Synthesis, Characterization, and Sensitivity of a CL-20/PNCB Spherical Composite for Security
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
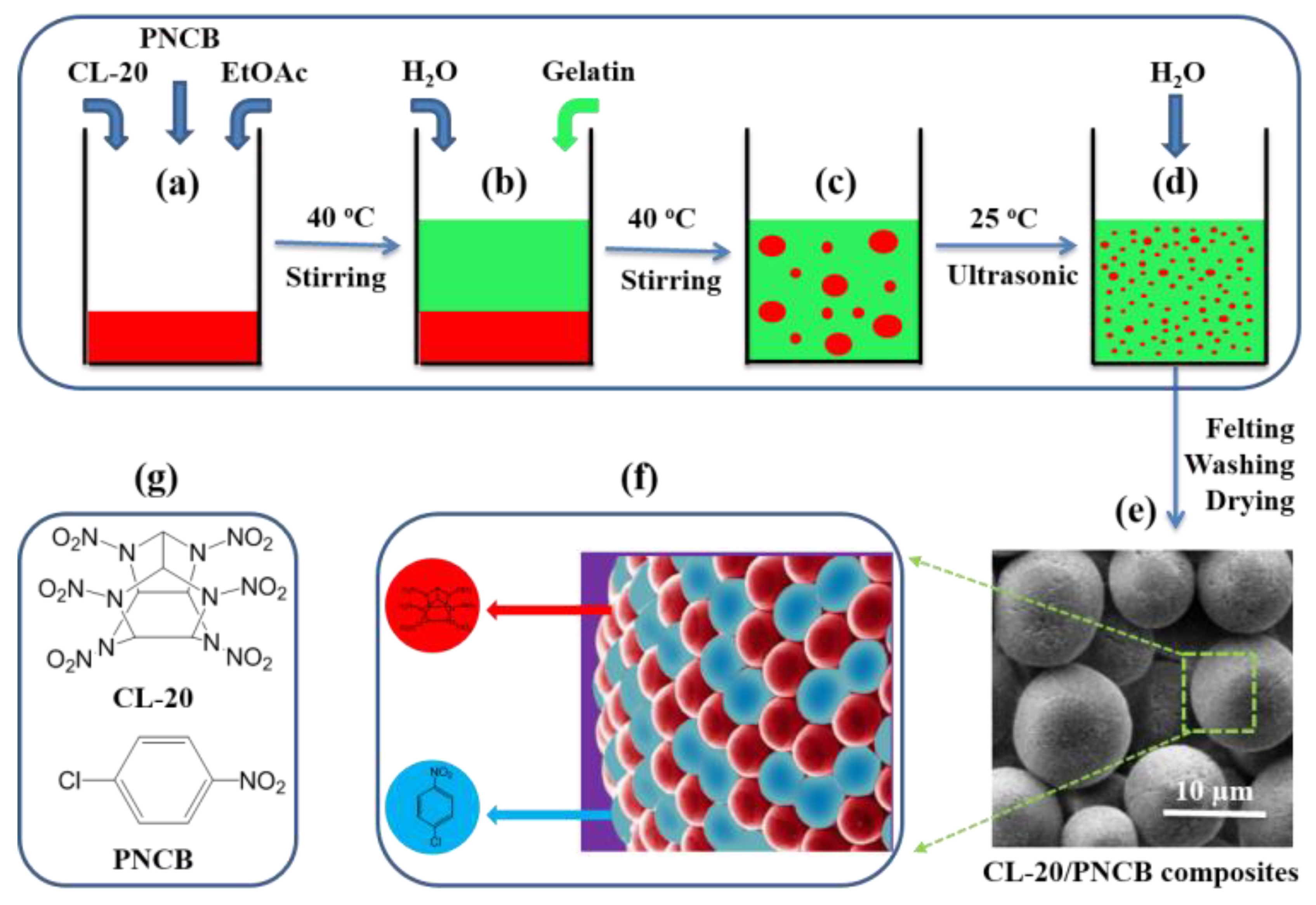
2.2. Preparation of Micro-Scale CL-20/PNCB Composite
2.3. Characterization
2.4. Impact Sensitivity Test
3. Results
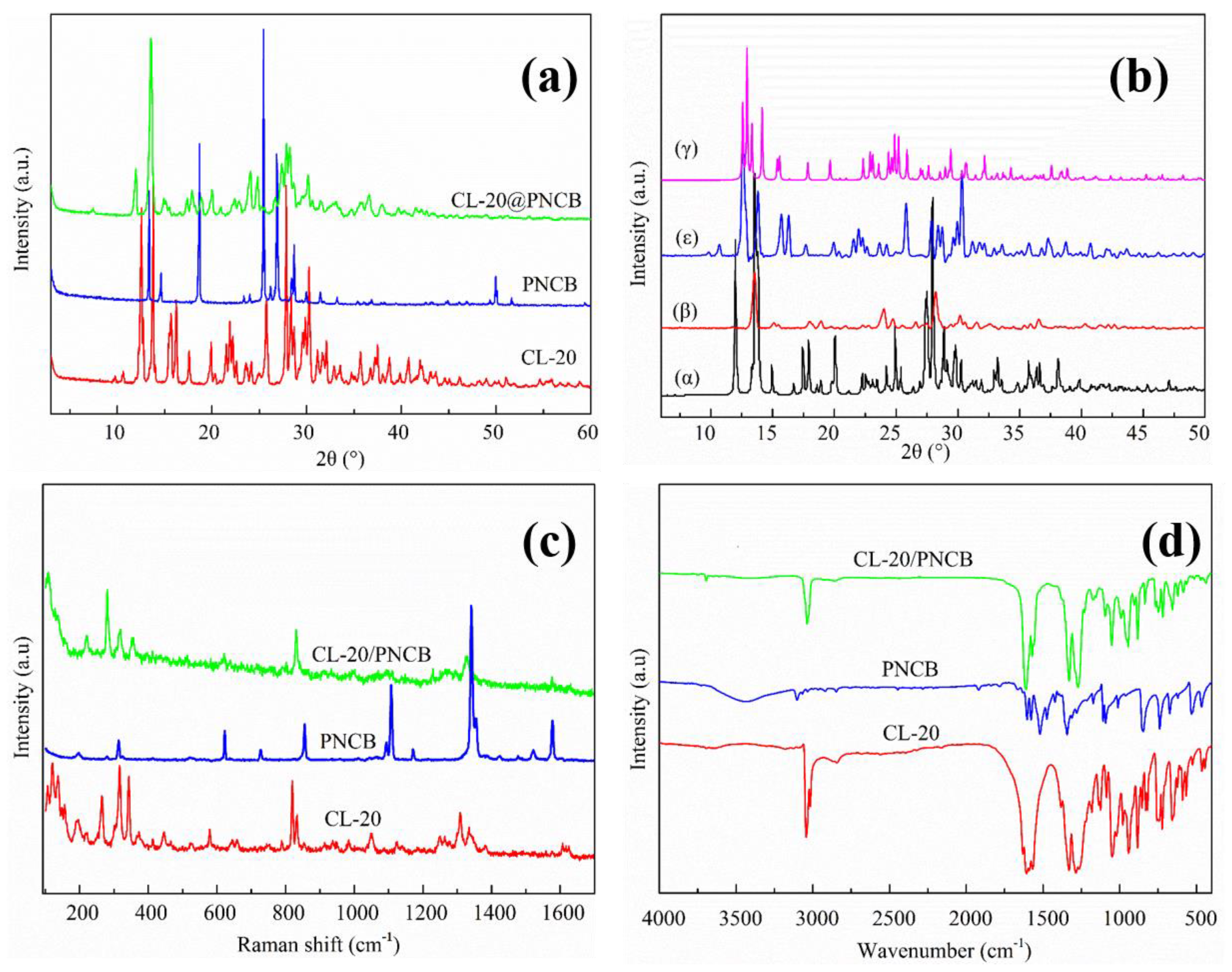
3.1. CL-20/PNCB Spherical Composite Formation Mechanism
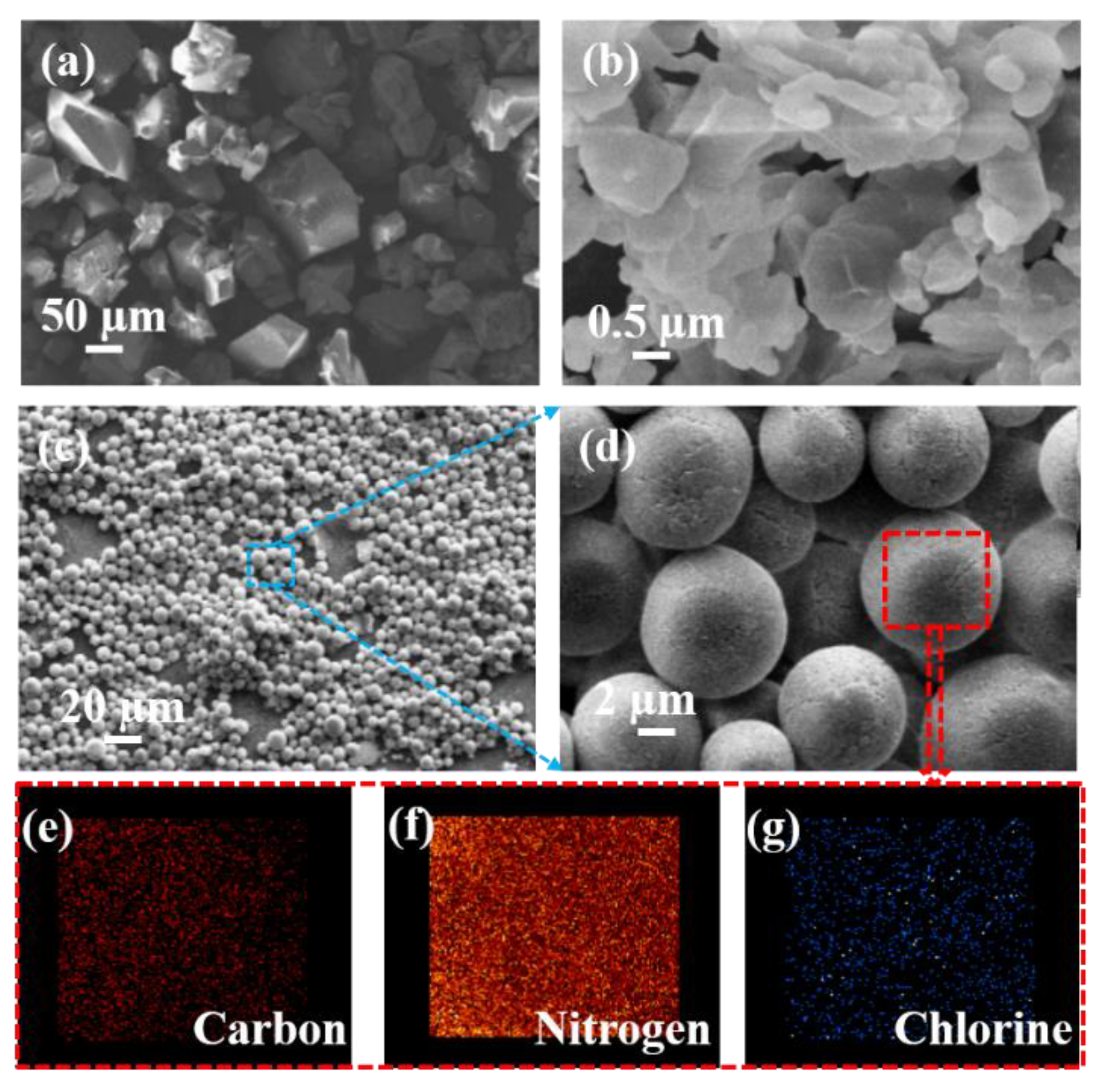
3.2. Morphology and Structure of the Micro-Scale CL-20/PNCB Composite
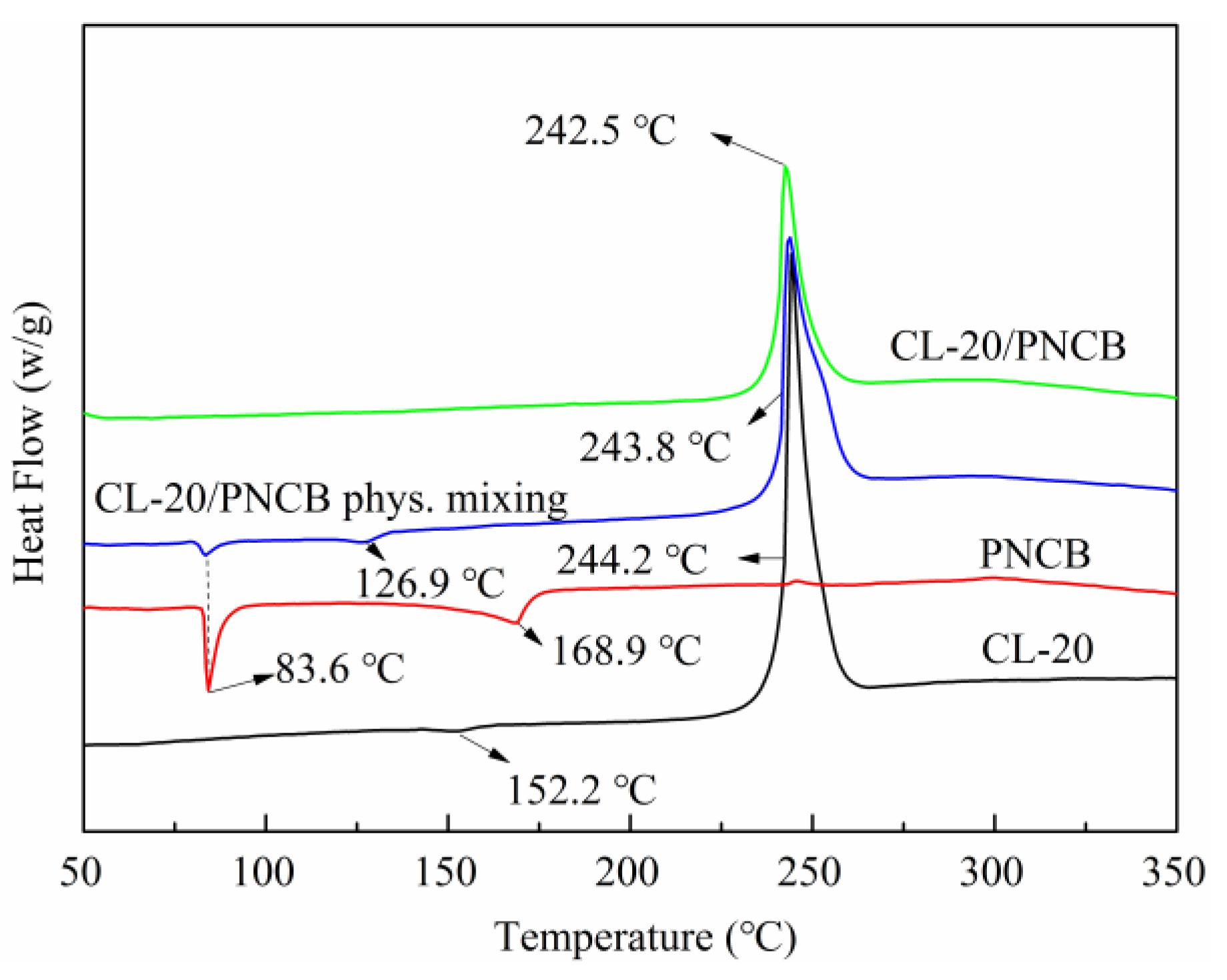
3.3. Thermodynamic Properties and the Sensitivity Performance
3.4. Detonation Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bolton, O.; Simke, L.R.; Pagoria, P.F.; Matzger, A.J. High power explosive with good sensitivity: A 2:1 cocrystal of CL-20: HMX. Cryst. Growth Des. 2012, 12, 4311–4314. [Google Scholar] [CrossRef]
- Gao, B.; Wang, D.J.; Zhang, J.; Hu, Y.J.; Shen, J.P.; Wang, J. Facile, continuous and large-scale synthesis of CL-20/HMX nano co-crystals with high-performance by ultrasonic spray-assisted electrostatic adsorption method. J. Mater. Chem. A 2014, 2, 19969–19974. [Google Scholar] [CrossRef]
- Bolton, O.; Matzger, A.J. Improved stability and smart-material functionality realized in an energetic cocrystal. Angew. Chem. Int. Ed. 2011, 50, 8960–8963. [Google Scholar] [CrossRef] [PubMed]
