Electrochemical and Stress Corrosion Mechanism of Submarine Pipeline in Simulated Seawater in Presence of Different Alternating Current Densities
Abstract
:1. Introduction
2. Experimental
2.1. Material and Solution
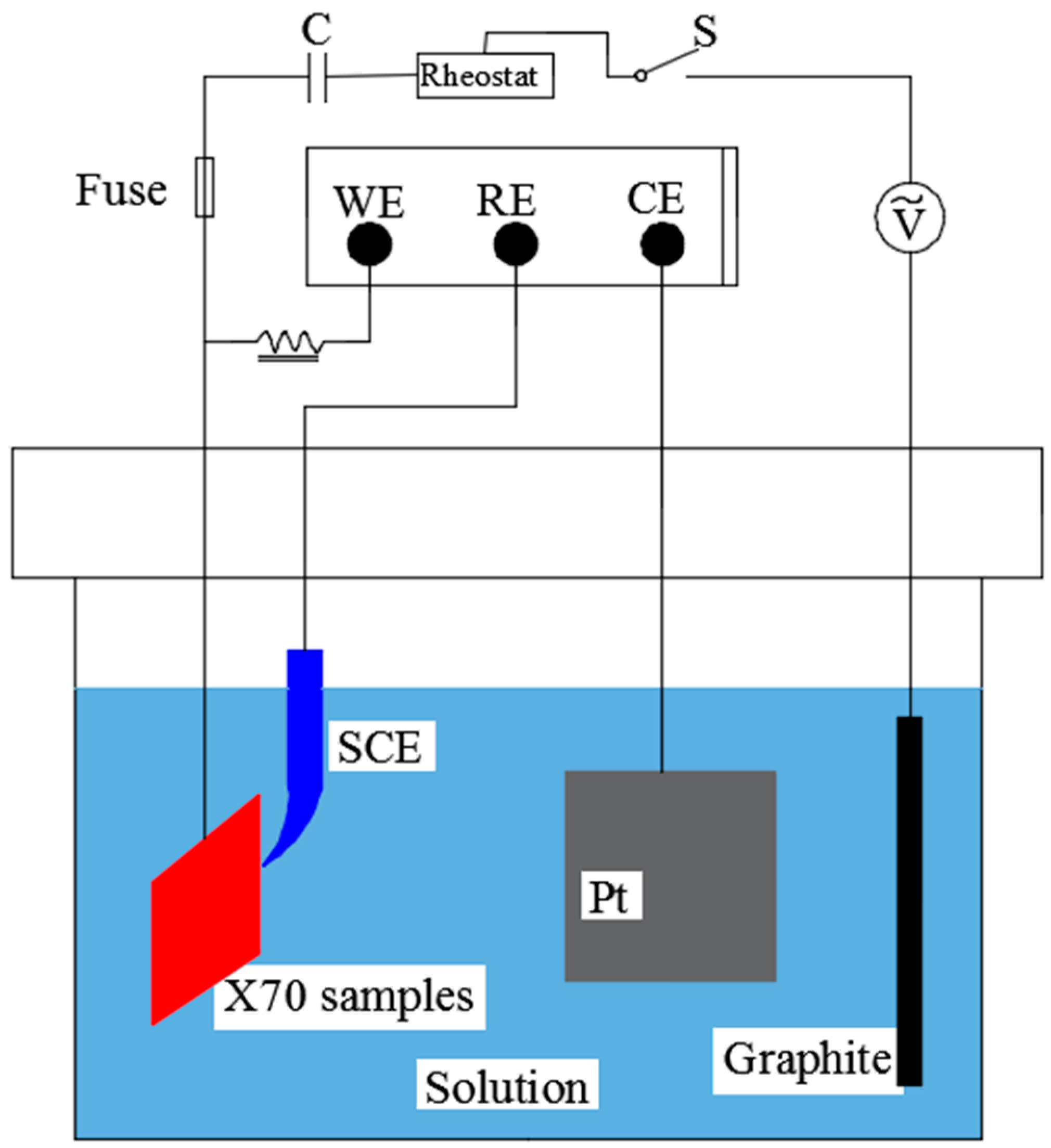
2.2. Electrochemical Measurements
2.3. Immersion Tests
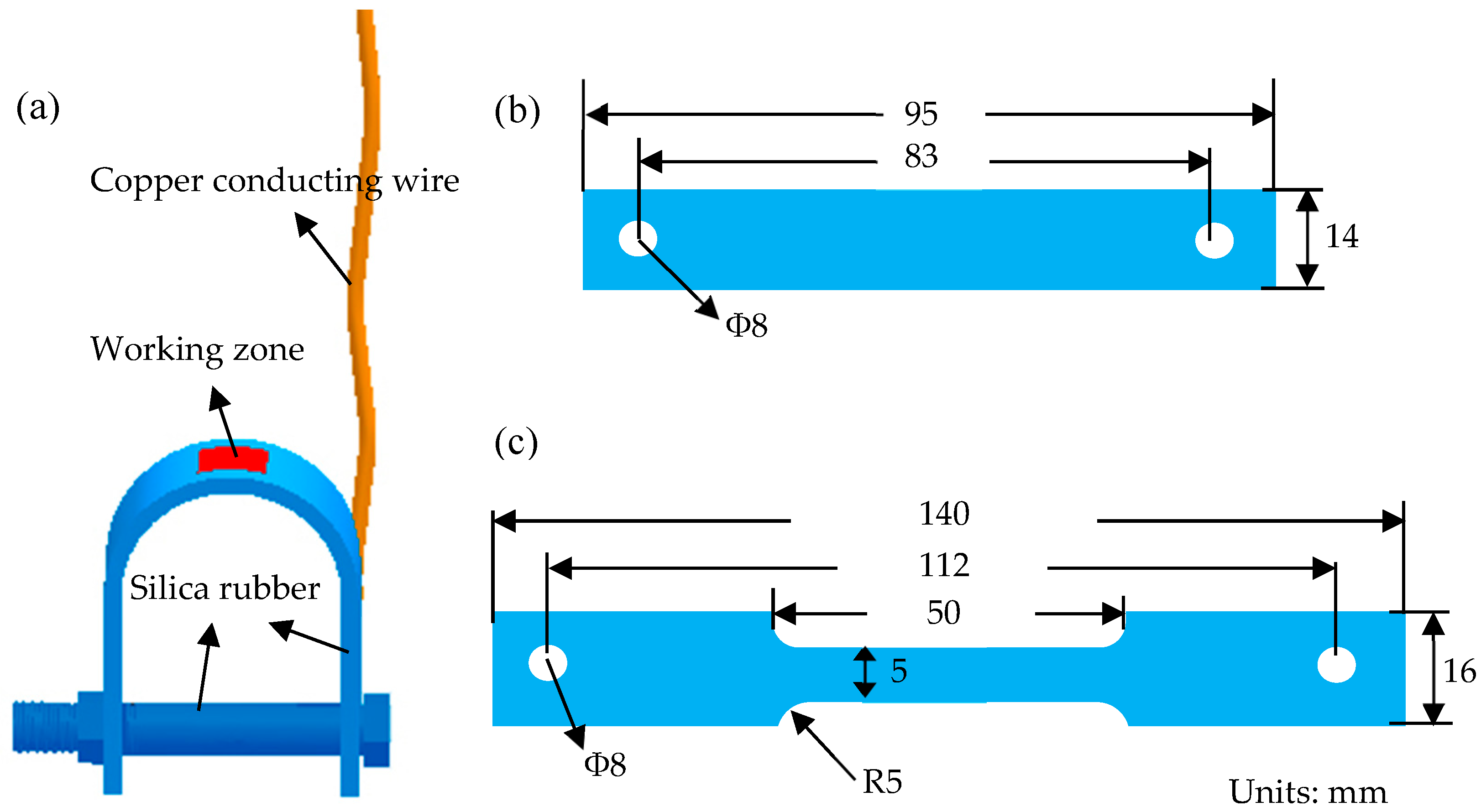
2.4. SSRT Tests
3. Results
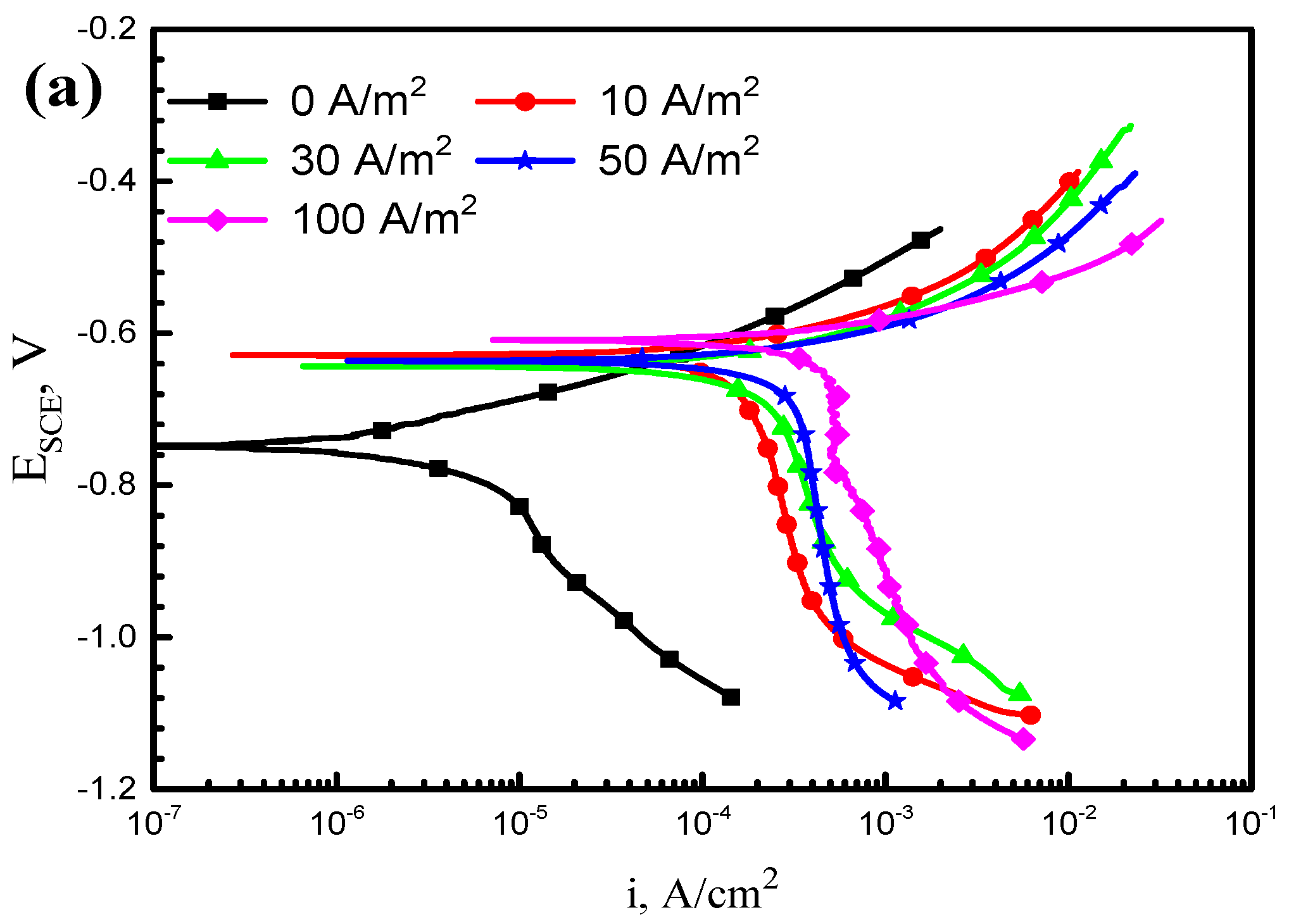
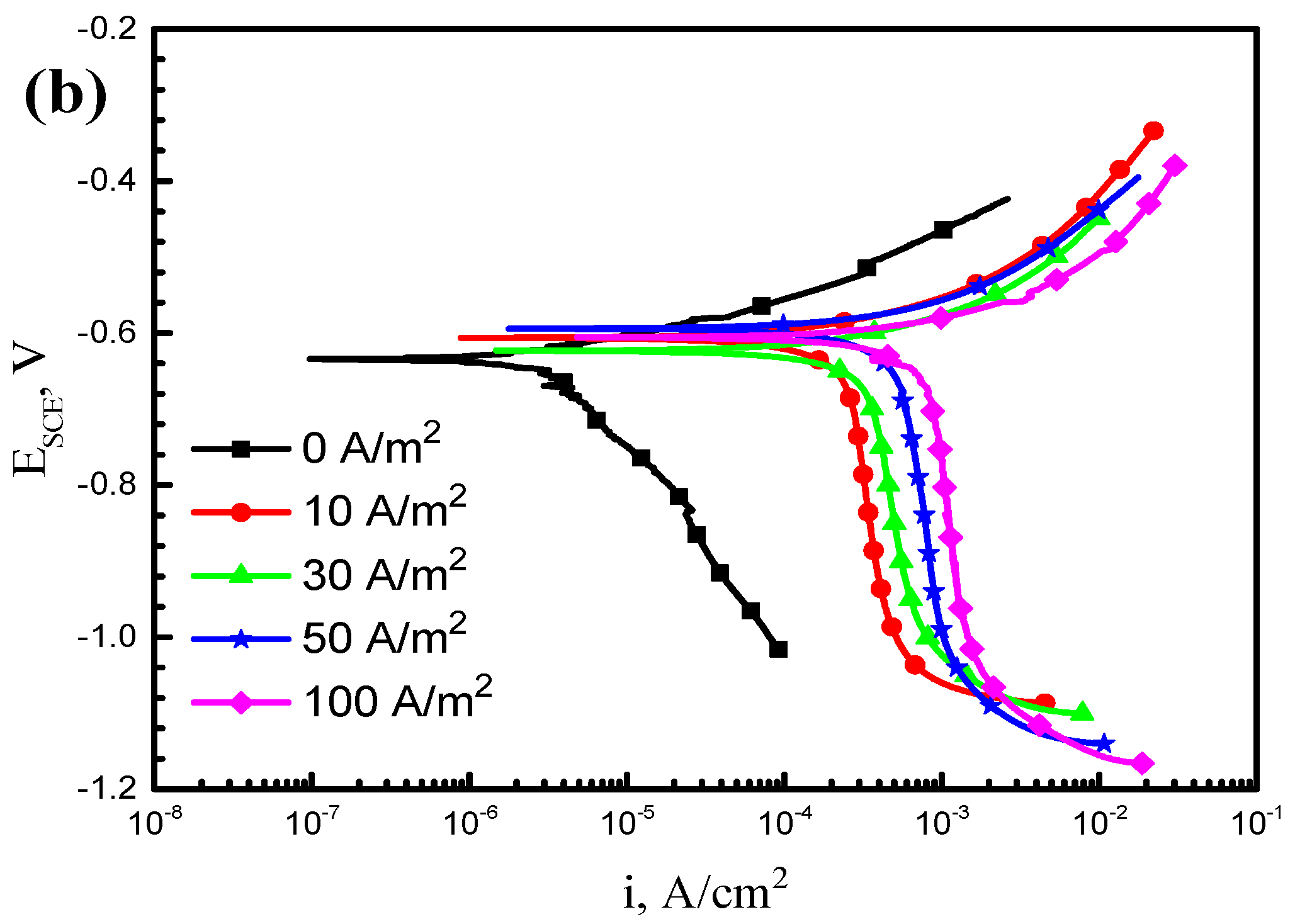
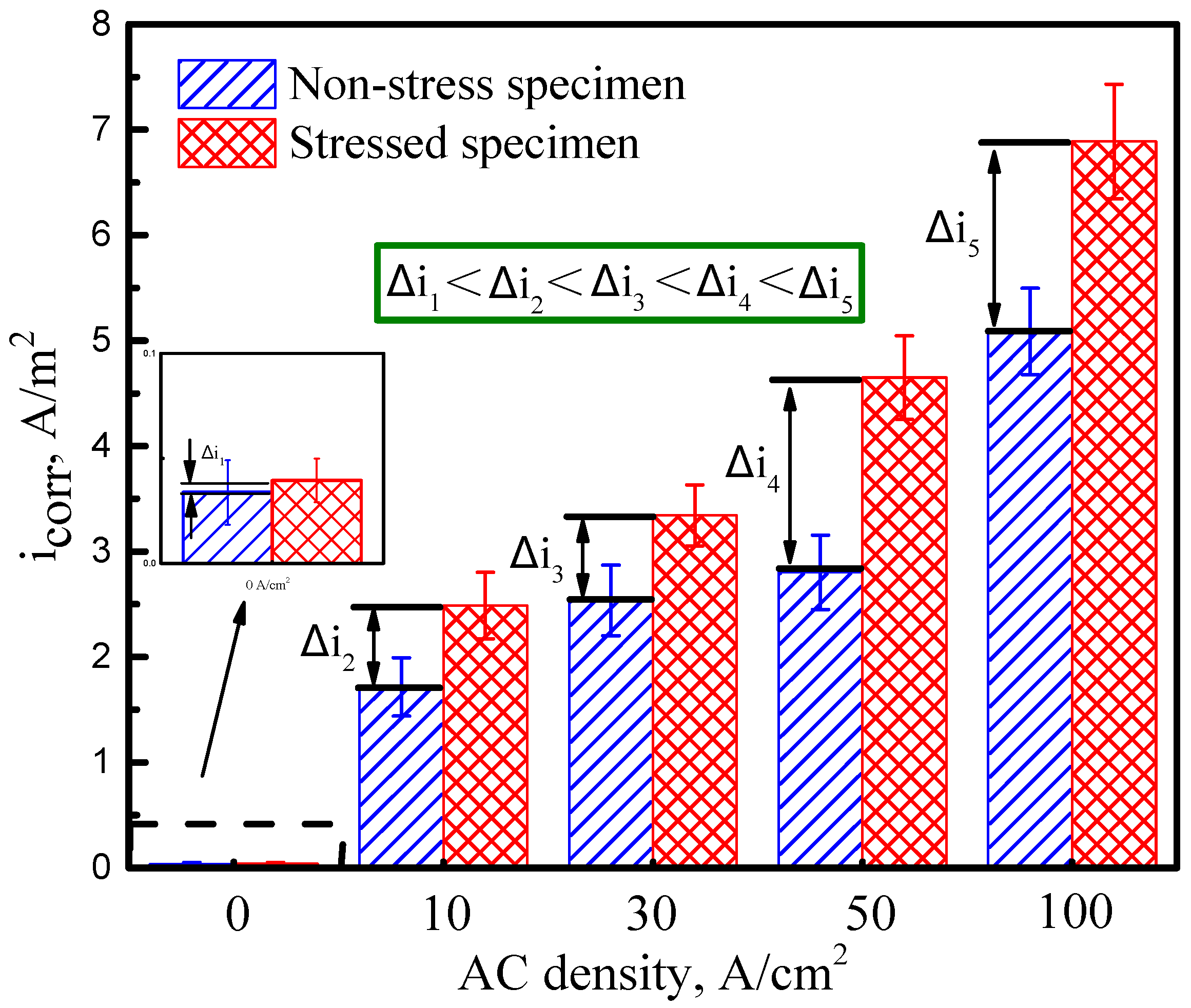
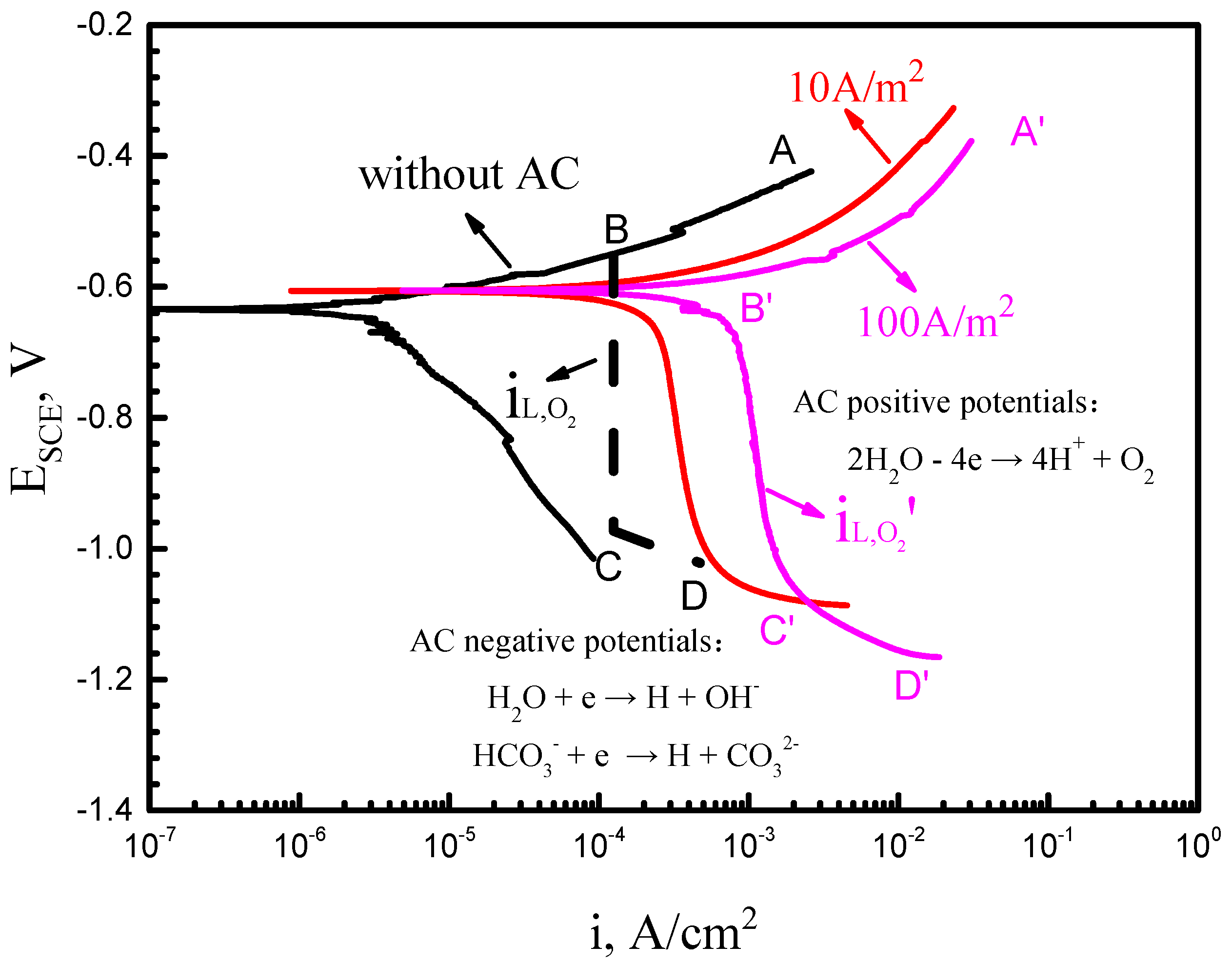
3.1. Potentiodynamic Polarization Tests
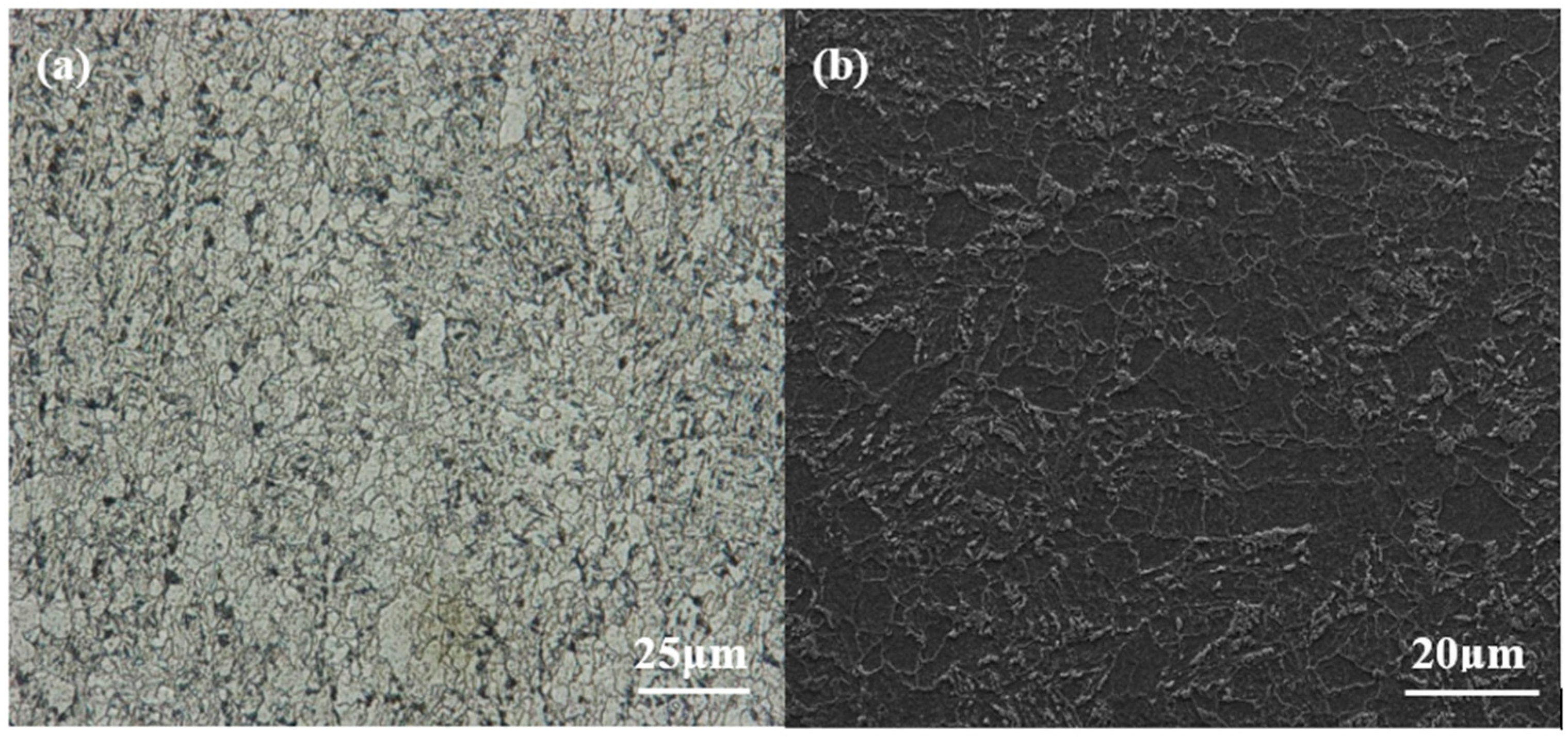
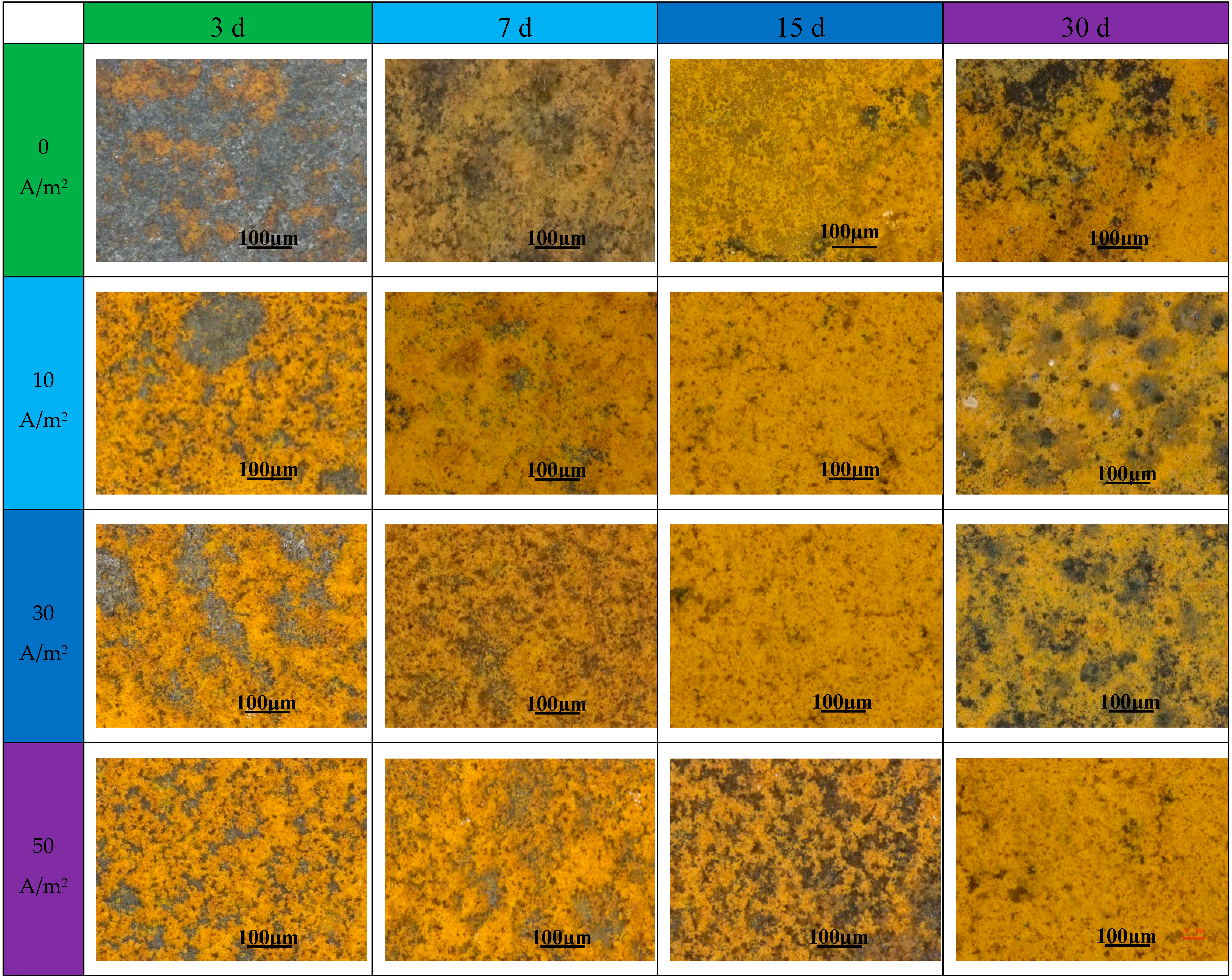
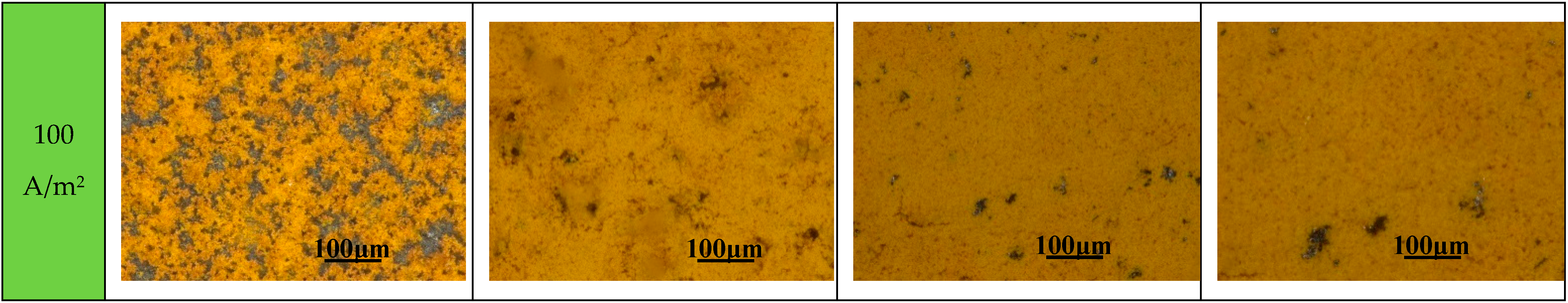
3.2. Immersion Tests
3.3. SCC Behavior
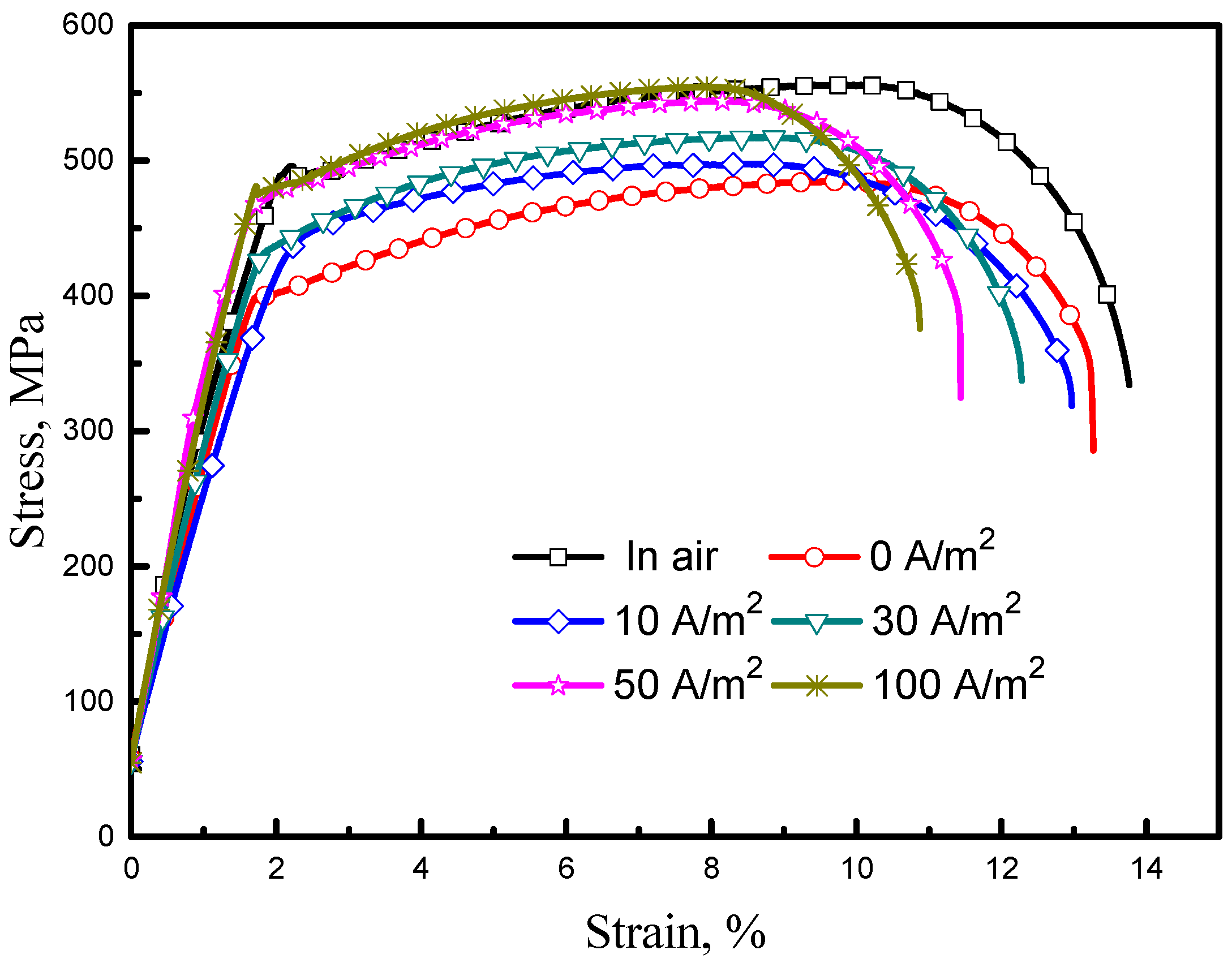
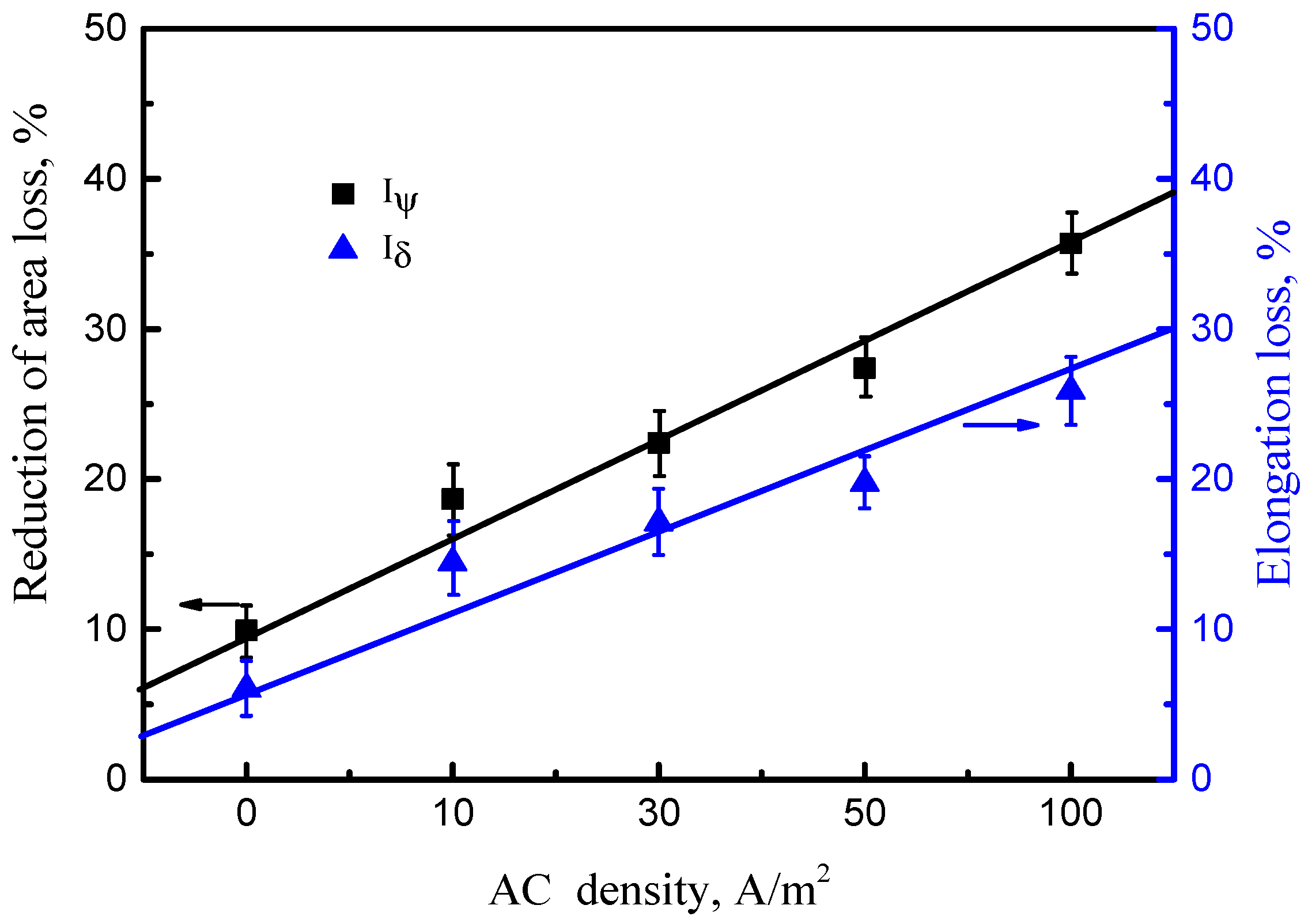
3.3.1. SSRT Curves and SCC Susceptibility
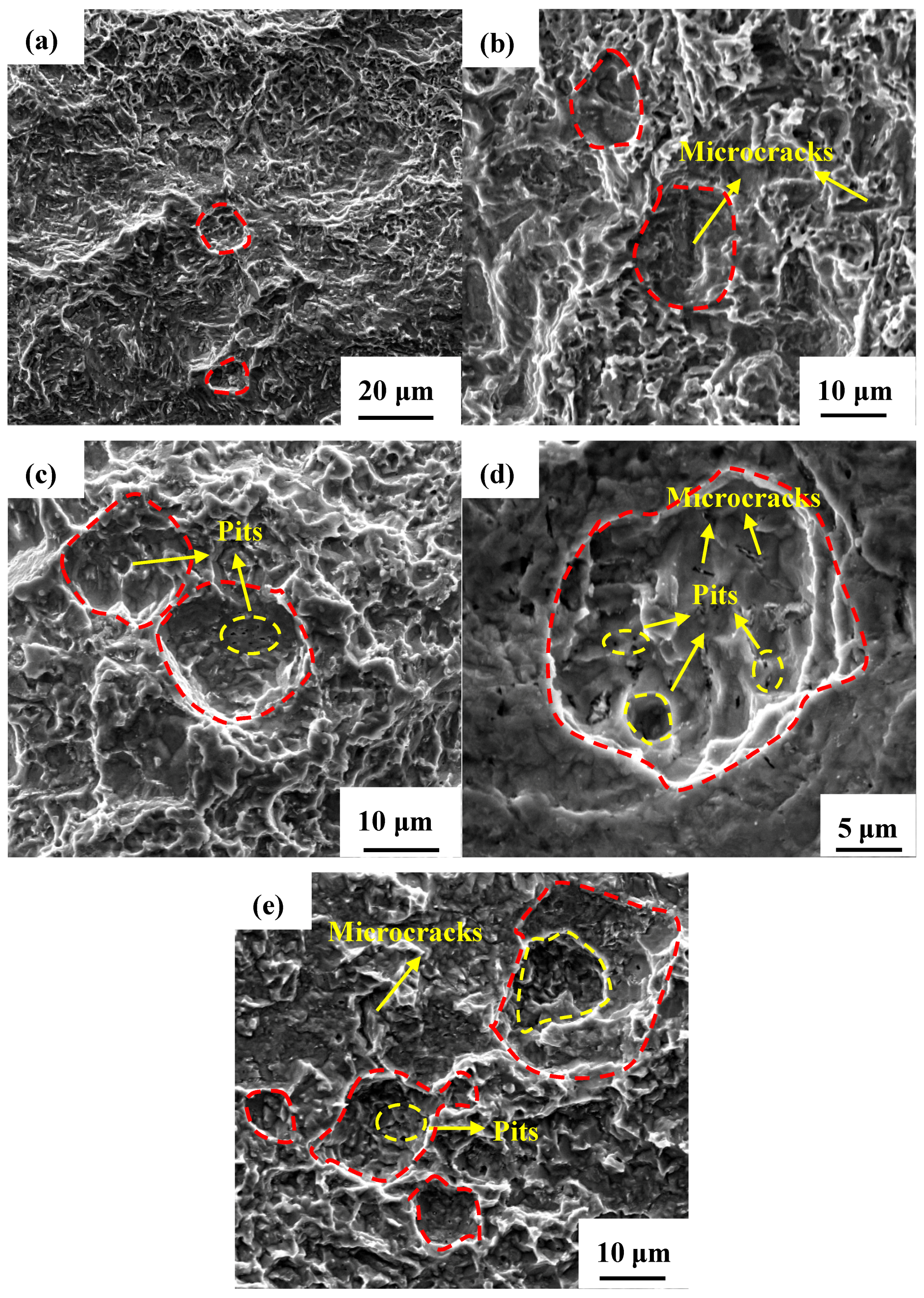
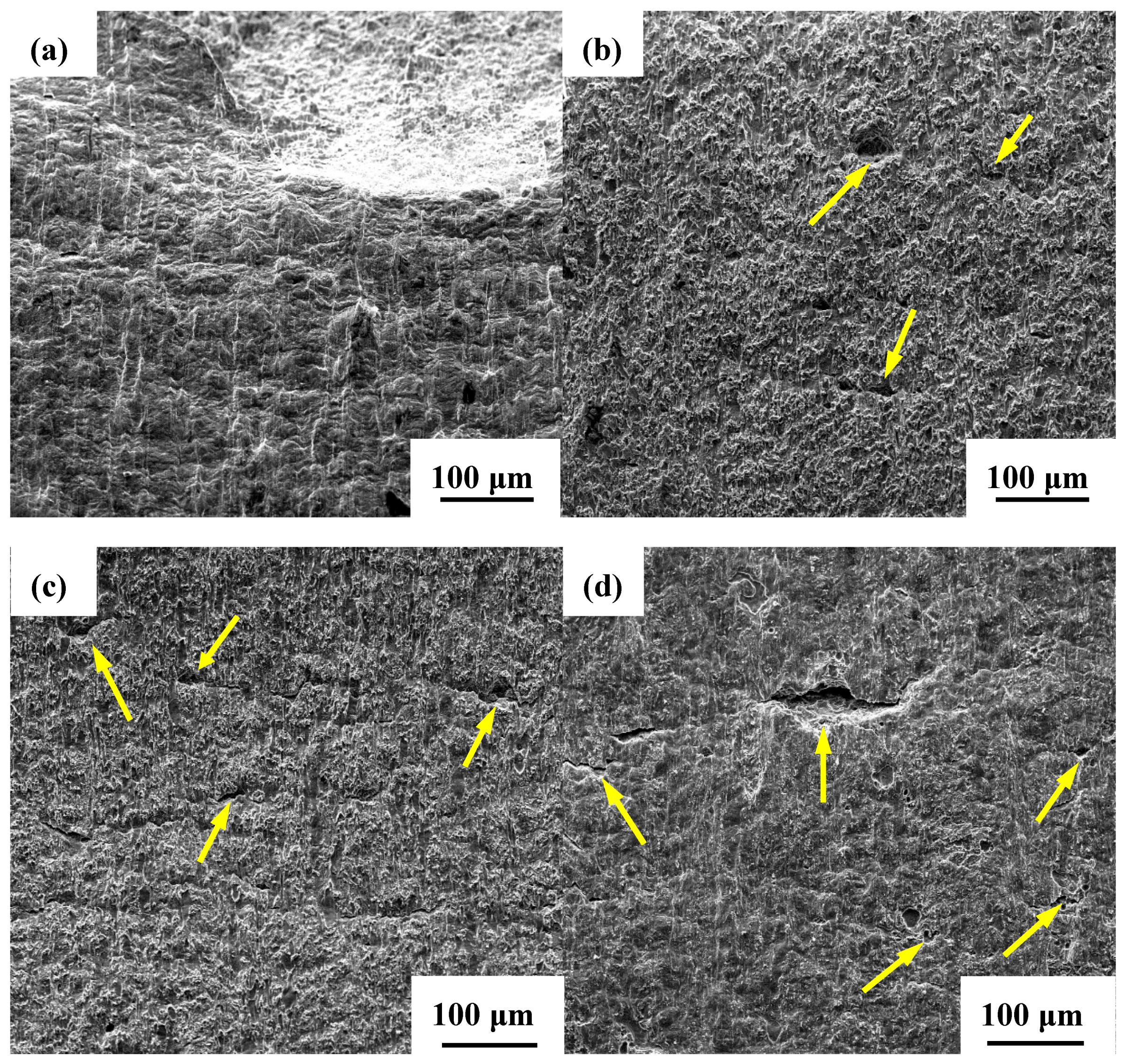
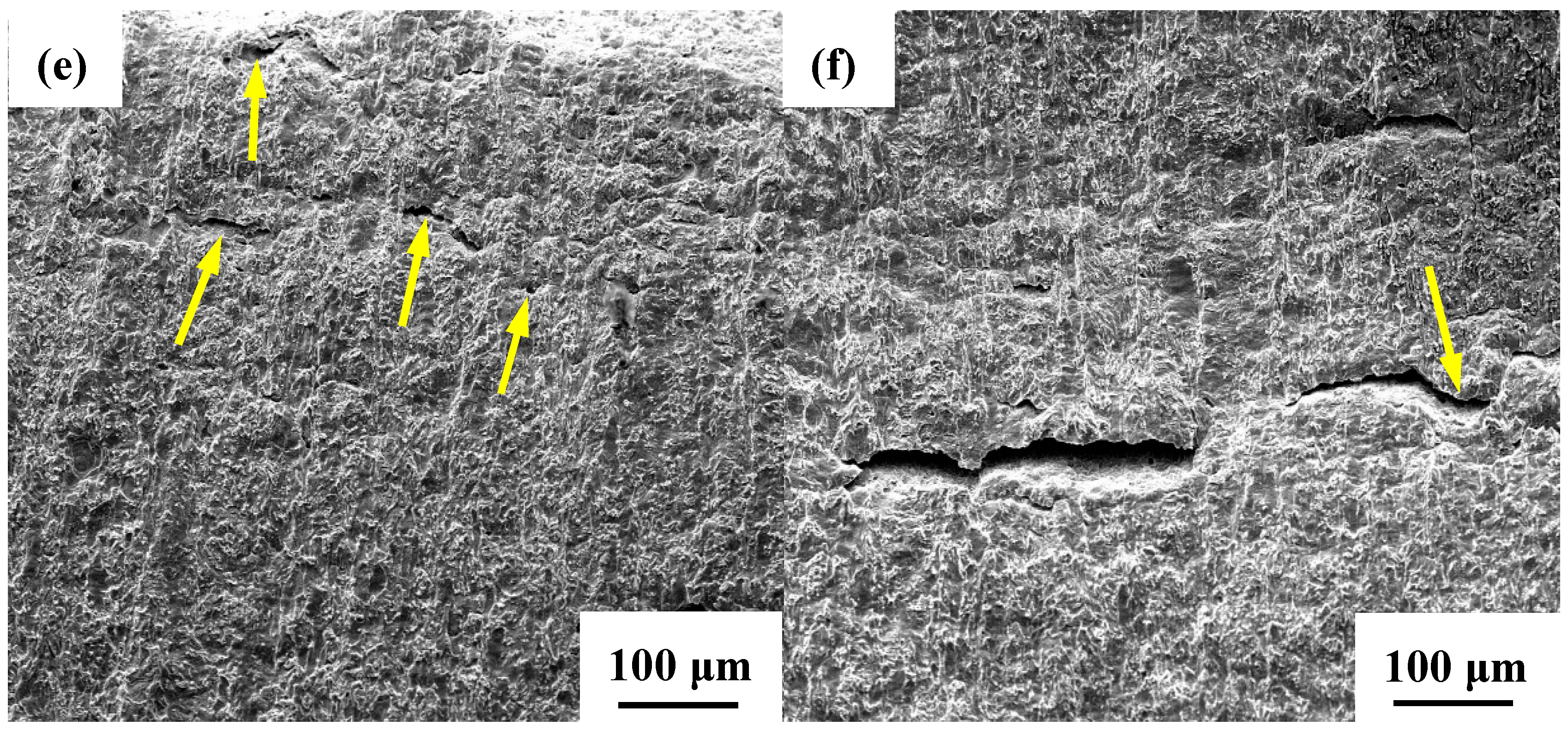
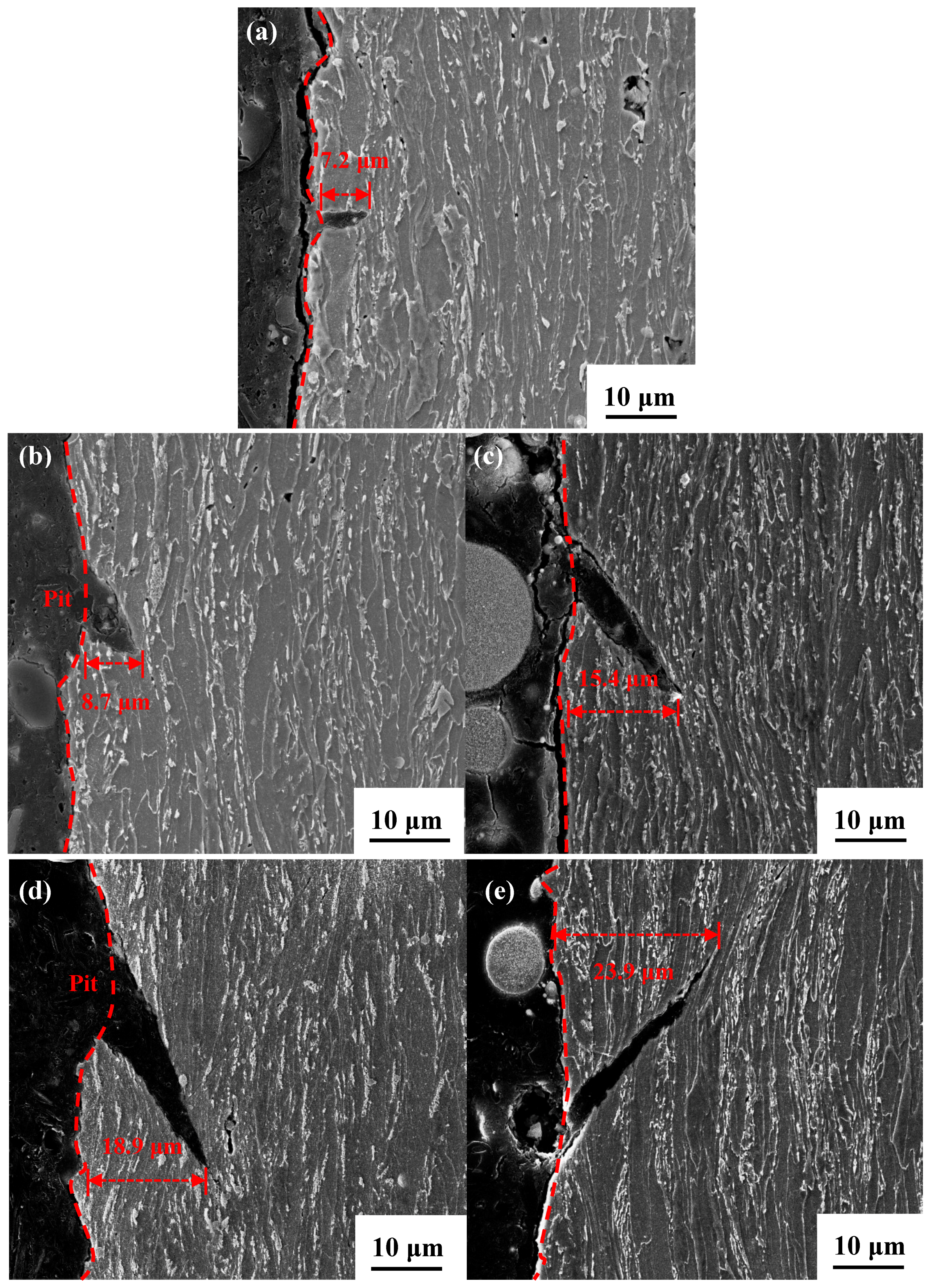
3.3.2. Profile of Cracks and Their Propagation Modes
4. Discussion
4.1. Effect of AC on Electrochemical Reactions of X70 Steel in Simulated Seawater
4.2. Effect of AC on the Stress Corrosion Behavior of X70 Steel in the Simulated Seawater
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hasan, S.; Sweet, L.; Hults, J.; Valbuena, G.; Singh, B. Corrosion risk-based subsea pipeline design. Int. J. Press. Vessels Pip. 2018, 159, 1–14. [Google Scholar] [CrossRef]
