Electronic Band Structure Variations in the Ceria Doped Zirconia: A First Principles Study
Abstract
1. Introduction
2. Methodology
3. Results and Discussion
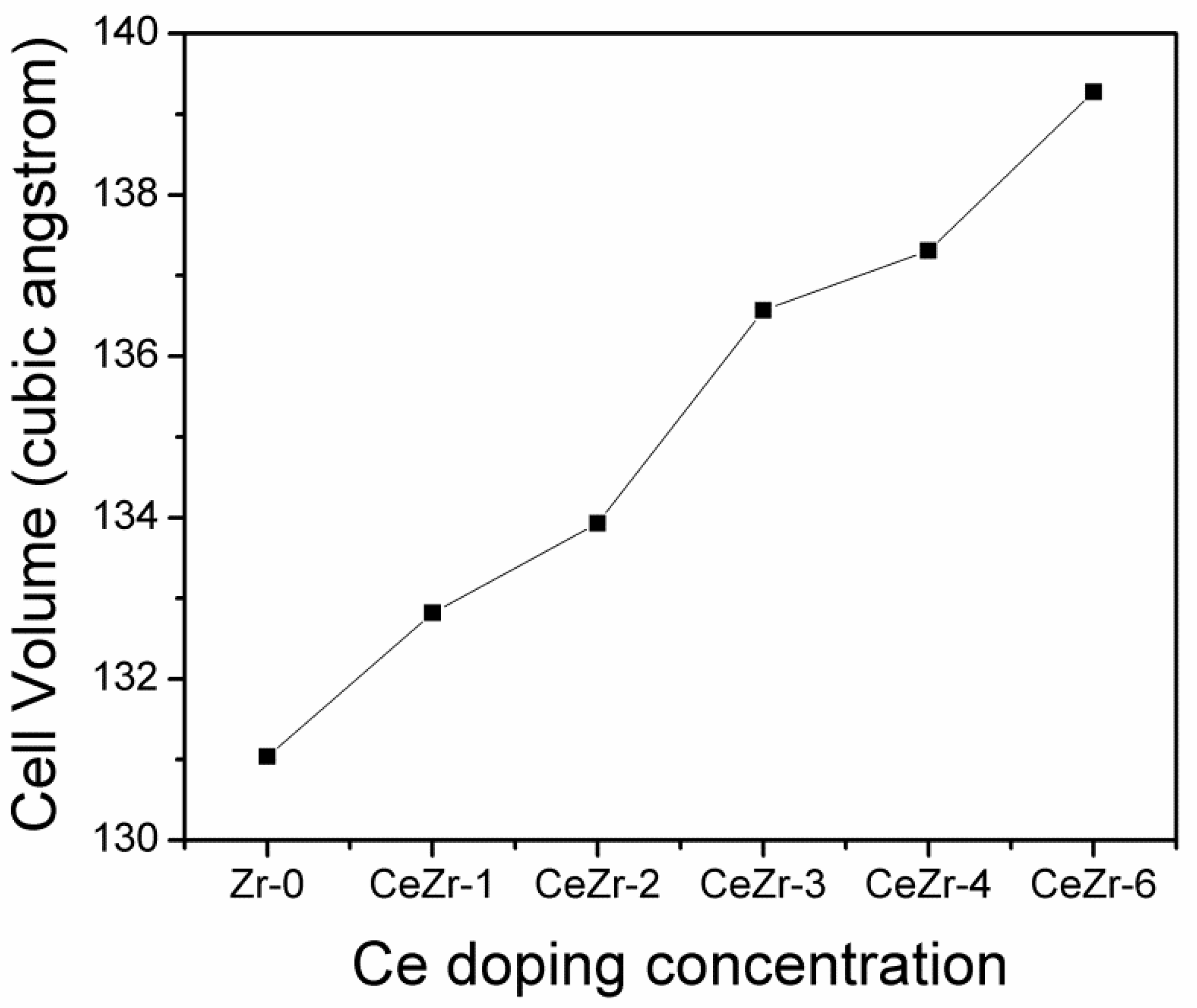
3.1. The Effect of Ce Inclusion on the ZrO2 Structure
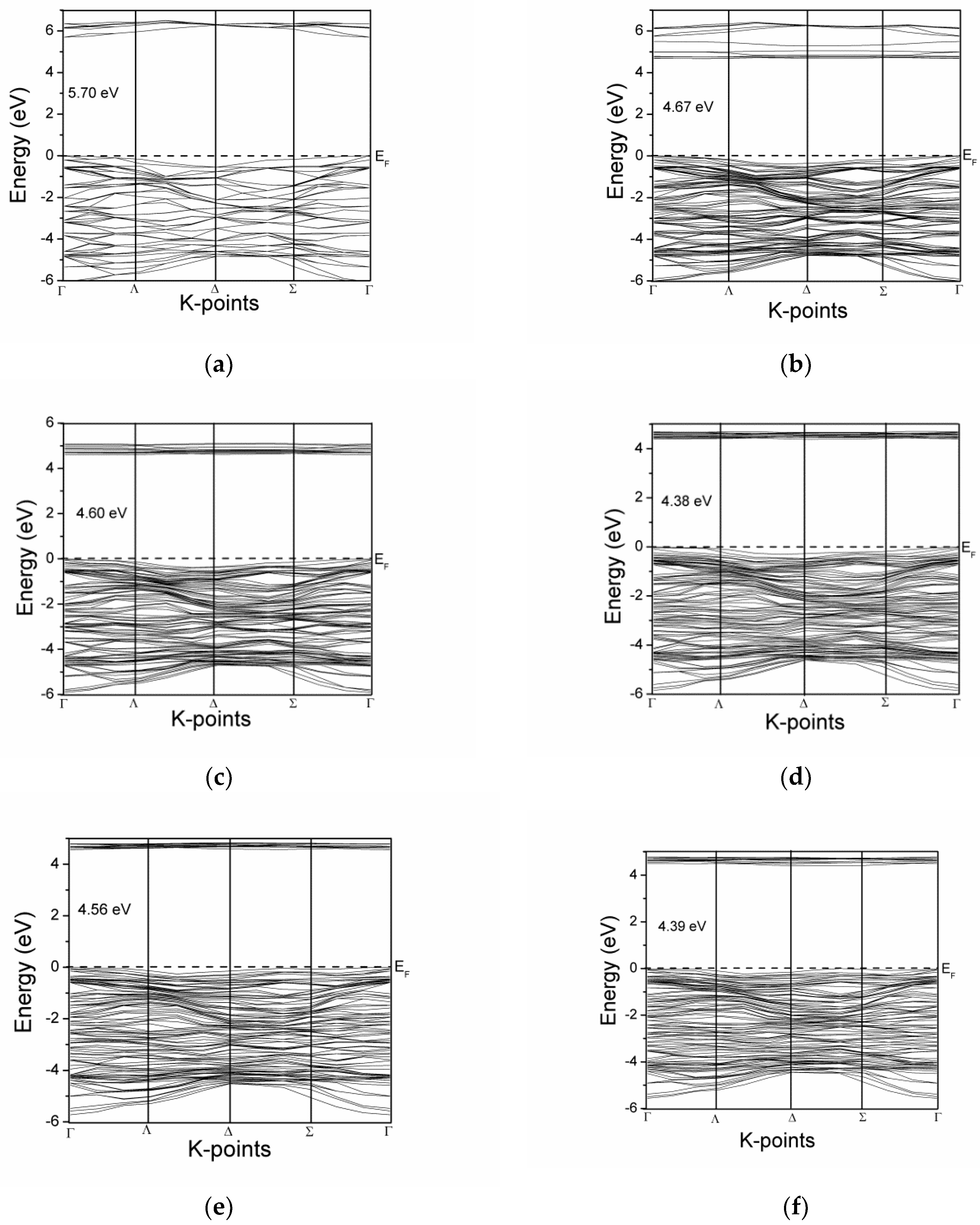
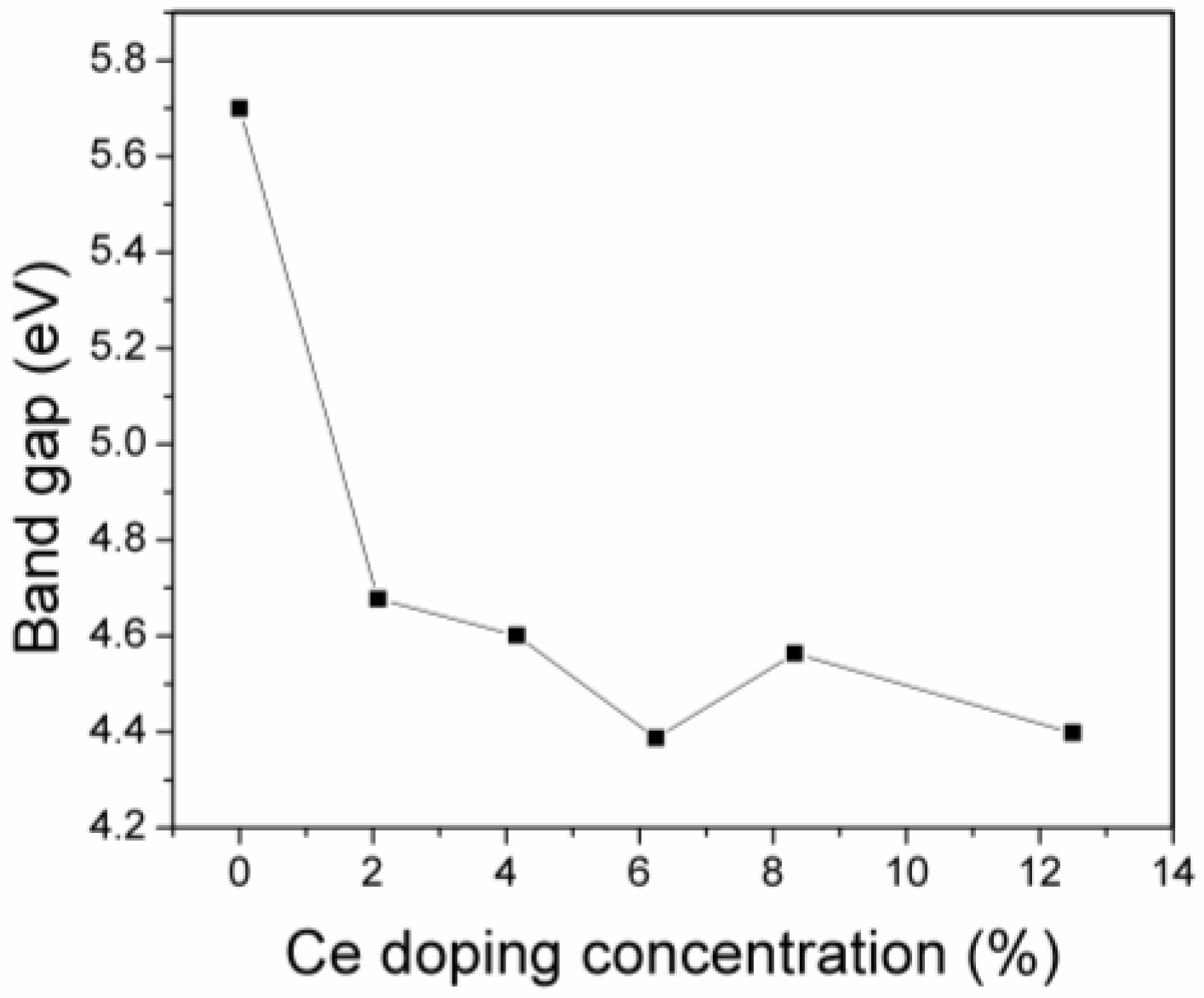
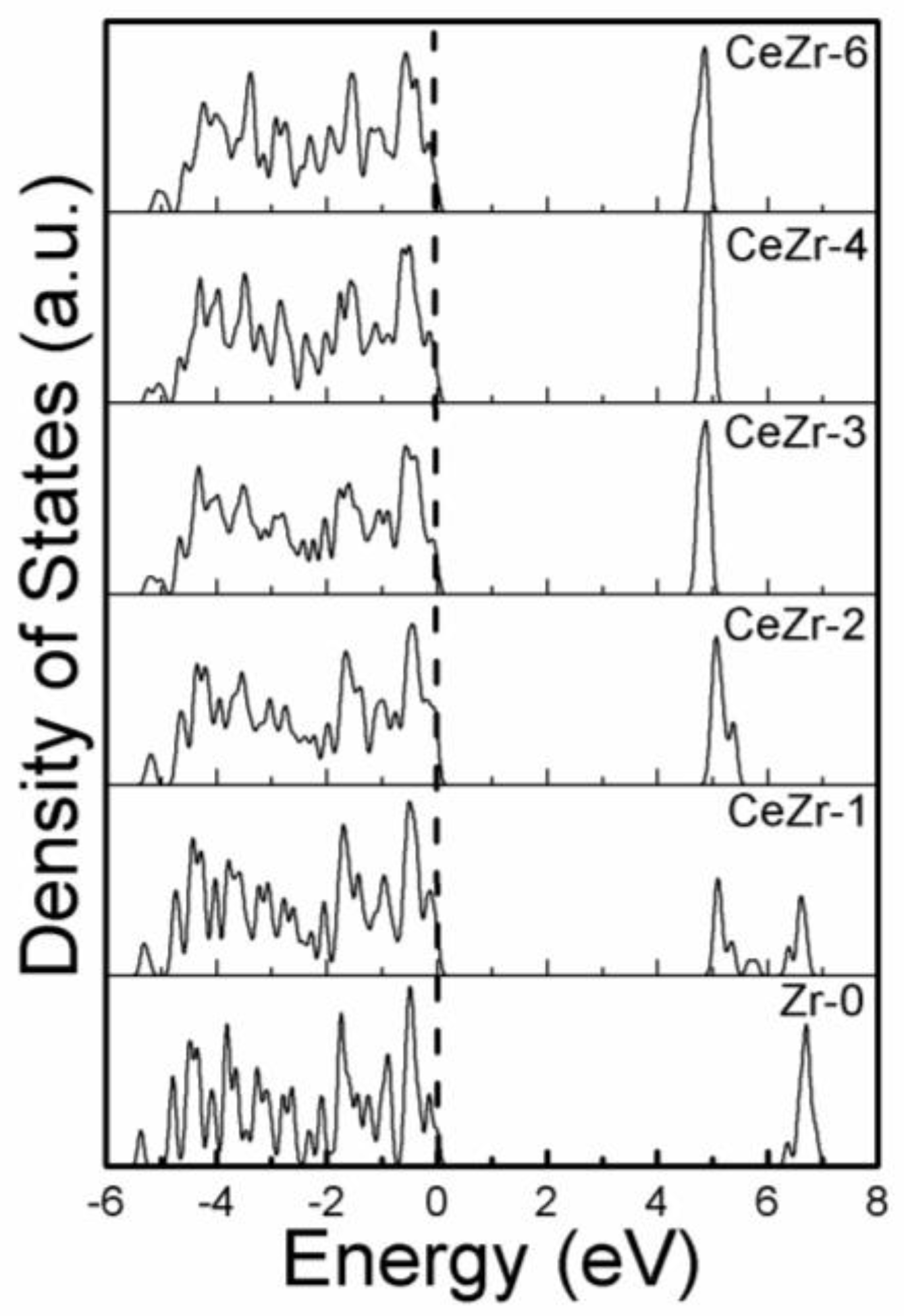
3.2. The Effect of Ce Inclusion on the Electronic Band Structure of Zirconia
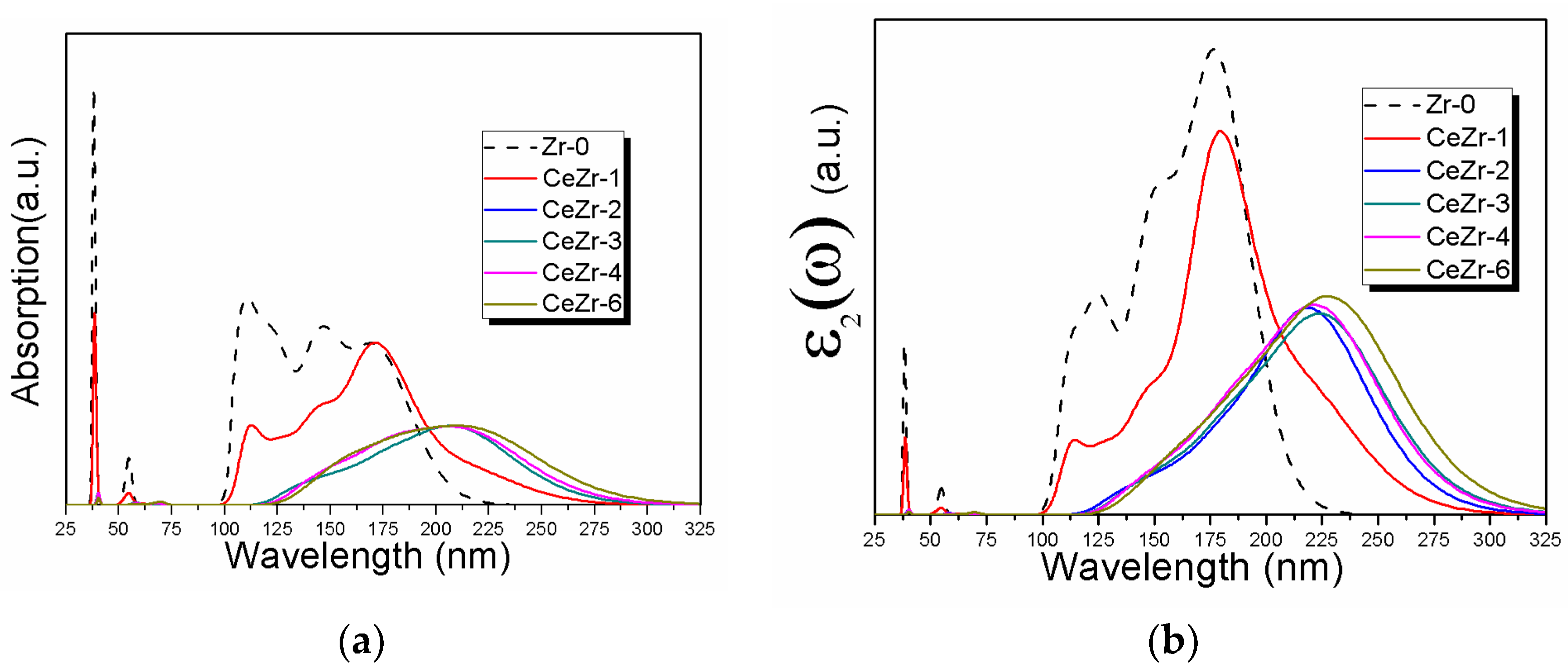
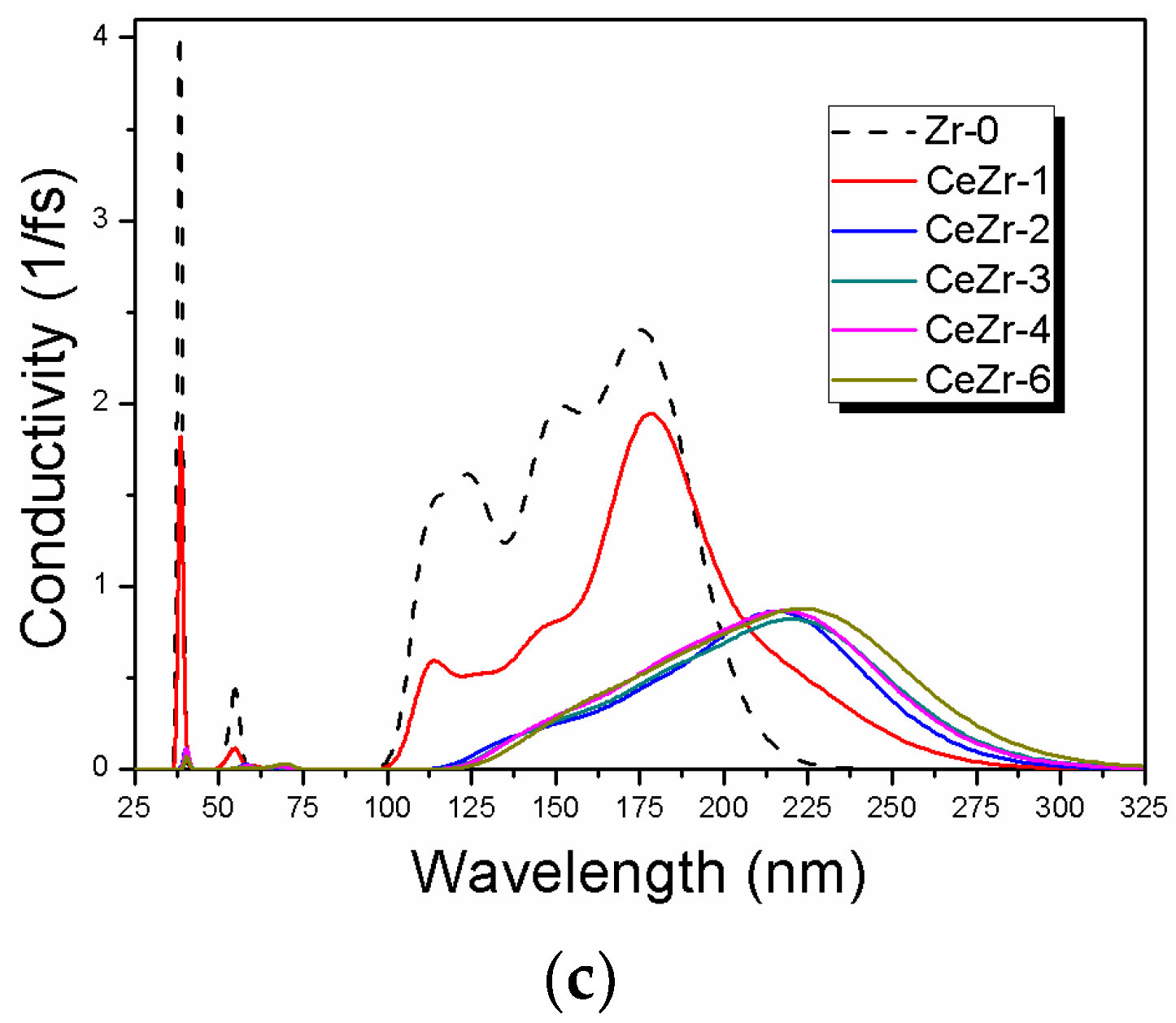
3.3. The Effect of Ce Inclusion on the Photo-Response of Zirconia
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Albert, M.I.; Yingna, D.; Yoshitaka, U. Effect of cation dopants in zirconia on interfacial properties in nickel/zirconia systems: An atomistic modeling study. J. Phys. Condens. Matter 2017, 29, 045001. [Google Scholar]
- Wang, Y.; Xu, F.; Gauvin, R.; Kong, M.; Khan, M.; Liu, Z.; Zeng, Y. Growth modes for monoclinic yttria-stabilized zirconia during the martensitic transformation. J. Am. Ceram. Soc. 2017, 100, 4874–4883. [Google Scholar] [CrossRef]