- Bayat, Y.; Zarandi, M.; Zarei, M.A.; Soleyman, R.; Zeynail, V. A novel approach for preparation of CL-20 nanoparticles by microemulsion method. J. Mol. Liq. 2014, 193, 83–86. [Google Scholar] [CrossRef]
- Vijayalakshmi, R.; Radhakrishnan, S.; Shitole, P.; Pawar, S.J.; Mishra, V.S.; Garg, R.K.; Talawar, M.B.; Sikder, A.K. Spherical 3-nitro-1,2,4-triazol-5-one (NTO) based melt-cast compositions: Heralding a new era of shock insensitive energetic materials. RSC Adv. 2015, 5, 101647–101655. [Google Scholar] [CrossRef]
- Li, J.; Wu, S.; Lu, K. Study on preparation of insensitive and spherical high bulk density nitroguanidine with controllable particle size. Propellants Explos. Pyrotech. 2016, 41, 312–320. [Google Scholar] [CrossRef]
- Pagire, S.K.; Korde, S.A.; Whiteside, B.R.; Kendrick, J.; Paradkar, A. Spherical crystallization of carbamazepine/saccharin co-crystals: Selective agglomeration and purification through surface interactions. Cryst. Growth Des. 2013, 13, 4162–4167. [Google Scholar] [CrossRef]
- Xu, H.; Guo, J.; Suslick, K.S. Porous carbon spheres from energetic carbon precursors using ultrasonic spray pyrolysis. Adv. Mater. 2012, 24, 6028–6033. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Fang, Z.Z.; Xia, Y.; Zhang, Y.; Zhou, C.S. A novel method for production of spherical Ti-6Al-4V powder for additive manufacturing. Powder Technol. 2016, 301, 331–335. [Google Scholar] [CrossRef]
