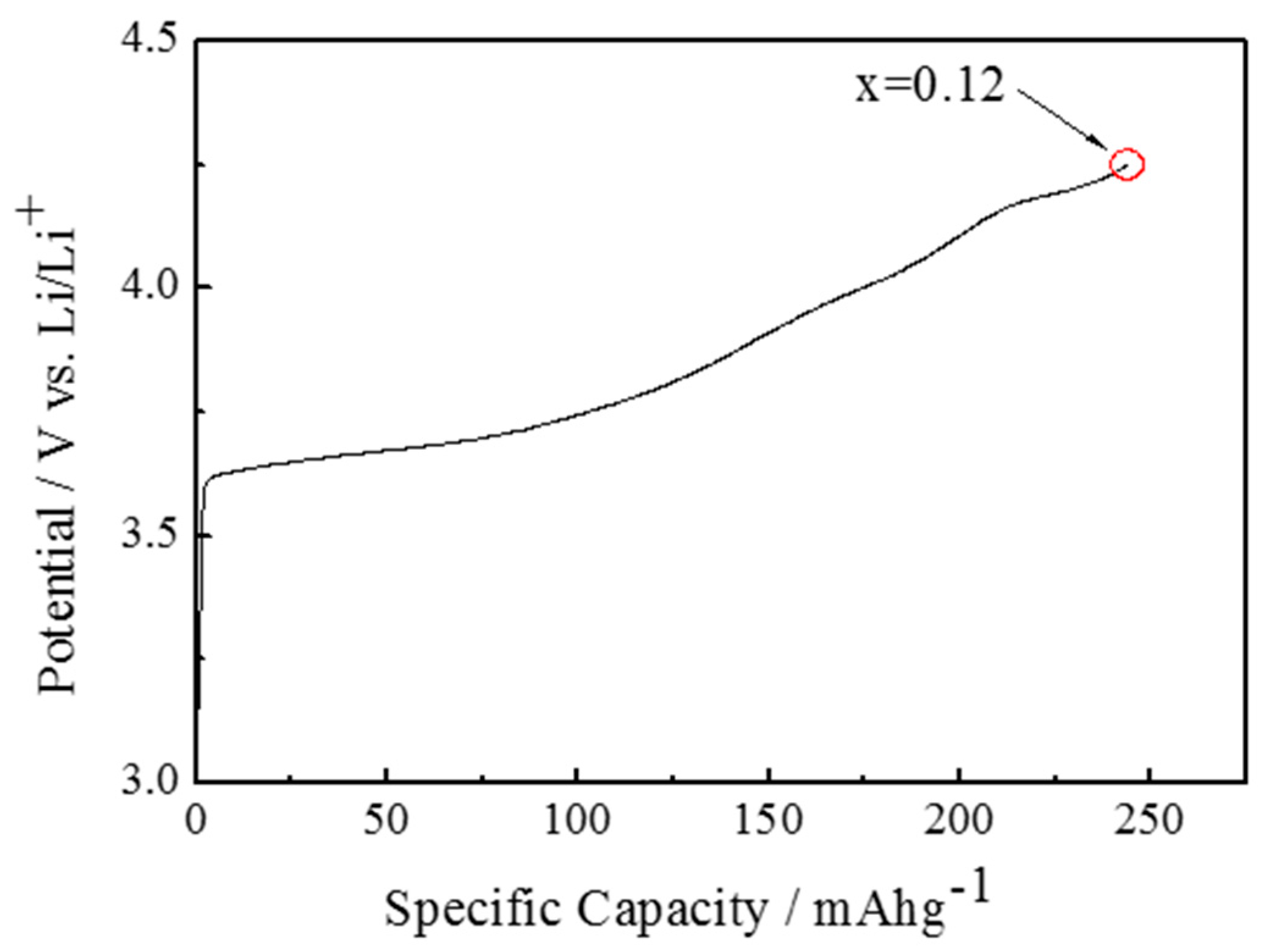
2.1. Electrochemical Lithium Extraction
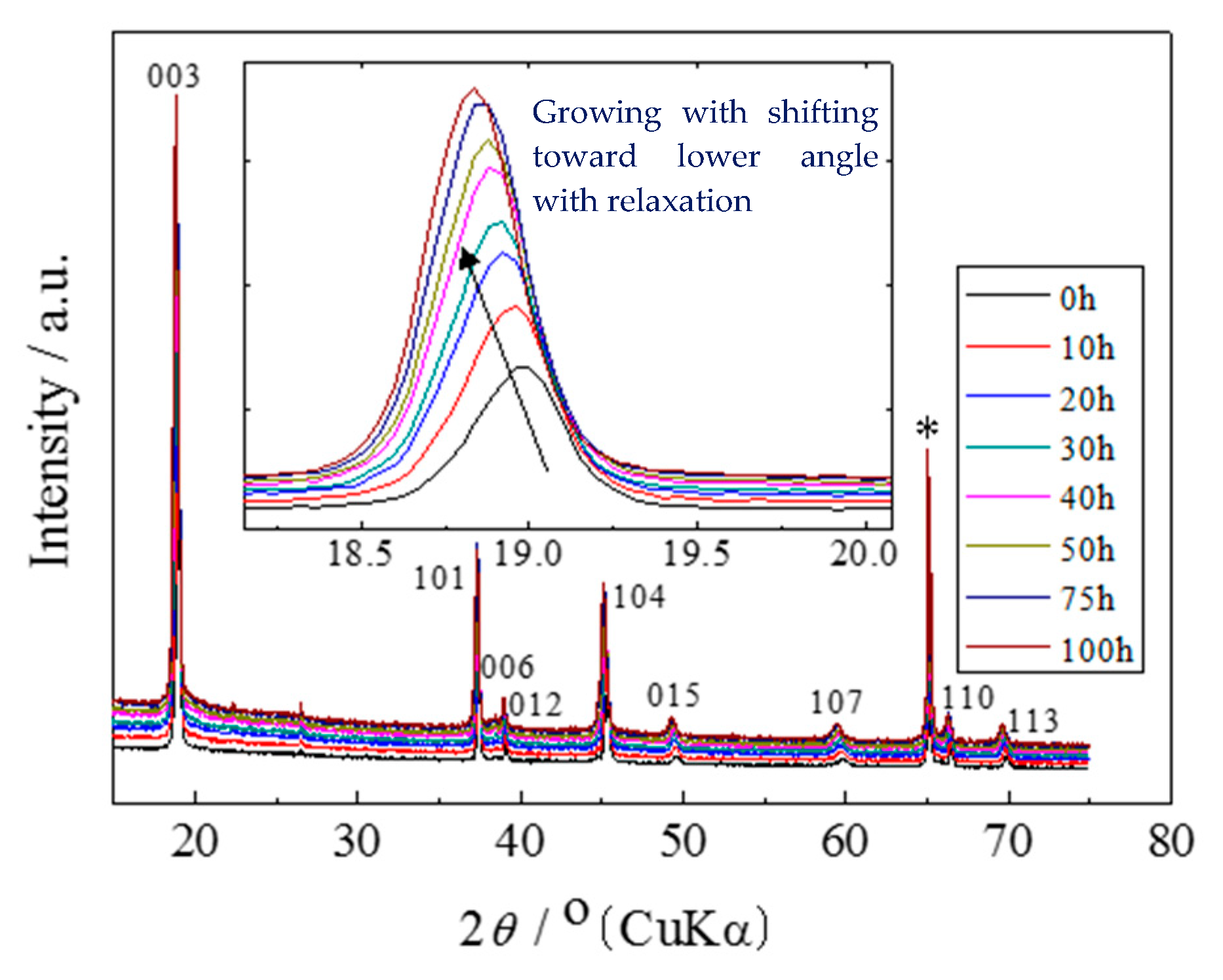
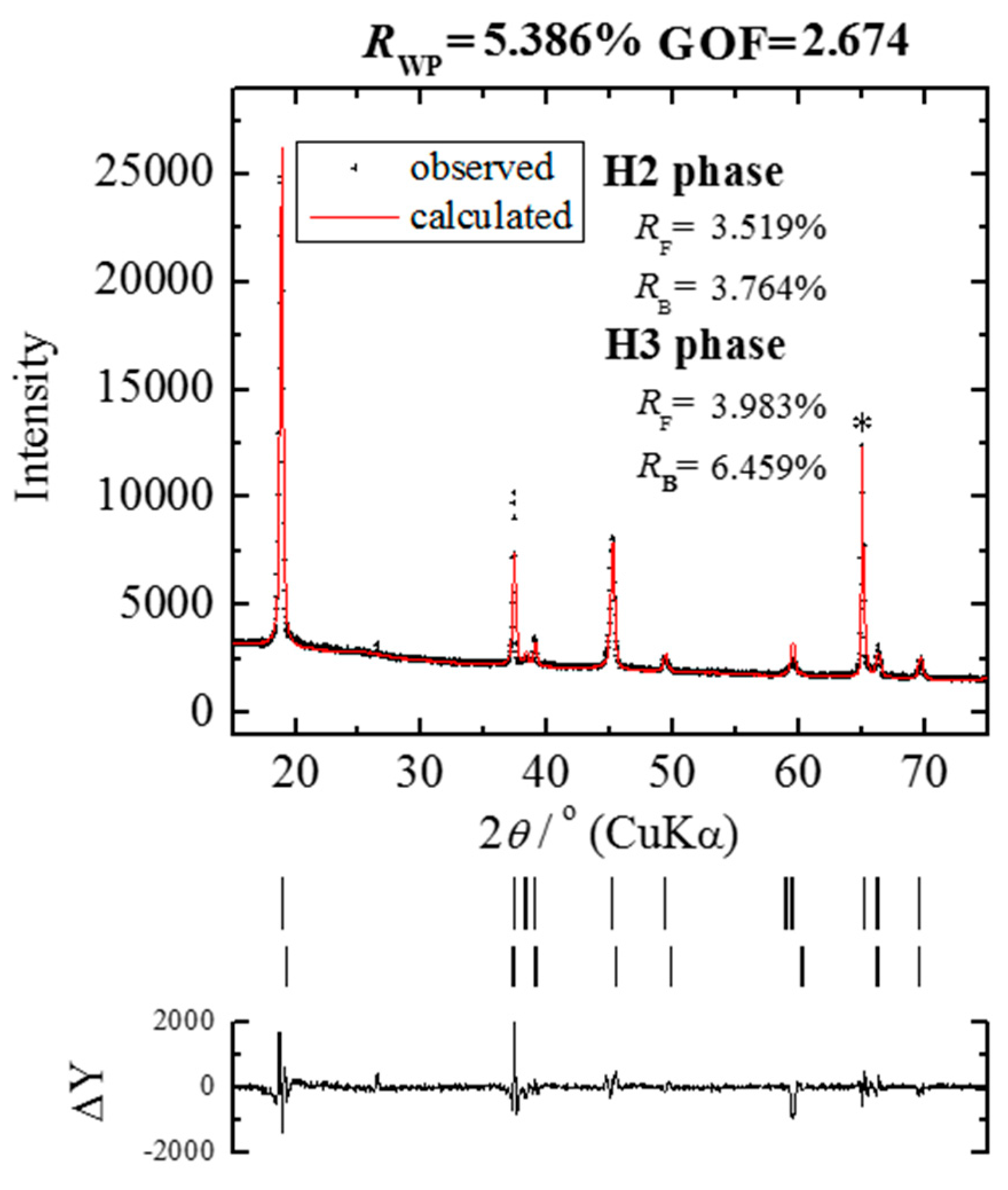
2.2. X-ray Diffraction and the Rietveld Analysis
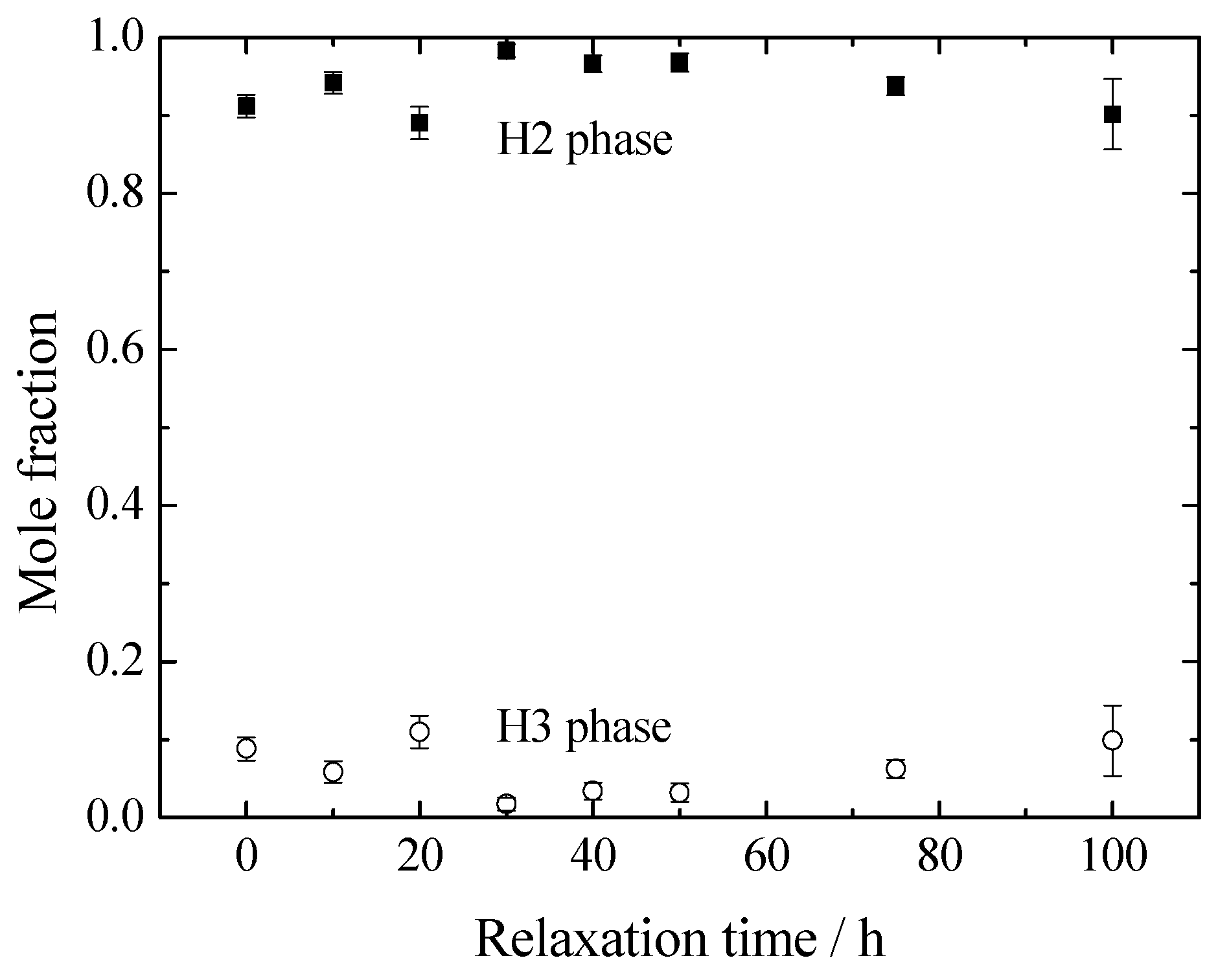
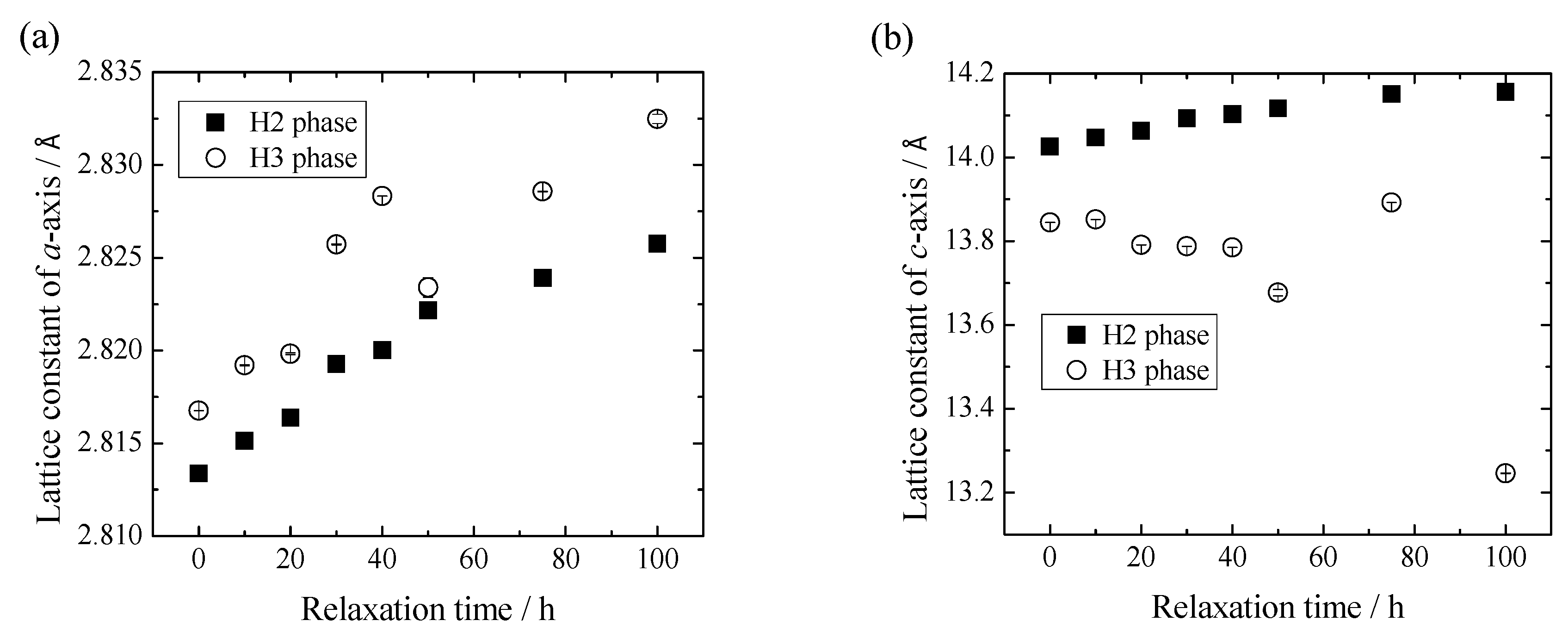
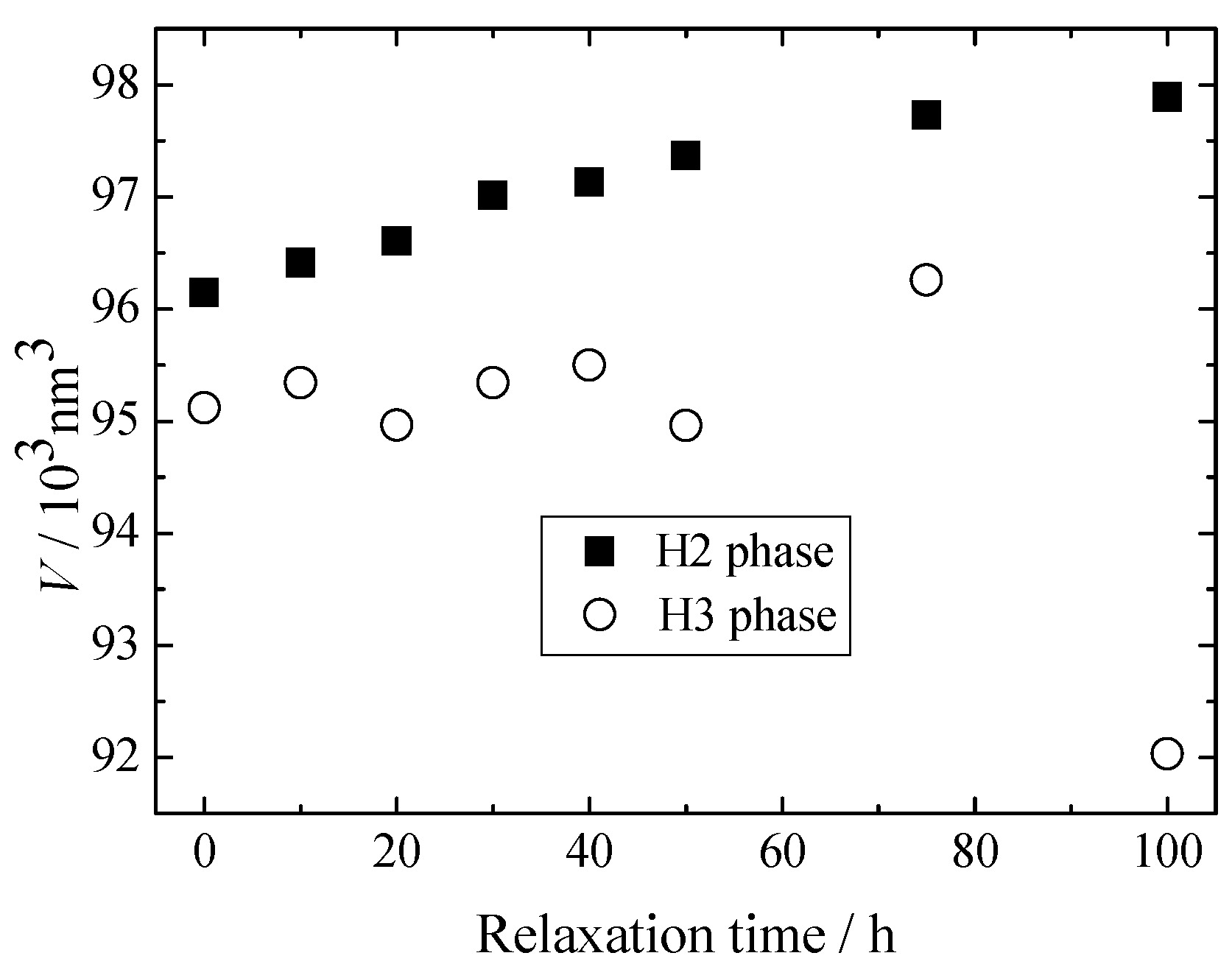
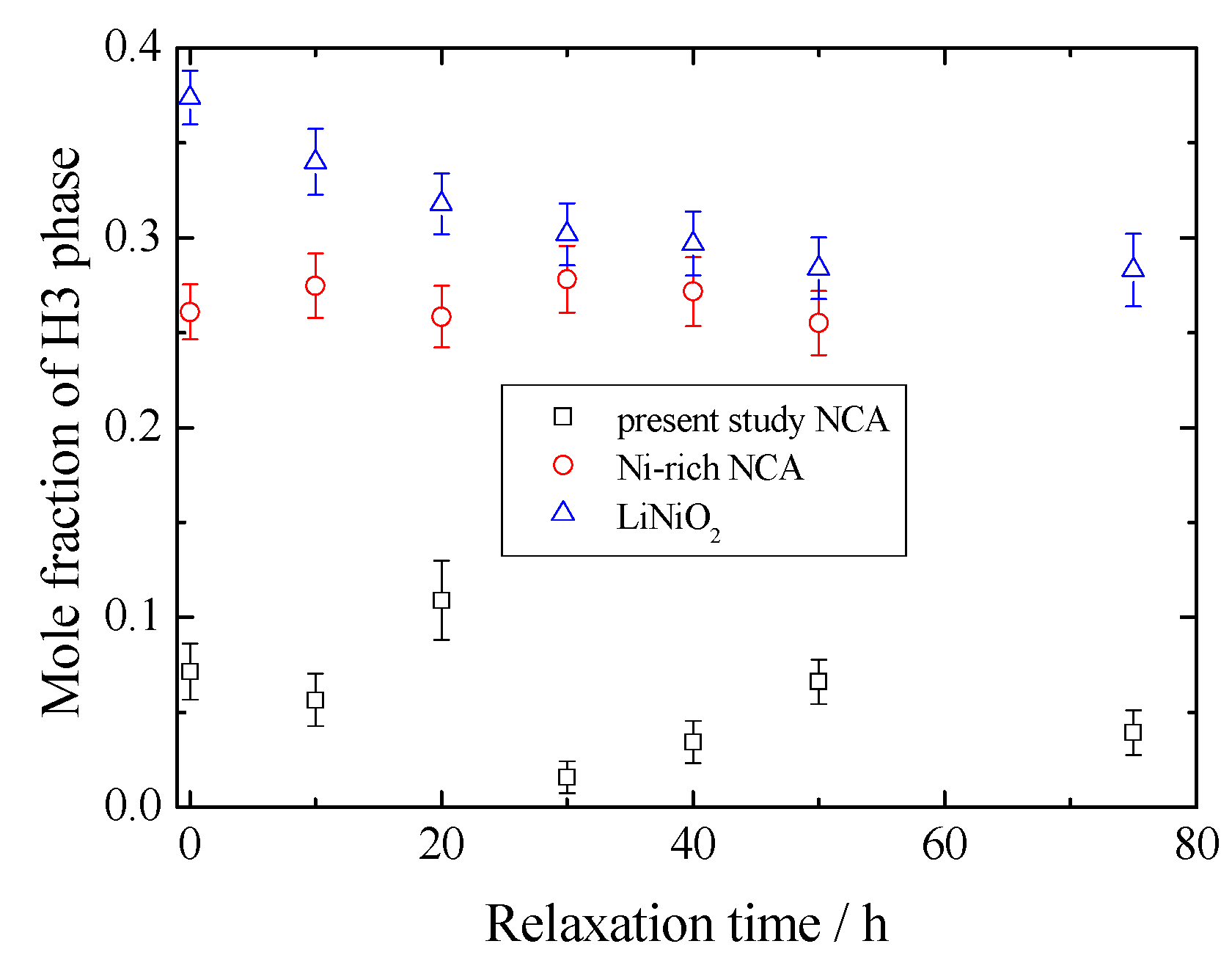
3. Results and Discussion
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.; Peng, H.; Liu, G.; Mcllwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2008, 3, 31–35. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Wanger, F.T.; Lakshmanan, B.; Mathias, M.F. Electrochemistry and the Future of the Automobile. J. Phys. Chem. Lett. 2010, 1, 2204–2219. [Google Scholar] [CrossRef]
- Kraytsberg, A.; Ein-Eli, Y. Higher, Stronger, Better. A Review of 5 Volt Cathode Materials for Advanced Lithium-Ion Batteries. Adv. Energy Mater. 2012, 2, 922–939. [Google Scholar] [CrossRef]
