Investigation of Three-Dimensional Microstructure of Tricalcium Silicate (C3S) by Electron Microscopy
Abstract
1. Introduction
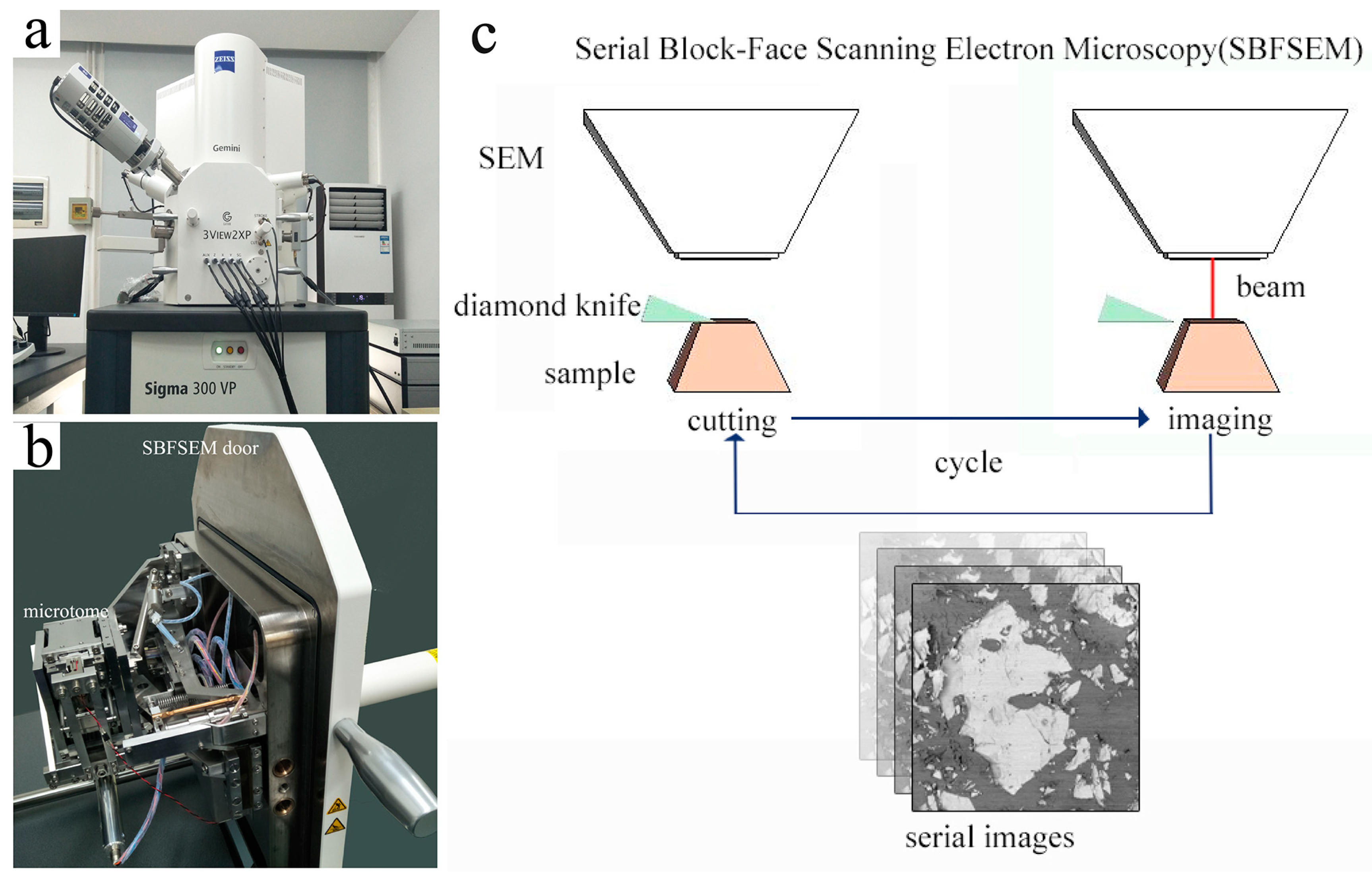
2. Materials and Methods
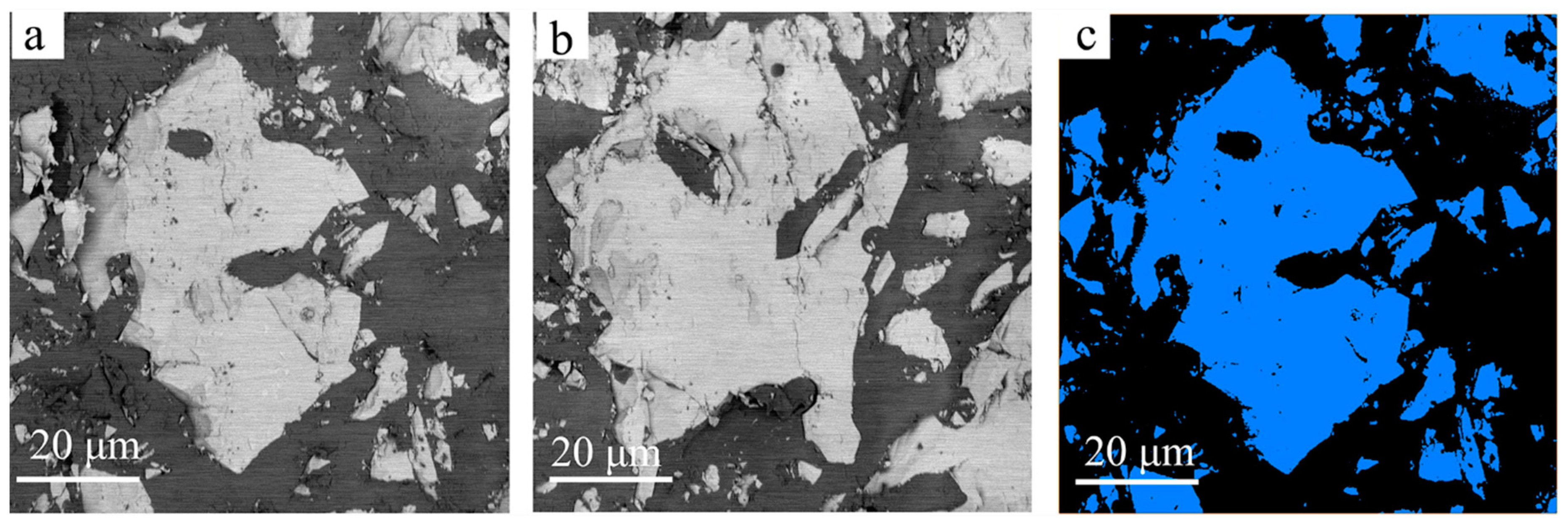
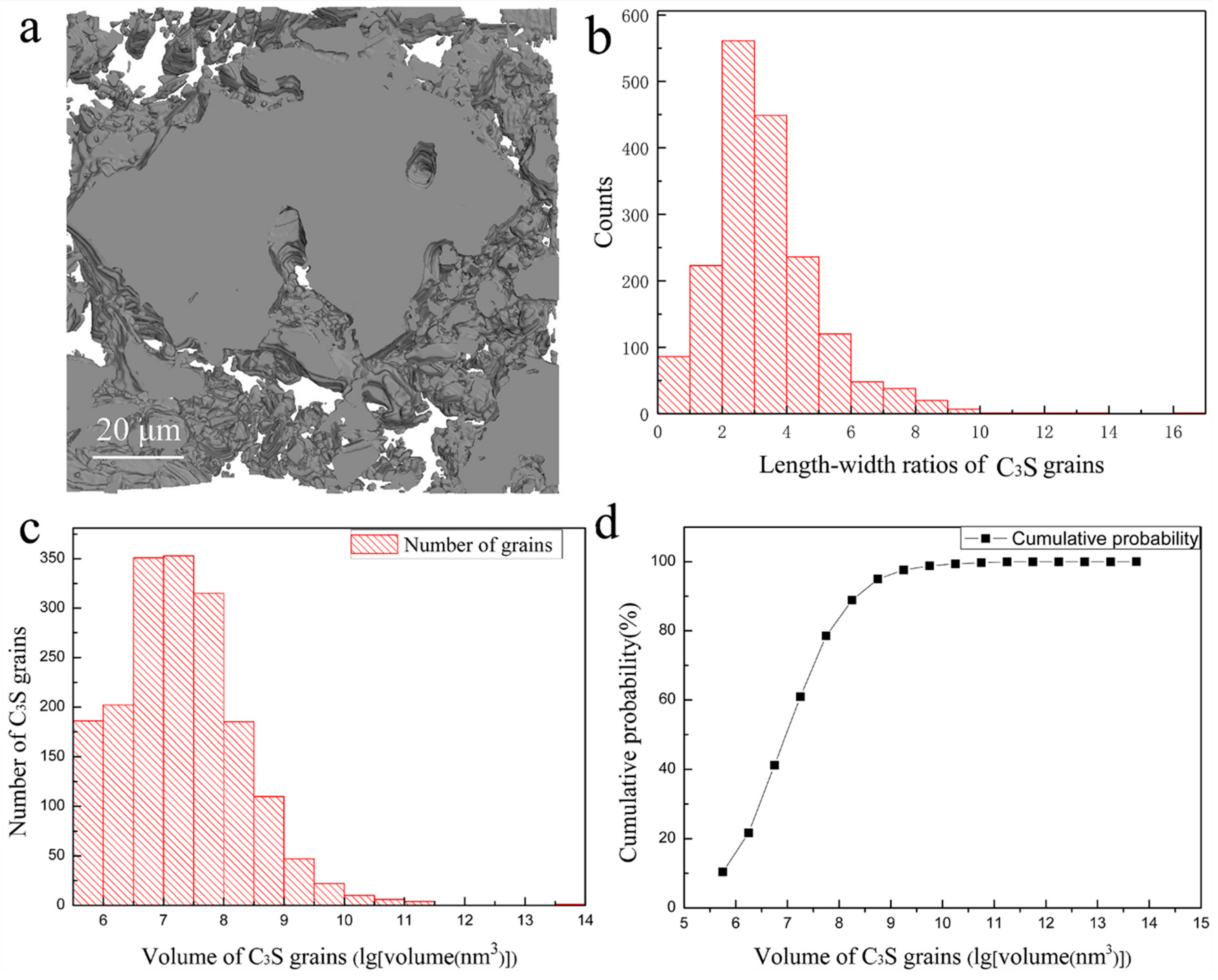
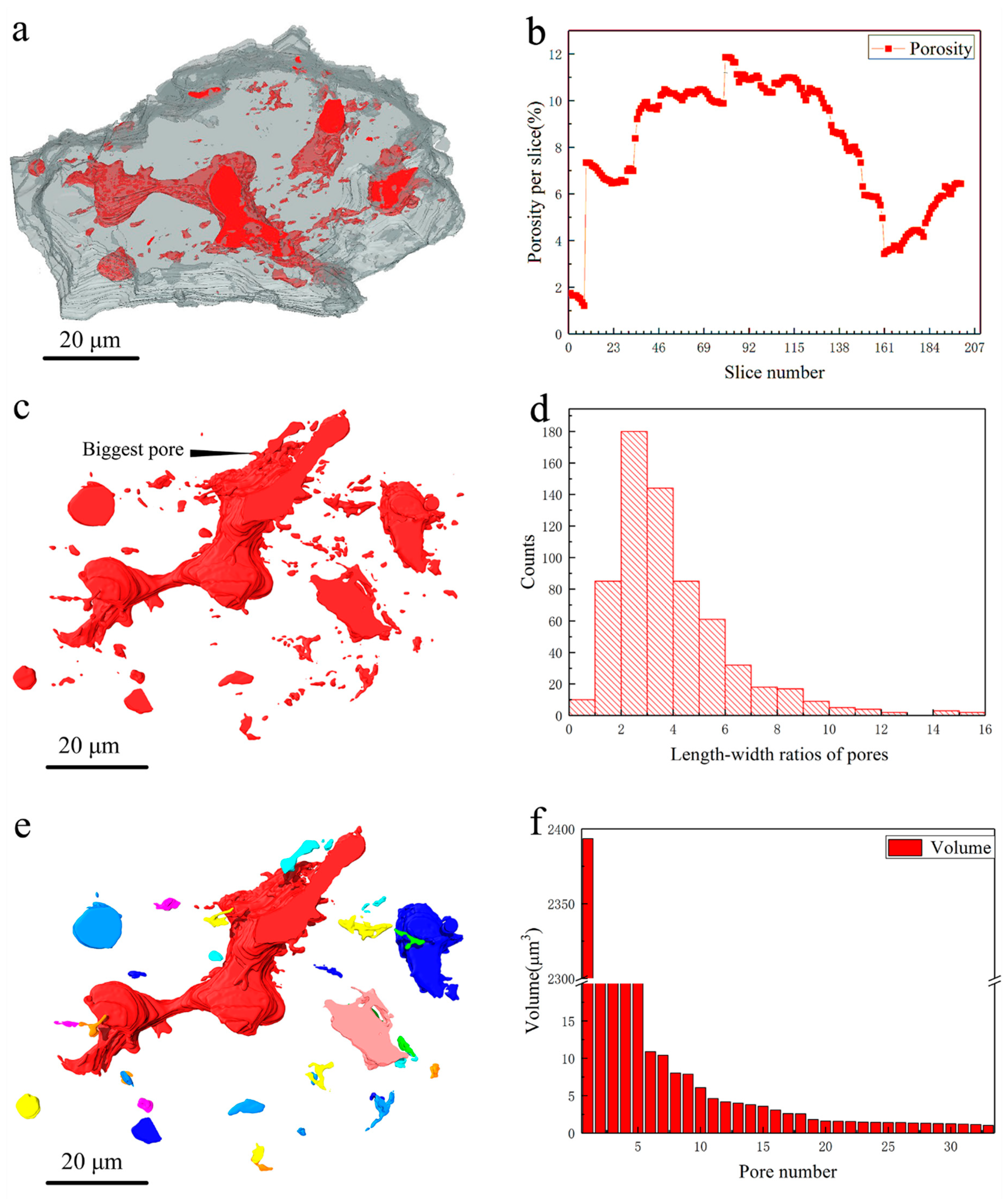
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alizadeh, R.; Raki, L.; Makar, J.M.; Beaudoin, J.J.; Moudrakovski, I. Hydration of tricalcium silicate in the presence of synthetic calcium–silicate–hydrate. J. Mater. Chem. 2009, 19, 7937–7946. [Google Scholar] [CrossRef]
- Dunstetter, F.; de Noirfontaine, M.N.; Courtial, M. Polymorphism of tricalcium silicate, the major compound of portland cement clinker. Cem. Concr. Res. 2006, 36, 39–53. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Monteiro, P.J.M.; Gartner, E.M.; Denbeaux, G.P. A soft X-ray microscope investigation into the effects of calcium chloride on tricalcium silicate hydration. Cem. Concr. Res. 2005, 35, 19–25. [Google Scholar] [CrossRef]
- De La Torre, A.; Bruque, S.; Campo, J.; Aranda, M. The superstructure of C3S from synchrotron and neutron powder diffraction and its role in quantitative phase analyses. Cem. Concr. Res. 2002, 32, 1347–1356. [Google Scholar] [CrossRef]
- Bae, S.; Kanematsu, M.; Hernández-Cruz, D.; Moon, J.; Kilcoyne, D.; Monteiro, P.J.M. In situ soft X-ray spectromicroscopy of early tricalcium silicate hydration. Materials 2016, 9, 976. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.; Bishnoi, S.; Van Balen, K.; Cizer, Ö. Modeling the effect of fineness and filler in early-age hydration of tricalcium silicate. J. Am. Ceram. Soc. 2017, 100, 1178–1194. [Google Scholar] [CrossRef]