- Vasilopoulou, M.; Georgiadou, D.G.; Soultati, A.; Boukos, N.; Gardelis, S.; Palilis, L.C.; Fakis, M.; Skoulatakis, G.; Kennou, S.; Botzakaki, M.; et al. Atomic-Layer-Deposited Aluminum and Zirconium Oxides for Surface Passivation of TiO2 in High-Efficiency Organic Photovoltaics. Adv. Energy Mater. 2014, 4, 1400214-n/a. [Google Scholar] [CrossRef]
- Padture, N.P.; Gell, M.; Jordan, E.H. Thermal Barrier Coatings for Gas-Turbine Engine Applications. Science 2002, 296, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.Q.; Vassen, R.; Stoever, D. Ceramic materials for thermal barrier coatings. J. Eur. Ceram. Soc. 2004, 24, 1–10. [Google Scholar] [CrossRef]
- Yang, Z.; Woo, T.K.; Hermansson, K. Effects of Zr doping on stoichiometric and reduced ceria: A first-principles study. J. Chem. Phys. 2006, 124, 224704. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-T.; Chang, J.-G. Oxygen vacancy formation and migration in Ce1−xZrxO2 catalyst: A DFT+U calculation. J. Chem. Phys. 2010, 132, 214702. [Google Scholar] [CrossRef] [PubMed]
