Analysis of Indium Oxidation State on the Electronic Structure and Optical Properties of TiO2
Abstract
1. Introduction
2. Method of Calculations
3. Results and Discussion
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Papadimitriou, V.C.; Stefanopoulos, V.G.; Romanias, M.N.; Papagiannakopoulos, P.; Sambani, K.; Tudose, V.; Kiriakidis, G. Determination of photo-catalytic activity of un-doped and Mn-doped TiO2 anatase powders on acetaldehyde under UV and visible light. Thin Solid Films 2011, 520, 1195–1201. [Google Scholar] [CrossRef]
- Murphy, A. Does carbon doping of TiO2 allow water splitting in visible light? Comments on “Nanotube enhanced photoresponse of carbon modified (CM)-n-TiO2 for efficient water splitting”. Sol. Energy Mater. Sol. Cells 2008, 92, 363–367. [Google Scholar] [CrossRef]
- Atsushiro Tanaka, K.H.; Kominami, H. A very simple method for the preparation of Au/TiO2 plasmonic photocatalysts working under irradiations of visible light in the range of 600–700 nm. Chem. Commun. 2017, 53, 4759–4762. [Google Scholar] [CrossRef] [PubMed]
- Timur, S.H.; Atabaev, M.A.H.; Lee, D.; Kim, H.K.; Hwang, Y.H. Pt-coated TiO2 nanorods for photoelectrochemical water splitting applications. Results Phys. 2016, 6, 373–376. [Google Scholar]
- Na Phattalung, S.; Limpijumnong, S.; Yu, J. Passivated co-doping approach to bandgap narrowing of titanium dioxide with enhanced photocatalytic activity. Appl. Catal. B Environ. 2017, 200, 1–9. [Google Scholar] [CrossRef]