- Nicoleau, L.; Nonat, A. A new view on the kinetics of tricalcium silicate hydration. Cem. Concr. Res. 2016, 86, 1–11. [Google Scholar] [CrossRef]
- Bailey, J.E.; Stewart, H.R. C3S hydration products viewed using a cryo stage in sem. J. Mater. Sci. Lett. 1984, 3, 411–414. [Google Scholar] [CrossRef]
- Glasser, L.D.; Lachowski, E.E.; Mohan, K.; Taylor, H.F.W. A multi-method study of C3S hydration. Cem. Concr. Res. 1978, 8, 733–740. [Google Scholar] [CrossRef]
- Slegers, P.A.; Genet, M.; Leonard, A.J.; Fripiat, J.J. Structural transformation of triealeium silicate during hydration. J. Appl. Crystallogr. 1977, 10, 270–276. [Google Scholar] [CrossRef]
- Wu, Z.; Yong, J. The hydration of tricalcium silicate in the presence of colloidal silica. J. Mater. Sci. 1984, 19, 3477–3486. [Google Scholar] [CrossRef]
- Fic, S.; Szeląg, M. Analysis of the development of cluster cracks caused by elevated temperatures in cement paste. Constr. Build. Mater. 2015, 83, 223–229. [Google Scholar] [CrossRef]
- Li, L.; Wang, Q.; Zhang, G.; Shi, L.; Dong, J.; Jia, P. A method of detecting the cracks of concrete undergo high-temperature. Constr. Build. Mater. 2018, 162, 345–358. [Google Scholar] [CrossRef]