- Bayat, Y.; Pourmortazavi, S.M.; Ahadi, H.; Iravani, H. Taguchi robust design to optimize supercritical carbon dioxide anti-solvent process for preparation of 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane nanoparticles. Chem. Eng. J. 2013, 230, 432–438. [Google Scholar] [CrossRef]
- Yang, Z.; Li, J.; Huang, B.; Liu, S.; Huang, Z.; Nie, F. Preparation and properties study of core-shell CL-20/TATB composites. Propellants Explos. Pyrotech. 2014, 39, 51–58. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Z.; Li, H.; Wang, J.; Zhou, X.; Zhang, Q. A novel cocrystal explosive of HNIW with good comprehensive properties. Propellants Explos. Pyrotech. 2014, 39, 590–596. [Google Scholar] [CrossRef]
- Chen, J.; He, S.; Huang, B.; Wu, P.; Qiao, Z.; Wang, J. Enhanced thermal decomposition properties of CL-20 through space-confining in three-dimensional hierarchically ordered porous carbon. ACS Appl. Mater. Interfaces 2017, 9, 10684–10691. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-J.; Kim, K.-M. Growth kinetics in seeded cooling crystallization of 3-nitro-1, 2, 4-triazol-5-one in water–N-methylpyrrolidone. Powder Technol. 2002, 122, 46–53. [Google Scholar] [CrossRef]