- Matiullah, K.; Zeng, Y.; Fawad, U.; Wazir, M.; Abdul, N.; Muhammad Iqbal, Z.; Asad, U. Enhancing the photoactivity of TiO2 by codoping with silver and molybdenum: The effect of dopant concentration on the photoelectrochemical properties. Mater. Res. Express 2017, 4, 045023. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Li, C.; Lu, S.; Gong, Y.Y.; Niu, L.Y.; Liu, X.J. Modulating TiO2 photocatalyst by Al doping: Density functional theory approach. Chem. Phys. Lett. 2016, 654, 13–17. [Google Scholar] [CrossRef]
- Lazzeri, M.; Vittadini, A.; Selloni, A. Structure and energetics of stoichiometric TiO2 anatase surfaces. Phys. Rev. B 2001, 63. [Google Scholar] [CrossRef]
- Long, M.; Cai, W.; Wang, Z.; Liu, G. Correlation of electronic structures and crystal structures with photocatalytic properties of undoped, N-doped and I-doped TiO2. Chem. Phys. Lett. 2006, 420, 71–76. [Google Scholar] [CrossRef]
- Khan, M.; Xu, J.; Chen, N.; Cao, W. Electronic and optical properties of pure and Mo doped anatase TiO2 using GGA and GGA+U calculations. Phys. B Condens. Matter 2012, 407, 3610–3616. [Google Scholar] [CrossRef]
- Khan, M.; Cao, W. Preparation of Y-doped TiO2 by hydrothermal method and investigation of its visible light photocatalytic activity by the degradation of methylene blue. J. Mol. Catal. A Chem. 2013, 376, 71–77. [Google Scholar] [CrossRef]