- Murota, T.; Hasegawa, T.; Aozasa, S.; Matsui, H.; Motoyama, M. Production method of cerium oxide with high storage capacity of oxygen and its mechanism. J. Alloys Compd. 1993, 193, 298–299. [Google Scholar] [CrossRef]
- Fornasiero, P.; Dimonte, R.; Rao, G.R.; Kaspar, J.; Meriani, S.; Trovarelli, A.; Graziani, M. Rh-Loaded CeO2-ZrO2 Solid-Solutions as Highly Efficient Oxygen Exchangers: Dependence of the Reduction Behavior and the Oxygen Storage Capacity on the Structural-Properties. J. Catal. 1995, 151, 168–177. [Google Scholar] [CrossRef]
- Andersson, D.A.; Simak, S.I.; Skorodumova, N.V.; Abrikosov, I.A.; Johansson, B. Redox properties of CeO2–MO2 (M=Ti, Zr, Hf, or Th) solid solutions from first principles calculations. Appl. Phys. Lett. 2007, 90, 031909. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, X.; Xiao, P. The effects of temperature and composition on the thermal conductivities of [(ZrO2)1−x(CeO2)x]0.92(Y2O3)0.08 (0 ≤ x ≤ 1) solid solutions. Acta Mater. 2012, 60, 914–922. [Google Scholar] [CrossRef]