- Stepanov, V.; Willey, T.M.; Ilavsky, J.; Gel, J.; Qiu, H. Structural Characterization of RDX-Based Explosive Nanocomposite. Propellants Explos. Pyrotech. 2013, 38, 386–393. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, A.; Ma, Y.; Xue, X.; Zhou, J.; Zhu, Y.; Zhang, C. Toward low-sensitive and high-energetic cocrystal III: Thermodynamics of energetic–energetic cocrystal formation. CrystEngComm 2015, 17, 9037–9047. [Google Scholar] [CrossRef]
- Yang, Z.; Li, H.; Huang, H.; Zhou, X.; Li, J.; Nie, F. Preparation and performance of a HNIW/TNT cocrystal explosive. Propellants Explos. Pyrotech. 2013, 38, 495–501. [Google Scholar] [CrossRef]
- Guo, D.; An, Q.; Zybin, S.V.; Goddard, W.A.; Huang, F.; Tang, B. The co-crystal of TNT/CL-20 leads to decreased sensitivity toward thermal decomposition from first principles based reactive molecular dynamics. J. Mater. Chem. A 2015, 34, 5409–5419. [Google Scholar] [CrossRef]
- Qiu, H.W.; Patel, R.B.; Damavarapu, R.; Stepanov, S.V. Nanoscale 2CL-20·HMX high explosive cocrystal synthesized by bead milling. CrystEngComm 2015, 6, 4080–4083. [Google Scholar] [CrossRef]
- Urbelis, J.H.; Young, V.G.; Swift, J.A. Using solvent effects to guide the design of a CL-20 cocrystal. CrystEngComm 2015, 34, 1564–1568. [Google Scholar] [CrossRef]