- Cui, Y.; Du, H.; Wen, L. Origin of visible-light-induced photocatalytic properties of S-doped anatase TiO2 by first-principles investigation. Solid State Commun. 2009, 149, 634–637. [Google Scholar] [CrossRef]
- Venditti, F.; Cuomo, F.; Ceglie, A.; Avino, P.; Russo, M.V.; Lopez, F. Visible Light Caffeic Acid Degradation by Carbon-Doped Titanium Dioxide. Langmuir 2015, 31, 3627–3634. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Ai, Z.; Jia, F.; Zhang, L.; Fan, X.; Zou, Z. Low temperature preparation and visible light photocatalytic activity of mesoporous carbon-doped crystalline TiO2. Appl. Catal. B Environ. 2007, 69, 138–144. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 for Environmental Photocatalytic Applications: A Review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- Khan, M.; Gul, S.R.; Li, J.; Cao, W. Variations in the structural, electronic and optical properties of N-doped TiO2 with increasing N doping concentration. Mod. Phys. Lett. B 2015, 29, 1550022. [Google Scholar] [CrossRef]
- Štengl, V.; Bakardjieva, S. Molybdenum-Doped Anatase and Its Extraordinary Photocatalytic Activity in the Degradation of Orange II in the UV and vis Regions. J. Phys. Chem. C 2010, 114, 19308–19317. [Google Scholar] [CrossRef]
- Etacheri, V.; Seery, M.K.; Hinder, S.J.; Pillai, S.C. Highly Visible Light Active TiO2−xNxHeterojunction Photocatalysts†. Chem. Mater. 2010, 22, 3843–3853. [Google Scholar] [CrossRef]