- Tian, D.; Zeng, C.; Wang, H.; Luo, H.; Cheng, X.; Xiang, C.; Wei, Y.; Li, K.; Zhu, X. Performance of cubic ZrO2 doped CeO2: First-principles investigation on elastic, electronic and optical properties of Ce1−xZrxO2. J. Alloys Compd. 2016, 671, 208–219. [Google Scholar] [CrossRef]
- Khan, M.; Cao, W.; Chen, N.; Iqbal, M.Z. Ab-initio calculations of synergistic chromium–nitrogen codoping effects on the electronic and optical properties of anatase TiO2. Vacuum 2013, 92, 32–38. [Google Scholar] [CrossRef]
- Skorodumova, N.V.; Baudin, M.; Hermansson, K. Surface properties of CeO2 from first principles. Phys. Rev. B 2004, 69, 075401. [Google Scholar] [CrossRef]
- Perdew, J.P.; Ruzsinszky, A.; Csonka, G.I.; Vydrov, O.A.; Scuseria, G.E.; Constantin, L.A.; Zhou, X.; Burke, K. Restoring the Density-Gradient Expansion for Exchange in Solids and Surfaces. Phys. Rev. Lett. 2008, 100, 136406. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Cao, W.; Ullah, M. Ab initiocalculations for the electronic and optical properties of Y-doped anatase TiO2. Phys. Status Solidi 2013, 250, 364–369. [Google Scholar] [CrossRef]