- Szelag, M. The Influence of Metakaolinite on the Development of Thermal Cracks in a Cement Matrix. Materials 2018, 11, 520. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Hills, C.D.; Yuan, M.; Liu, H.; Tyrer, M. Characterization of carbonated tricalcium silicate and its sorption capacity for heavy metals: A micron-scale composite adsorbent of active silicate gel and calcite. J. Hazard. Mater. 2008, 153, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Yan, P.; Liu, R. Study on the hydration product of cement in early age using TEM. Sci. China Technol. Sci. 2012, 55, 2284–2290. [Google Scholar] [CrossRef]
- Kjellsen, K.O.; Justnes, H. Revisiting the microstructure of hydrated tricalcium silicate—A comparison to portland cement. Cem. Concr. Compos. 2004, 26, 947–956. [Google Scholar] [CrossRef]
- Kolovos, K.; Tsivilis, S.; Kakali, G. SEM examination of clinkers containing foreign elements. Cem. Concr. Compos. 2005, 27, 163–170. [Google Scholar] [CrossRef]
- Ylmén, R.; Jäglid, U.; Steenari, B.-M.; Panas, I. Early hydration and setting of portland cement monitored by IR, SEM and VICAT techniques. Cem. Concr. Res. 2009, 39, 433–439. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, X.; Hou, D.; Guo, S. Hydration for the alite mineral: Morphology evolution, reaction mechanism and the compositional influences. Constr. Build. Mater. 2017, 155, 413–426. [Google Scholar] [CrossRef]
- Hansen, T.C. Physical structure of hardened cement paste. A classical approach. Mater. Struct. 1986, 19, 423–436. [Google Scholar] [CrossRef]
- Xi, Y.; Siemer, D.D.; Scheetz, B.E. Strength development, hydration reaction and pore structure of autoclaved slag cement with added silica fume. Cem. Concr. Res. 1997, 26, 75–82. [Google Scholar] [CrossRef]