- Yang, Y.S.; Khan, F.; Thodi, P.; Abbassi, R. Corrosion induced failure analysis of subsea pipelines. Reliab. Eng. Syst. Saf. 2017, 159, 214–222. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Song, M.D. Failure analysis of a natural gas pipeline. Eng. Fail. Anal. 2016, 63, 61–71. [Google Scholar] [CrossRef]
- Lei, X.W.; Feng, Y.R.; Fu, A.Q.; Zhang, J.X.; Bai, Z.Q.; Yin, C.X.; Lu, C.H. Investigation of stress corrosion cracking behavior of super 13Cr tubing by full-scale tubular goods corrosion test system. Eng. Fail. Anal. 2015, 50, 62–70. [Google Scholar] [CrossRef]
- Qiao, Q.; Cheng, G.X.; Wu, W.; Li, Y.; Huang, H.; Wei, Z.F. Failure analysis of corrosion at an inhomogeneous welded joint in a natural gas gathering pipeline considering the combined action of multiple factors. Eng. Fail. Anal. 2016, 64, 126–143. [Google Scholar] [CrossRef]
- Mansoori, H.; Mirzaee, R.; Esmaeilzadeh, F.; Vojood, A.; Dowrani, A.S. Pitting corrosion failure analysis of a wet gas pipeline. Eng. Fail. Anal. 2017, 82, 16–25. [Google Scholar] [CrossRef]
- Mustapha, A.; Charles, E.A.; Hardie, D. Evaluation of environment-assisted cracking susceptibility of a grade X100 pipeline steel. Corros. Sci. 2012, 54, 5–9. [Google Scholar] [CrossRef]
- Oskuie, A.A.; Shahrabi, T.; Shahriari, A.; Saebnoori, E. Electrochemical impedance spectroscopy analysis of X70 pipeline steel stress corrosion cracking in high pH carbonate solution. Corros. Sci. 2012, 61, 111–122. [Google Scholar] [CrossRef]