- Di Valentin, C.; Finazzi, E.; Pacchioni, G.; Selloni, A.; Livraghi, S.; Paganini, M.C.; Giamello, E. N-doped TiO2: Theory and experiment. Chem. Phys. 2007, 339, 44–56. [Google Scholar] [CrossRef]
- Shchukin, D.; Poznyak, S.; Kulak, A.; Pichat, P. TiO2-In2O3 photocatalysts: Preparation, characterisations and activity for 2-chlorophenol degradation in water. J. Photochem. Photobiol. A Chem. 2004, 162, 423–430. [Google Scholar] [CrossRef]
- Rodríguez-González, V.; Moreno-Rodríguez, A.; May, M.; Tzompantzi, F.; Gómez, R. Slurry photodegradation of 2,4-dichlorophenoxyacetic acid: A comparative study of impregnated and sol–gel In2O3–TiO2 mixed oxide catalysts. J. Photochem. Photobiol. A Chem. 2008, 193, 266–270. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Xu, L.; Yu, X.; Guo, Y. Silver and Indium Oxide Codoped TiO2 Nanocomposites with Enhanced Photocatalytic Activity. J. Phys. Chem. C 2008, 112, 11481–11489. [Google Scholar] [CrossRef]
- Wang, E.; Yang, W.; Cao, Y. Unique Surface Chemical Species on Indium Doped TiO2 and Their Effect on the Visible Light Photocatalytic Activity. J. Phys. Chem. C 2009, 113, 20912–20917. [Google Scholar] [CrossRef]
- Jaffe, J.E.; Snyder, J.A.; Lin, Z.; Hess, A.C. LDA and GGA calculations for high-pressure phase transitions in ZnO and MgO. Phys. Rev. B 2000, 62, 1660–1665. [Google Scholar] [CrossRef]