- Li, C.; Zhang, H.P.; Fu, L.J.; Liu, H.; Wu, Y.P.; Rahm, E.; Holze, R.; Wu, H.Q. Cathode materials modified by surface coating for lithium ion batteries. Electrochim. Acta 2006, 51, 3872–3883. [Google Scholar] [CrossRef]
- Wu, Y.P.; Rahm, E.; Holze, R. Effects of heteroatoms on electrochemical performance of electrode materials for lithium ion batteries. Electrochim. Acta 2002, 47, 3491–3507. [Google Scholar] [CrossRef]
- Ebner, W.; Fouchard, L.; Xie, L. The LiNiO2/carbon lithium-ion battery. Solid State Ionics 1994, 69, 238–256. [Google Scholar] [CrossRef]
- Kalyani, P.; Kalaiselvi, N. Various aspects of LiNiO2 chemistry: A review. Sci. Technol. Adv. Mater. 2005, 6, 689–703. [Google Scholar] [CrossRef]
- Ohzuku, T.; Ueda, A.; Nagayama, M. Electrochemistry and Structural Chemistry of LiNiO2 () for 4 Volt Secondary Lithium Cells. J. Electrochem. Soc. 1993, 140, 1862–1870. [Google Scholar] [CrossRef]
- Amin, R.; Ravansbæk, D.B.; Chiang, Y. Characterization of Electronic and Ionic Transport in Li1-xNi0.8Co0.15Al0.05O2 (NCA). J. Electrochem. Soc. 2015, 162, A1163–A1169. [Google Scholar] [CrossRef]
- Jo, M.; Noh, M.; Oh, P.; Kim, Y.; Cho, J. A New High Power LiNi0.81Co0.1Al0.09O2 Cathode Material for Lithium-Ion Batteries. Adv. Energy Mater. 2014, 4. [Google Scholar] [CrossRef]
- Chen, C.H.; Liu, J.; Stoll, M.E.; Henriksen, G.; Vissers, D.R.; Amine, K. Aluminum-doped lithium nickel cobalt oxide electrodes for high-power lithium-ion batteries. J. Power Sources 2004, 128, 278–285. [Google Scholar] [CrossRef]
- Castro-García, S.; Castro-Couceiro, A.; Senarís-Rodríguez, M.A.; Soulette, F.; Julien, C. Influence of aluminum doping on the properties of LiCoO2 and LiNi0.5Co0.5O2 oxides. Solid State Ionics 2003, 156, 15–26. [Google Scholar] [CrossRef]