- Arafin, M.A.; Szpunar, J.A. Effect of bainitic microstructure on the susceptibility of pipeline steels to hydrogen induced cracking. Mater. Sci. Eng. A 2011, 528, 4927–4940. [Google Scholar] [CrossRef]
- Lu, B.T.; Luo, J.L.; Norton, P.R. Environmentally assisted cracking mechanism of pipeline steel in near-neutral pH groundwater. Corros. Sci. 2010, 52, 1787–1795. [Google Scholar] [CrossRef]
- Kang, Y.W.; Chen, W.X.; Kania, R.; Boven, G.V.; Worthingham, R. Simulation of crack growth during hydrostatic testing of pipeline steel in near-neutral pH environment. Corros. Sci. 2011, 53, 968–975. [Google Scholar] [CrossRef]
- Javidi, M.; Bahalaou Horeh, S. Investigating the mechanism of stress corrosion cracking in near-neutral and high pH environments for API 5L X52 steel. Corros. Sci. 2014, 80, 213–220. [Google Scholar] [CrossRef]
- Wakelin, R.G.; Gummow, R.A.; Segall, S.M. AC corrosion-case histories, test procedures, mitigation. In Proceedings of the CORROSION/1998, San Diego, CA, USA, 22–27 March 1998; Paper No. 565. NACE: Houston, TX, USA, 1998. [Google Scholar]
- Gummow, R.A.; Wakelin, R.G.; Seggal, S.M. AC corrosion-a new challenge to pipeline integrity. In Proceedings of the CORROSION/1998, San Diego, CA, USA, 22–27 March 1998; Paper No. 566. NACE: Houston, TX, USA, 1998. [Google Scholar]