- Pfrommer, B.G.; Côté, M.; Louie, S.G.; Cohen, M.L. Relaxation of Crystals with the Quasi-Newton Method. J. Comput. Phys. 1997, 131, 233–240. [Google Scholar] [CrossRef]
- Jia, L.; Wu, C.; Han, S.; Yao, N.; Li, Y.; Li, Z.; Chi, B.; Pu, J.; Jian, L. Theoretical study on the electronic and optical properties of (N, Fe)-codoped anatase TiO2 photocatalyst. J. Alloys Compd. 2011, 509, 6067–6071. [Google Scholar] [CrossRef]
- Hou, Y.D.; Wang, X.C.; Wu, L.; Chen, X.F.; Ding, Z.X.; Wang, X.X.; Fu, X.Z. N-doped SiO2/TiO2 mesoporous nanoparticles with enhanced photocatalytic activity under visible-light irradiation. Chemosphere 2008, 72, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Jia, Q.; Wang, Y.; Zhang, W.; Xu, J. The Electronic Structure and Optical Properties of Anatase TiO2 with Rare Earth Metal Dopants from First-Principles Calculations. Materials 2018, 11, 179. [Google Scholar] [CrossRef] [PubMed]
- Treacy, J.P.W.; Hussain, H.; Torrelles, X.; Grinter, D.C.; Cabailh, G.; Bikondoa, O.; Nicklin, C.; Selcuk, S.; Selloni, A.; Lindsay, R.; et al. Geometric structure of anatase TiO2 (101). Phys. Rev. B 2017, 95, 075416. [Google Scholar] [CrossRef]
- Khan, M.; Cao, W.; Ullah, M. Ab initiocalculations for the electronic and optical properties of Y-doped anatase TiO2. Phys. Status Solidi (B) 2013, 250, 364–369. [Google Scholar] [CrossRef]