- Xue, X.G.; Ma, Y.; Zeng, Q.; Zhang, C. Initial Decay Mechanism of the Heated CL-20/HMX Cocrystal: A Case of the Cocrystal Mediating the Thermal Stability of the Two Pure Components. J. Phys. Chem. C 2017, 121, 4899–4908. [Google Scholar] [CrossRef]
- Katin, K.P.; Maslov, M.M. Toward CL-20 crystalline covalent solids: On the dependence of energy and electronic properties on the effective size of CL-20 chains. J. Phys. Chem. Solids 2017, 108, 82–87. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Lu, Y.W.; Guo, C.P. Ultrasonic-assisted emulsion synthesis of well-distributed spherical composite CL-20@PNA with enhanced high sensitivity. Mater. Lett. 2017, 205, 94–97. [Google Scholar] [CrossRef]
- GJB772A-1997 Explosive Experiment Method; National Defense Science and Technology Commission Military Standard Press: Beijing, China, 1997.
- Freitas, R.A.; Nicolai, T.; Chassenieux, C.; Benyahia, L. Stabilization of water-in-water emulsions by polysaccharide-coated protein particles. Langmuir 2016, 32, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Binks, B.P.; Tyowua, A.T. Particle-stabilized powdered water-in-oil emulsions. Langmuir 2016, 32, 3110–3115. [Google Scholar] [CrossRef] [PubMed]
- Asano, I.; So, S.; Lodge, T.P. Oil-in-oil emulsions stabilized by asymmetric polymersomes formed by AC+ BC block polymer co-assembly. J. Am. Chem. Soc. 2016, 138, 4714–4717. [Google Scholar] [CrossRef] [PubMed]