- Pfrommer, B.G.; Côté, M.; Louie, S.G.; Cohen, M.L. Relaxation of Crystals with the Quasi-Newton Method. J. Comput. Phys. 1997, 131, 233–240. [Google Scholar] [CrossRef]
- Ramin, G.S.; Matiullah, K.; Zeng, Y.; Bo, W. Structural, electronic and optical properties of non-compensated and compensated models of yttrium stabilized zirconia. Mater. Res. Express 2017, 4, 126304. [Google Scholar]
- Garcia, J.C.; Scolfaro, L.M.R.; Lino, A.T.; Freire, V.N.; Farias, G.A.; Silva, C.C.; Alves, H.L.; Rodrigues, S.C.P.; da Silva, E.F., Jr. Structural, electronic, and optical properties of ZrO2 from ab initio calculations. J. Appl. Phys. 2006, 100, 104103. [Google Scholar] [CrossRef]
- Stefanovich, E.V.; Shluger, A.L.; Catlow, C.R.A. Theoretical study of the stabilization of cubic-phase ZrO2 by impurities. Phys. Rev. B 1994, 49, 11560–11571. [Google Scholar] [CrossRef]
- French, R.H.; Glass, S.J.; Ohuchi, F.S.; Xu, Y.N.; Ching, W.Y. Experimental and theoretical determination of the electronic structure and optical properties of three phases of ZrO2. Phys. Rev. B 1994, 49, 5133–5142. [Google Scholar] [CrossRef]
- Khan, M.; Cao, W. Cationic (V, Y)-codoped TiO2 with enhanced visible light induced photocatalytic activity: A combined experimental and theoretical study. J. Appl. Phys. 2013, 114, 183514. [Google Scholar] [CrossRef]
- Khan, M.; Xu, J.; Chen, N.; Cao, W. First principle calculations of the electronic and optical properties of pure and (Mo, N) co-doped anatase TiO2. J. Alloys Compd. 2012, 513, 539–545. [Google Scholar] [CrossRef]
- Sun, J.; Wang, H.-T.; He, J.; Tian, Y. Ab initio investigations of optical properties of the high-pressure phases of ZnO. Phys. Rev. B 2005, 71, 125132. [Google Scholar] [CrossRef]
- Khan, M.; Xu, J.; Chen, N.; Cao, W. Electronic and optical properties of pure and Mo doped anatase TiO2 using GGA and GGA+U calculations. Phys. B Condens. Matter 2012, 407, 3610–3616. [Google Scholar] [CrossRef]
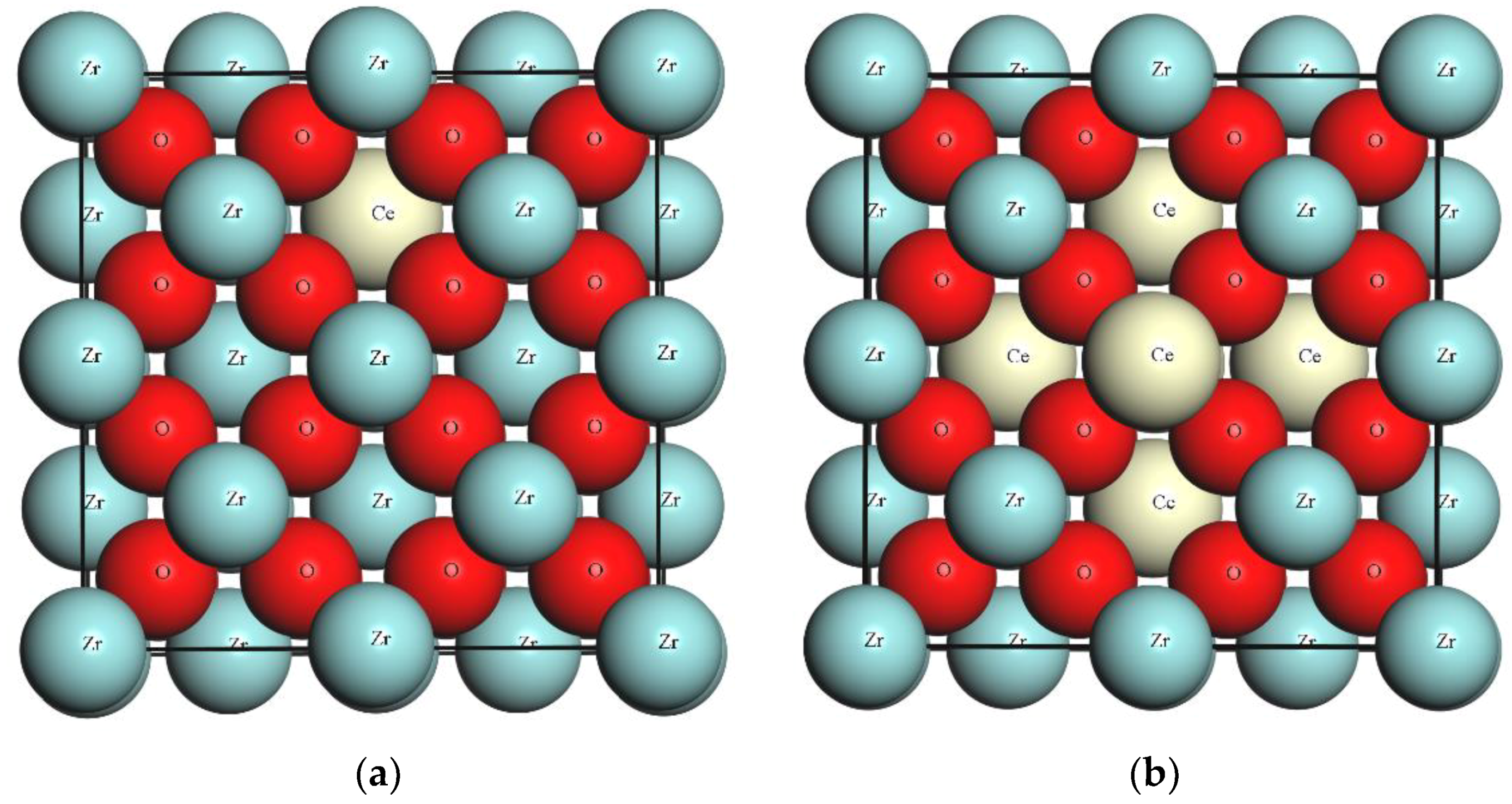