- Hiroshi, O.; Takeo, S. Selective and Active Transport of In3+ through N-Nitroso-N-p-octadecylphenylhydroxylamine Ammonium Salt Impregnated Membrane. Bull. Chem. Soc. Japan 1990, 63, 920–925. [Google Scholar]
- Khan, M.; Yi, Z.; Gul, S.R.; Wang, Y.; Fawad, U. Visible-light-active silver- , vanadium-codoped TiO2 with improved photocatalytic activity. J. Mater. Sci. 2017, 52, 5634–5640. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Yang, H.; Xue, X.; Liu, Z. Doping TiO2 with boron or/and cerium elements: Effects on photocatalytic antimicrobial activity. Vacuum 2016, 131, 58–64. [Google Scholar] [CrossRef]
- Yu, Q.; Jin, L.; Zhou, C. Ab initio study of electronic structures and absorption properties of pure and Fe3+ doped anatase TiO2. Sol. Energy Mater. Sol. Cells 2011, 95, 2322–2326. [Google Scholar] [CrossRef]
- Long, R.; English, N.J. Electronic properties of F/Zr co-doped anatase TiO2 photocatalysts from GGA+U calculations. Chem. Phys. Lett. 2010, 498, 338–344. [Google Scholar] [CrossRef]
- Rubio-Ponce, A.; Conde-Gallardo, A.; Olguín, D. First-principles study of anatase and rutile TiO2 doped with Eu ions: A comparison of GGA and LDA+U calculations. Phys. Rev. B 2008, 78, doi–10. [Google Scholar] [CrossRef]
- Chen, Q.; Tang, C.; Zheng, G. First-principles study of anatase (101) surfaces doped with N. Phys. B Condens. Matter 2009, 404, 1074–1078. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
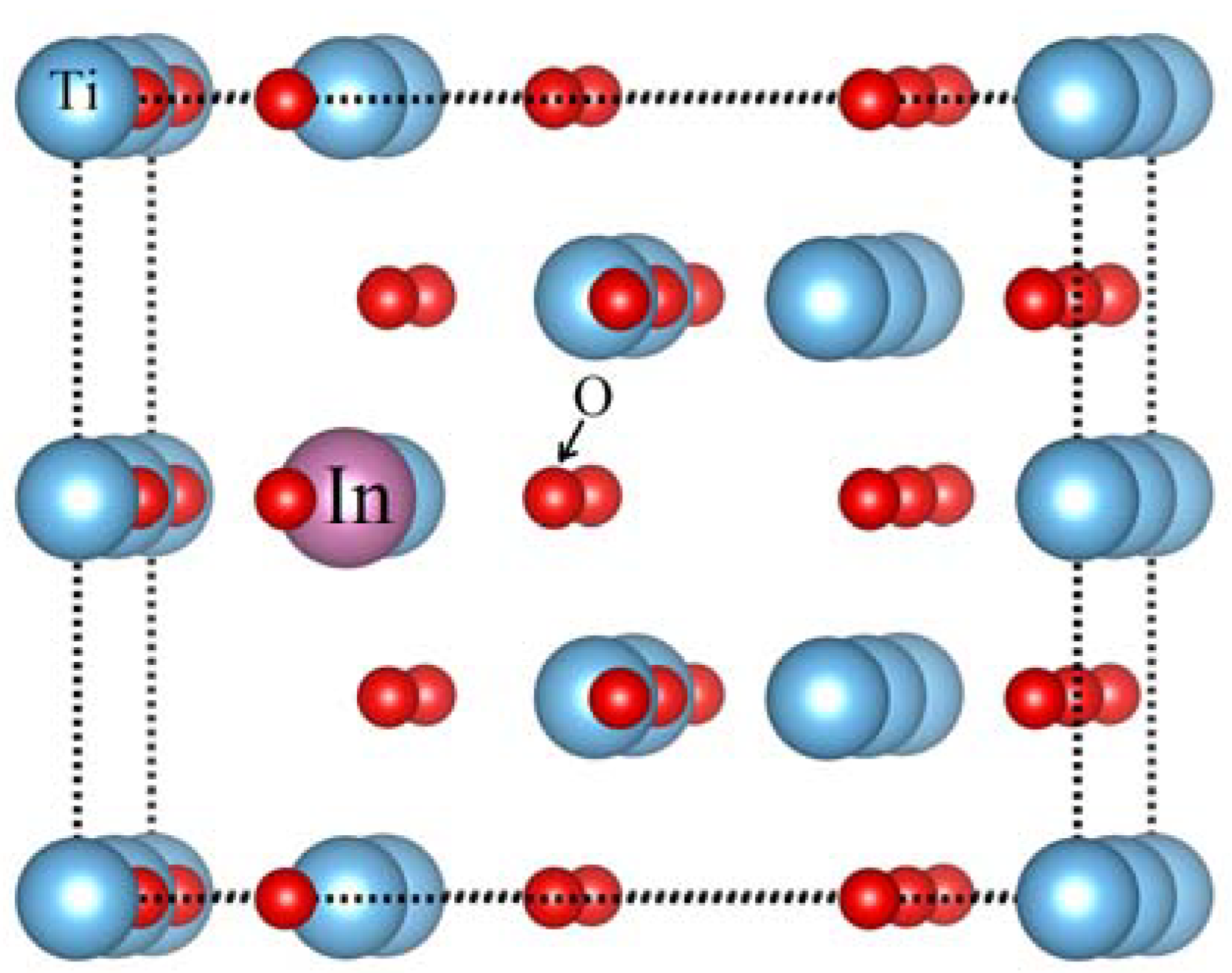
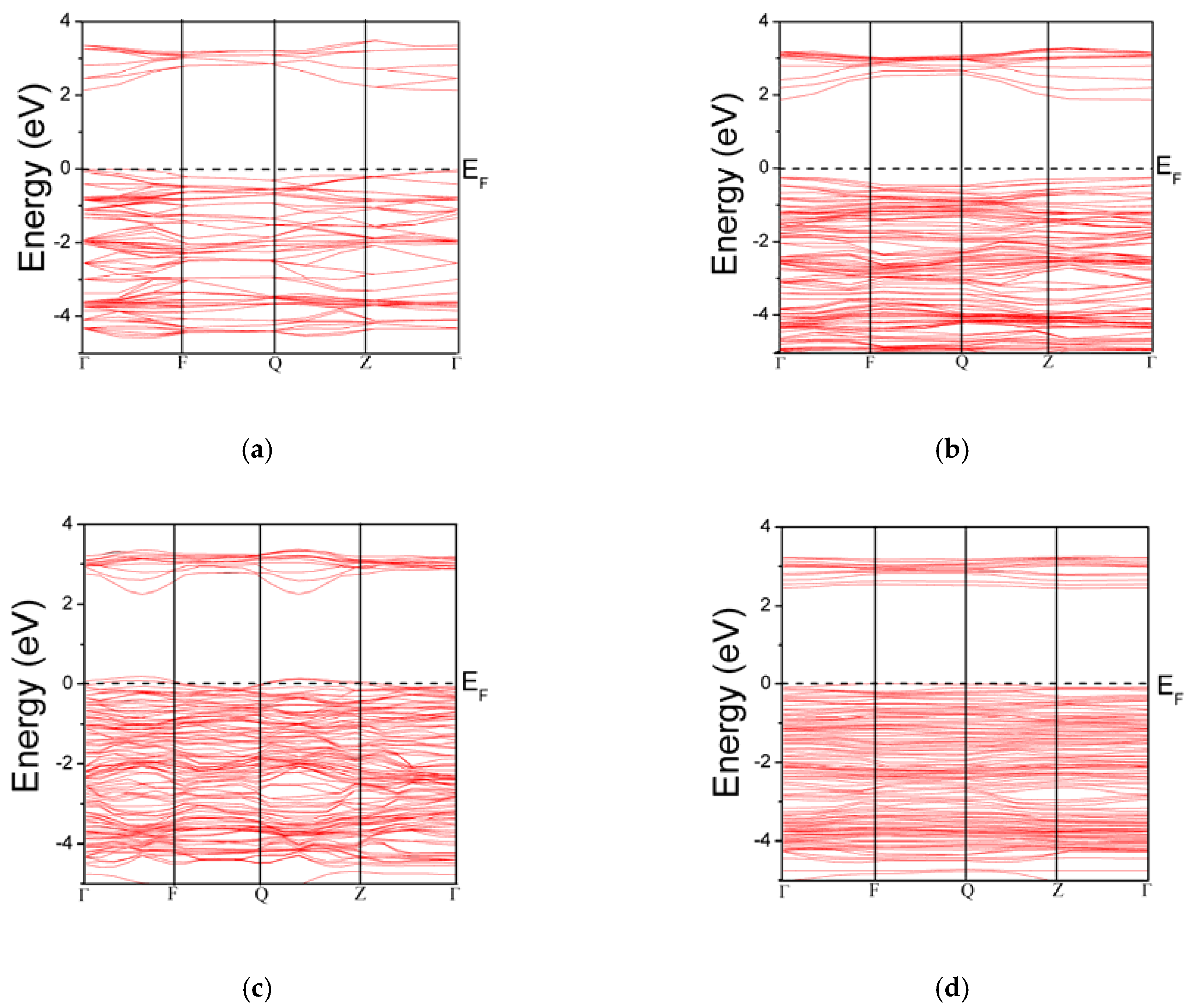