- Roger, F. Testing and mitigation of AC corrosion on 8 line: A field study. In Proceedings of the CORROSION/2004, New Orleans, LA, USA, 28 March–1 April 2004; Paper No. 210. NACE: Houston, TX, USA, 2004. [Google Scholar]
- Goidanich, S.; Lazzari, L.; Ormellese, M.; Pedeferri, M. Influence of AC on corrosion kinetics for carbon steel, zinc and copper. In Proceedings of the CORROSION/2005, Houston, TX, USA, 3–7 April 2005; Paper No. 189. NACE: Houston, TX, USA, 2005. [Google Scholar]
- Wan, H.X.; Song, D.D.; Liu, Z.Y.; Du, C.W.; Zeng, Z.P.; Wang, Z.G.; Ding, D.; Li, X.G. Effect of negative half-wave alternating current on stress corrosion cracking behavior and mechanism of X80 pipeline steel in near-neutral solution. Constr. Build. Mater. 2017, 154, 580–589. [Google Scholar] [CrossRef]
- Kuang, D.; Cheng, Y.F. Understand the AC induced pitting corrosion on pipelines in both high pH and neutral pH carbonate/bicarbonate solutions. Corros. Sci. 2014, 85, 304–310. [Google Scholar] [CrossRef]
- Guo, Y.B.; Meng, T.; Wang, D.G.; Tan, H.; He, R.Y. Experimental research on the corrosion of X series pipeline steels under alternating current interference. Eng. Fail. Anal. 2017, 78, 87–98. [Google Scholar] [CrossRef]
- Guo, Y.B.; Tan, H.; Meng, T.; Wang, D.G.; Liu, S.H. Effects of alternating current interference on the cathodic protection for API 5L X60 pipeline steel. J. Nat. Gas Sci. Eng. 2016, 36, 414–423. [Google Scholar] [CrossRef]