| S. No. | Model | Representation | Ce Doping Concentration (%) | Ce/Zr Ratio (%) |
|---|---|---|---|---|
| 1 | Zr16O32 | Zr-0 | 0 | 100 |
| 2 | CeZr15O32 | CeZr-1 | 2.08 | 6.25 |
| 3 | Ce2Zr14O32 | CeZr-2 | 4.16 | 12.5 |
| 4 | Ce3Zr13O32 | CeZr-3 | 6.25 | 18.75 |
| 5 | Ce4Zr12O32 | CeZr-4 | 8.33 | 25.0 |
| 6 | Ce6Zr10O32 | CeZr-6 | 12.5 | 37.5 |
| Model | a (Å) | b (Å) | c (Å) | Cell Volume (Å3) |
|---|---|---|---|---|
| Zr-0 | 5.0790 | 5.0790 | 5.0796 | 131.0392 |
| Zr-0 (calculations [12]) | 5.0654 | 5.0654 | 5.0654 | 129.974 |
| Zr-0 (experiments [20]) | - | - | 5.090 | - |
| CeZr-1 | 5.0959 | 5.0945 | 5.0925 | 132.8176 |
| CeZr-2 | 5.1179 | 5.1179 | 5.1132 | 133.9314 |
| CeZr-3 | 5.1489 | 5.1520 | 5.1482 | 136.5704 |
| CeZr-4 | 5.1582 | 5.1582 | 5.1604 | 137.3086 |
| CeZr-6 | 5.1886 | 5.1886 | 5.1733 | 139.2799 |
| S. No. | Model | O-Zr (Å) | O-O (Å) | O-Ce (Å) |
|---|---|---|---|---|
| 1 | Zr-0 | 2.1993 | 2.5395 | - |
| 2 | CeZr-1 | 2.2004 | 2.5430 | 2.2885 |
| 3 | CeZr-2 | 2.2049 | 2.5507 | 2.2919 |
| 4 | CeZr-3 | 2.2127 | 2.5643 | 2.2992 |