- Yoon, W.S.; Chung, K.Y.; McBreen, J.; Yang, X.Q. A comparative study on structural changes of LiCo1/3Ni1/3Mn1/3O2 and LiNi0.8Co0.15Al0.05O2 during first charge using in situ XRD. Electrochem. Commun. 2006, 8, 1257–1262. [Google Scholar] [CrossRef]
- Robert, R.; Novak, P. Structural Changes and Microstrain Generated on LiNi0.80Co0.15Al0.05O2 during Cycling: Effects on the Electrochemical Performance. J. Electrochem. Soc. 2015, 162, A1823–A1828. [Google Scholar] [CrossRef]
- Robert, R.; Bünzli, C.; Berg, E.J.; Novak, P. Activation Mechanism of LiNi0.80Co0.15Al0.05O2: Surface and Bulk Operando Electrochemical, Differential Electrochemical Mass Spectrometry, and X-ray Diffraction Analyses. Chem. Mater. 2015, 27, 526–536. [Google Scholar] [CrossRef]
- Tamura, T.; Takai, S.; Yabutsuka, T.; Yao, T. Relaxation Analysis of LixNiO2 and Lix(NCA)O2 in the Deeply Lithium Extracted Region (x ≤ 0.12). J. Electrochem. Soc. 2017, 164, A1514–A1519. [Google Scholar] [CrossRef]