- Denkov, N.; Cholakova, D.; Tcholakova, S.; Smoukov, S.K. On the mechanism of drop self-shaping in cooled emulsions. Langmuir 2016, 32, 7985–7991. [Google Scholar] [CrossRef] [PubMed]
- Kamlet, M.J.; Jacobs, S.J. Chemistry of detonations. I. a simple method for calculating detonation properties of C-H-N-O explosives. J. Chem. Phys. 1968, 48, 23–35. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Lu, Y.; Gao, B.; Wang, D.; Guo, C.; Yang, G. Synthesis, Characterization, and Sensitivity of a CL-20/PNCB Spherical Composite for Security. Materials 2018, 11, 1130. https://doi.org/10.3390/ma11071130
Zhu Y, Lu Y, Gao B, Wang D, Guo C, Yang G. Synthesis, Characterization, and Sensitivity of a CL-20/PNCB Spherical Composite for Security. Materials. 2018; 11(7):1130. https://doi.org/10.3390/ma11071130
Chicago/Turabian StyleZhu, Yanfang, Yuewen Lu, Bing Gao, Dunju Wang, Changping Guo, and Guangcheng Yang. 2018. "Synthesis, Characterization, and Sensitivity of a CL-20/PNCB Spherical Composite for Security" Materials 11, no. 7: 1130. https://doi.org/10.3390/ma11071130
APA StyleZhu, Y., Lu, Y., Gao, B., Wang, D., Guo, C., & Yang, G. (2018). Synthesis, Characterization, and Sensitivity of a CL-20/PNCB Spherical Composite for Security. Materials, 11(7), 1130. https://doi.org/10.3390/ma11071130