| 5 | CeZr-4 | 2.2144 | 2.5650 | 2.2944 |
| 6 | CeZr-6 | 2.2199 | 2.5813 | 2.3006 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramin Gul, S.; Khan, M.; Zeng, Y.; Lin, M.; Wu, B.; Tsai, C.-T. Electronic Band Structure Variations in the Ceria Doped Zirconia: A First Principles Study. Materials 2018, 11, 1238. https://doi.org/10.3390/ma11071238
Ramin Gul S, Khan M, Zeng Y, Lin M, Wu B, Tsai C-T. Electronic Band Structure Variations in the Ceria Doped Zirconia: A First Principles Study. Materials. 2018; 11(7):1238. https://doi.org/10.3390/ma11071238
Chicago/Turabian StyleRamin Gul, Sahar, Matiullah Khan, Yi Zeng, Maohua Lin, Bo Wu, and Chi-Tay Tsai. 2018. "Electronic Band Structure Variations in the Ceria Doped Zirconia: A First Principles Study" Materials 11, no. 7: 1238. https://doi.org/10.3390/ma11071238
APA StyleRamin Gul, S., Khan, M., Zeng, Y., Lin, M., Wu, B., & Tsai, C.-T. (2018). Electronic Band Structure Variations in the Ceria Doped Zirconia: A First Principles Study. Materials, 11(7), 1238. https://doi.org/10.3390/ma11071238