- Park, S.; Oda, M.; Yao, T. Relaxation structure analysis of Li inserted γ-Fe2O3. Solid State Ionics 2011, 203, 29–32. [Google Scholar] [CrossRef]
- Park, S.; Ito, S.; Takasu, K.; Yao, T. Multistage Li Insertion and Extraction Relaxation Analysis of γ-Fe2O3. Electrochemistry 2012, 80, 804–807. [Google Scholar] [CrossRef]
- Seo, I.S.; Park, S.; Yao, T. Relaxation Phase Analysis of Li Inserted Li-Mn-O Cathode for Secondary Li Ion Batteries. ECS Electrochem. Lett. 2013, 2, A6–A9. [Google Scholar] [CrossRef]
- Park, S.; Kameyama, K.; Yao, T. Relaxation Crystal Analysis of LiFePO4 Cathode for Li-Ion Secondary Battery. Electrochem. Solid State Lett. 2012, 15, A49–A52. [Google Scholar] [CrossRef]
- Seo, I.; Nagashima, S.; Takai, S.; Yao, T. Relaxation Analysis of Li-Co-O Cathode Material for Li Ion Secondary Batteries. ECS Electrochem. Lett. 2013, 2, A72–A74. [Google Scholar] [CrossRef]
- Satou, Y.; Komine, S.; Park, S.; Yao, T. Relaxation analysis of LiMnPO4-based olivine-type material. Solid State Ionics 2014, 262, 35–38. [Google Scholar] [CrossRef]
- Satou, Y.; Komine, S.; Takai, S.; Yao, T. Relaxation Structure Analysis of the Single-Phase Reaction of LiMn0.75Fe0.25PO4. J. Electrochem. Soc. 2014, 161, A1759–A1763. [Google Scholar] [CrossRef]
- Satou, Y.; Komine, S.; Takai, S.; Yao, T. Non-Equilibrium Li Insertion Paths in LiMn0.75Fe0.25PO4 Observed during the Relaxation Process. ECS Electrochem. Lett. 2015, 4, A37–A40. [Google Scholar] [CrossRef]
- Yamada, K.; Takai, S.; Yabutsuka, T.; Yao, T. Relaxation Analysis of LiNi0.5Mn1.5O4 5 V Cathode Material by Means of the Rietveld Refinement. Electrochemistry 2016, 84, 808–811. [Google Scholar] [CrossRef]