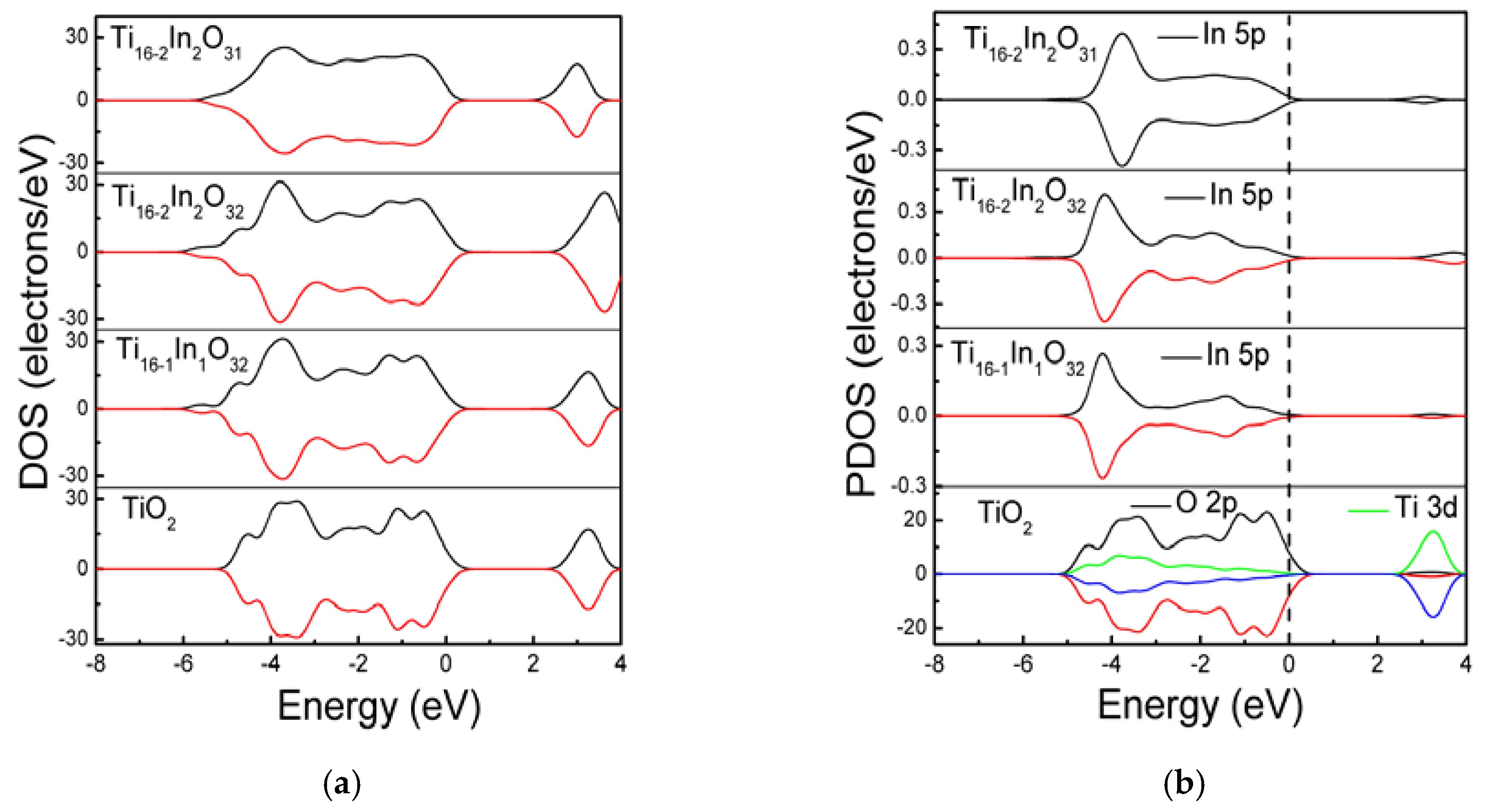
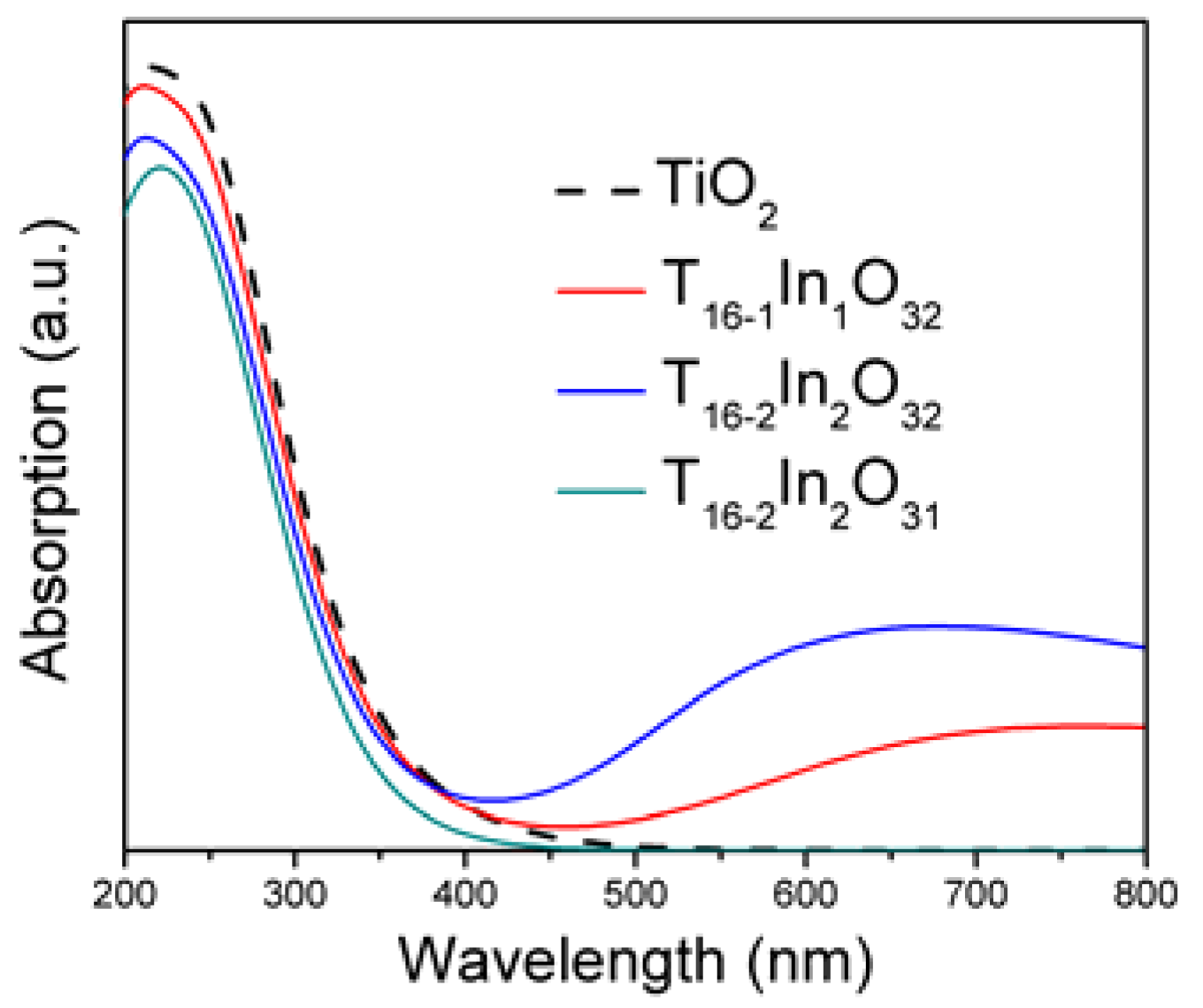
| Bond Length | TiO2 | Ti16-1In1O32 | Ti16-2In2O32 | Ti16-2In2O31 |
|---|---|---|---|---|
| DO-Ti (Å) | 1.9651 | 1.9721 | 1.9846 | 1.9755 |
| DO-O (Å) | 2.7034 | 2.7152 | 2.6886 | 2.7547 |
| DO-In (Å) | --- | 2.1294 | 2.1707 | 2.1723 |
| TiO2 | Ti16-1In1O32 | Ti16-2In2O32 | Ti16-2In2O31 | |
|---|---|---|---|---|
| Fermi level (eV) | Above VBM | 0.25 eV above VBM | 0.1 eV below VBM | 0.1 eV above VBM |
| VBM (eV) | 0 | −0.75 | 0.1 | −0.92 |
| CBM (eV) | 2.13 | 1.85 | 2.75 | 2.40 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.; Lan, Z.; Zeng, Y. Analysis of Indium Oxidation State on the Electronic Structure and Optical Properties of TiO2. Materials 2018, 11, 952. https://doi.org/10.3390/ma11060952
Khan M, Lan Z, Zeng Y. Analysis of Indium Oxidation State on the Electronic Structure and Optical Properties of TiO2. Materials. 2018; 11(6):952. https://doi.org/10.3390/ma11060952
Chicago/Turabian StyleKhan, Matiullah, Zhenghua Lan, and Yi Zeng. 2018. "Analysis of Indium Oxidation State on the Electronic Structure and Optical Properties of TiO2" Materials 11, no. 6: 952. https://doi.org/10.3390/ma11060952
APA StyleKhan, M., Lan, Z., & Zeng, Y. (2018). Analysis of Indium Oxidation State on the Electronic Structure and Optical Properties of TiO2. Materials, 11(6), 952. https://doi.org/10.3390/ma11060952