- Chen, B.; Guizar-Sicairos, M.; Xiong, G.; Shemilt, L.; Diaz, A.; Nutter, J.; Burdet, N.; Huo, S.; Mancuso, J.; Monteith, A.; et al. Three-dimensional structure analysis and percolation properties of a barrier marine coating. Sci. Rep. 2013, 3, 1177. [Google Scholar] [CrossRef] [PubMed]
- Denk, W.; Horstmann, H. Serial block-face scanning electron microscopy to reconstruct three-dimensional tissue nanostructure. PLoS Biol. 2004, 2, e329. [Google Scholar] [CrossRef] [PubMed]
- Pollreisz, A.; Messinger, J.D.; Sloan, K.R.; Mittermueller, T.J.; Weinhandl, A.S.; Benson, E.K.; Kidd, G.J.; Schmidt-Erfurth, U.; Curcio, C.A. Visualizing melanosomes, lipofuscin, and melanolipofuscin in human retinal pigment epithelium using serial block face scanning electron microscopy. Exp. Eye Res. 2018, 166, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Briggman, K.L.; Helmstaedter, M.; Denk, W. Wiring specificity in the direction-selectivity circuit of the retina. Nature 2011, 471, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Yusuf, M.; Hashimoto, T.; Estandarte, A.K.; Thompson, G.; Robinson, I. Three-dimensional positioning and structure of chromosomes in a human prophase nucleus. Sci. Adv. 2017, 3, e1602231. [Google Scholar] [CrossRef] [PubMed]
- Jurrus, E.; Hardy, M.; Tasdizen, T.; Fletcher, P.T.; Koshevoy, P.; Chien, C.B.; Denk, W.; Whitaker, R. Axon tracking in serial block-face scanning electron microscopy. Med. Image Anal. 2009, 13, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, K.; Clark, H.R.; Chavan, V.; Benson, E.K.; Kidd, G.J.; Srivastava, S. Analysis of brain mitochondria using serial block-face scanning electron microscopy. JoVE-J. Vis. Exp. 2016, e54214. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Hashimoto, T.; Vergeer, F.; Burgess, A.; Thompson, G.; Robinson, I. Three-dimensional analysis of the spatial distribution of iron oxide particles in a decorative coating by electron microscopic imaging. Prog. Org. Coat. 2014, 77, 1069–1072. [Google Scholar] [CrossRef]
- Thompson, G.E.; Hashimoto, T.; Zhong, X.L.; Curioni, M.; Zhou, X.; Skeldon, P.; Withers, P.J.; Carr, J.A.; Monteith, A.G. Revealing the three dimensional internal structure of aluminium alloys. Surf. Interface Anal. 2013, 45, 1536–1542. [Google Scholar] [CrossRef]
- Miranda, K.; Girard-Dias, W.; Attias, M.; de Souza, W.; Ramos, I. Three dimensional reconstruction by electron microscopy in the life sciences: An introduction for cell and tissue biologists. Mol. Reprod. Dev. 2015, 82, 530–547. [Google Scholar] [CrossRef] [PubMed]
- Titze, B.; Genoud, C. Volume scanning electron microscopy for imaging biological ultrastructure. Biol. Cell 2016, 108, 307–323. [Google Scholar] [CrossRef] [PubMed]
- Torre, A.G.D.l.; Aranda, M.A.G. Accuracy in rietveld quantitative phase analysis of portland cements. J. Appl. Crystallogr. 2003, 36, 1169–1176. [Google Scholar] [CrossRef]