- Fu, A.Q.; Cheng, Y.F. Effects of alternating current on corrosion of a coated pipeline steel in a chloride-containing carbonate/bicarbonate solution. Corros. Sci. 2010, 52, 612–619. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Li, X.G.; Cheng, Y.F. Understand the occurrence of pitting corrosion of pipeline carbon steel under cathodic polarization. Electrochim. Acta 2012, 60, 259–263. [Google Scholar] [CrossRef]
- Mai, W.; Soghrati, S. A phase field model for simulating the stress corrosion cracking initiated from pits. Corros. Sci. 2017, 125, 87–98. [Google Scholar] [CrossRef]
- Meo, D.D.; Russo, L.; Oterkus, E. Modeling of the onset, propagation, and interaction of multiple cracks generated from corrosion pits by using peridynamics. J. Eng. Mater.-Technol. 2017, 139, 041001. [Google Scholar] [CrossRef]
- Zhu, M.; Du, C.W.; Li, X.G.; Liu, Z.Y.; Li, H.; Zhang, D.W. Effect of AC on stress corrosion cracking behavior and mechanism of X80 pipeline steel in carbonate/bicarbonate solution. Corros. Sci. 2014, 87, 224–232. [Google Scholar] [CrossRef]
- Xu, L.Y.; Su, X.; Cheng, Y.F. Effect of alternating current on cathodic protection on pipelines. Corros. Sci. 2013, 66, 263–268. [Google Scholar] [CrossRef]
- Wang, L.W.; Wang, X.H.; Cui, Z.Y.; Liu, Z.Y.; Du, C.W.; Li, X.G. Effect of alternating voltage on corrosion of X80 and X100 steels in a chloride containing solution-Investigated by AC voltammetry technique. Corros. Sci. 2014, 86, 213–222. [Google Scholar] [CrossRef]