- Iwai, T.; Kitajima, D.; Takai, S.; Yabutsuka, T.; Yao, T. α-PbO2 Formation on the Cathode of Lead Acid Battery due to the Local Cell Reaction. J. Electrochem. Soc. 2016, 163, A3087–A3090. [Google Scholar] [CrossRef]
- Yao, T.; Ozawa, N.; Aikawa, T.; Yoshinaga, S. Analysis of layered structures of lithium–graphite intercalation compounds by one-dimensional Rietveld method. Solid State Ionics 2004, 175, 199–202. [Google Scholar] [CrossRef]
| Relaxation Time | Mole Fraction | c/Å | Oxide Ion Coordinates | Rwp | |||
|---|---|---|---|---|---|---|---|
| H2 Phase | H3 Phase | H2 Phase | H3 Phase | H2 Phase | H3 Phase | ||
| 0 h | 0.91 (1) | 0.09 (1) | 14.062 (2) | 13.843 (9) | 0.2316 (7) | 0.268 (4) | 5.09 |
| 50 h | 0.97 (1) | 0.03 (1) | 14.116 (1) | 13.755 (8) | 0.2341 (8) | 0.218 (10) | 5.71 |
| 100 h | 0.90 (4) | 0.10 (4) | 14.156 (10) | 13.246 (8) | 0.2329 (7) | 0.298 (9) | 5.40 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.; Takai, S.; Yabutsuka, T.; Yao, T. Structural Relaxation of Lix(Ni0.874Co0.090Al0.036)O2 after Lithium Extraction down to x = 0.12. Materials 2018, 11, 1299. https://doi.org/10.3390/ma11081299
Kang J, Takai S, Yabutsuka T, Yao T. Structural Relaxation of Lix(Ni0.874Co0.090Al0.036)O2 after Lithium Extraction down to x = 0.12. Materials. 2018; 11(8):1299. https://doi.org/10.3390/ma11081299
Chicago/Turabian StyleKang, Jian, Shigeomi Takai, Takeshi Yabutsuka, and Takeshi Yao. 2018. "Structural Relaxation of Lix(Ni0.874Co0.090Al0.036)O2 after Lithium Extraction down to x = 0.12" Materials 11, no. 8: 1299. https://doi.org/10.3390/ma11081299
APA StyleKang, J., Takai, S., Yabutsuka, T., & Yao, T. (2018). Structural Relaxation of Lix(Ni0.874Co0.090Al0.036)O2 after Lithium Extraction down to x = 0.12. Materials, 11(8), 1299. https://doi.org/10.3390/ma11081299