- Briggman, K.L.; Denk, W. Towards neural circuit reconstruction with volume electron microscopy techniques. Curr. Opin. Neurobiol. 2006, 16, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; King, H.; van Huis, M.; Drury, M.; Plümper, O. Nano-tomography of porous geological materials using focused ion beam-scanning electron microscopy. Minerals 2016, 6, 104. [Google Scholar] [CrossRef]
- Barbieri, D.; Yuan, H.; Ismailoglu, A.S.; de Bruijn, J.D. Comparison of two moldable calcium phosphate-based bone graft materials in a noninstrumented canine interspinous implantation model. Tissue Eng. Part A 2017, 23, 1310–1320. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Liu, X.; Zhao, Y.; Zhang, Y.; Wang, P.; Robinson, I.; Chen, B. Investigation of Three-Dimensional Microstructure of Tricalcium Silicate (C3S) by Electron Microscopy. Materials 2018, 11, 1110. https://doi.org/10.3390/ma11071110
Yang F, Liu X, Zhao Y, Zhang Y, Wang P, Robinson I, Chen B. Investigation of Three-Dimensional Microstructure of Tricalcium Silicate (C3S) by Electron Microscopy. Materials. 2018; 11(7):1110. https://doi.org/10.3390/ma11071110
Chicago/Turabian StyleYang, Fei, Xianping Liu, Yongjuan Zhao, Yongming Zhang, Peiming Wang, Ian Robinson, and Bo Chen. 2018. "Investigation of Three-Dimensional Microstructure of Tricalcium Silicate (C3S) by Electron Microscopy" Materials 11, no. 7: 1110. https://doi.org/10.3390/ma11071110
APA StyleYang, F., Liu, X., Zhao, Y., Zhang, Y., Wang, P., Robinson, I., & Chen, B. (2018). Investigation of Three-Dimensional Microstructure of Tricalcium Silicate (C3S) by Electron Microscopy. Materials, 11(7), 1110. https://doi.org/10.3390/ma11071110