- Kim, D.K.; Muralidharan, S.; Ha, T.H.; Bae, J.H.; Ha, Y.C.; Lee, H.G.; Scantlebury, J.D. Electrochemical studies on the alternating current corrosion of mild steel under cathodic protection condition in marine environments. Electrochim. Acta 2006, 51, 5259–5267. [Google Scholar] [CrossRef]
- Muralidharan, S.; Kim, D.K.; Ha, T.H.; Bae, J.H.; Ha, Y.C.; Lee, H.G.; Scantlebury, J.D. Influence of alternating, direct and superimposed alternating and direct current on the corrosion of mild steel in marine environments. Desalination 2007, 216, 103–115. [Google Scholar] [CrossRef]
- Sofronis, P.; Liang, Y.; Aravas, N. Hydrogen induced shear localization of the plastic flow in metals and alloys. Eur. J. Mech. A/Solids 2001, 20, 857–872. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, W.; Pan, Y.; Liu, Z.Y.; Zhou, X.C.; Li, X.G. Electrochemical mechanism of stress corrosion cracking of API X70 pipeline steel under different AC frequencies. Constr. Build. Mater. 2018, 171, 622–633. [Google Scholar] [CrossRef]
- Zhu, M.; Du, C.W.; Li, X.G.; Liu, Z.Y.; Wang, S.R.; Li, J.K.; Zhang, D.W. Effect of AC density on stress corrosion cracking behavior of X80 pipeline steel in high pH carbonate/bicarbonate solution. Electrochim. Acta 2014, 117, 351–359. [Google Scholar] [CrossRef]
- Wu, W.; Liu, Z.Y.; Hu, S.S.; Li, X.G.; Du, C.W. Effect of pH and hydrogen on the stress corrosion cracking behavior of duplex stainless steel in marine atmosphere environment. Ocean Eng. 2017, 146, 311–323. [Google Scholar] [CrossRef]
- Lavignea, O.; Gamboa, E.; Luzin, V.; Law, M. Analysis of intergranular stress corrosion crack paths in gas pipeline steels; straight or inclined? Eng. Fail. Anal. 2018, 85, 26–35. [Google Scholar] [CrossRef]
- Li, X.G. Corrosion and Protection of Materials; Central South University: Changsha, China, 2009; ISBN 978-7-81105-697-6. (In Chinese) [Google Scholar]
- Xu, L.Y.; Su, X.; Yin, Z.X.; Tang, Y.H.; Cheng, Y.F. Development of a real-time AC/DC data acquisition technique for studies of AC corrosion of pipelines. Corros. Sci. 2012, 61, 215–223. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Li, X.G.; Du, C.W.; Cheng, Y.F. Local additional potential model for effect of strain rate on SCC of pipeline steel in an acidic soil solution. Corros. Sci. 2009, 51, 2863–2871. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Li, X.G.; Cheng, Y.F. Mechanistic aspect of near-neutral pH stress corrosion cracking of pipelines under cathodic polarization. Corros. Sci. 2012, 55, 54–60. [Google Scholar] [CrossRef]
- Branko, N.P. Hydrogen Permeation and Hydrogen-Induced Cracking; Corrosion Engineering: New York, NY, USA, 2015; pp. 327–364. [Google Scholar]
- Yan, Y.J.; Yan, Y.; He, Y. Hydrogen-induced cracking mechanism of precipitation strengthened austenitic stainless steel weldment. Int. J. Hydrog. Energy 2015, 40, 2404–2414. [Google Scholar] [CrossRef]
- Akiyama, E.J.; Li, S.J. Electrochemical hydrogen permeation tests under galvanostatic hydrogen charging conditions conventionally used for hydrogen embrittlement study. Corros. Rev. 2016, 34, 103–112. [Google Scholar] [CrossRef]
- Mohtadi-Bonab, M.A.; Eskandari, M.; Rahman, K.M.M. An extensive study of hydrogen-induced cracking susceptibility in an API X60 sour service pipeline steel. Int. J. Hydrog. Energy 2016, 41, 4185–4197. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.; Pan, Y.; Liu, Z.; Du, C.; Li, X. Electrochemical and Stress Corrosion Mechanism of Submarine Pipeline in Simulated Seawater in Presence of Different Alternating Current Densities. Materials 2018, 11, 1074. https://doi.org/10.3390/ma11071074
Wu W, Pan Y, Liu Z, Du C, Li X. Electrochemical and Stress Corrosion Mechanism of Submarine Pipeline in Simulated Seawater in Presence of Different Alternating Current Densities. Materials. 2018; 11(7):1074. https://doi.org/10.3390/ma11071074
Chicago/Turabian StyleWu, Wei, Yue Pan, Zhiyong Liu, Cuiwei Du, and Xiaogang Li. 2018. "Electrochemical and Stress Corrosion Mechanism of Submarine Pipeline in Simulated Seawater in Presence of Different Alternating Current Densities" Materials 11, no. 7: 1074. https://doi.org/10.3390/ma11071074
APA StyleWu, W., Pan, Y., Liu, Z., Du, C., & Li, X. (2018). Electrochemical and Stress Corrosion Mechanism of Submarine Pipeline in Simulated Seawater in Presence of Different Alternating Current Densities. Materials, 11(7), 1074. https://doi.org/10.3390/ma11071074






