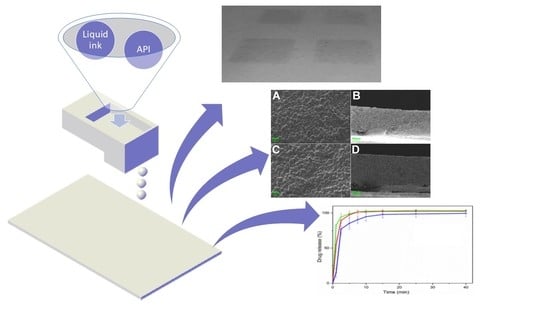
In Vitro Evaluation of 2D-Printed Edible Films for the Buccal Delivery of Diclofenac Sodium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solubility Studies
2.3. Development and Printability of Inks
2.4. Printing of Buccal Films
2.5. Drug Loading and Water Uptake
2.6. Physicochemical Characterization
2.7. In Vitro Studies
2.7.1. Drug Release
2.7.2. Permeation Studies
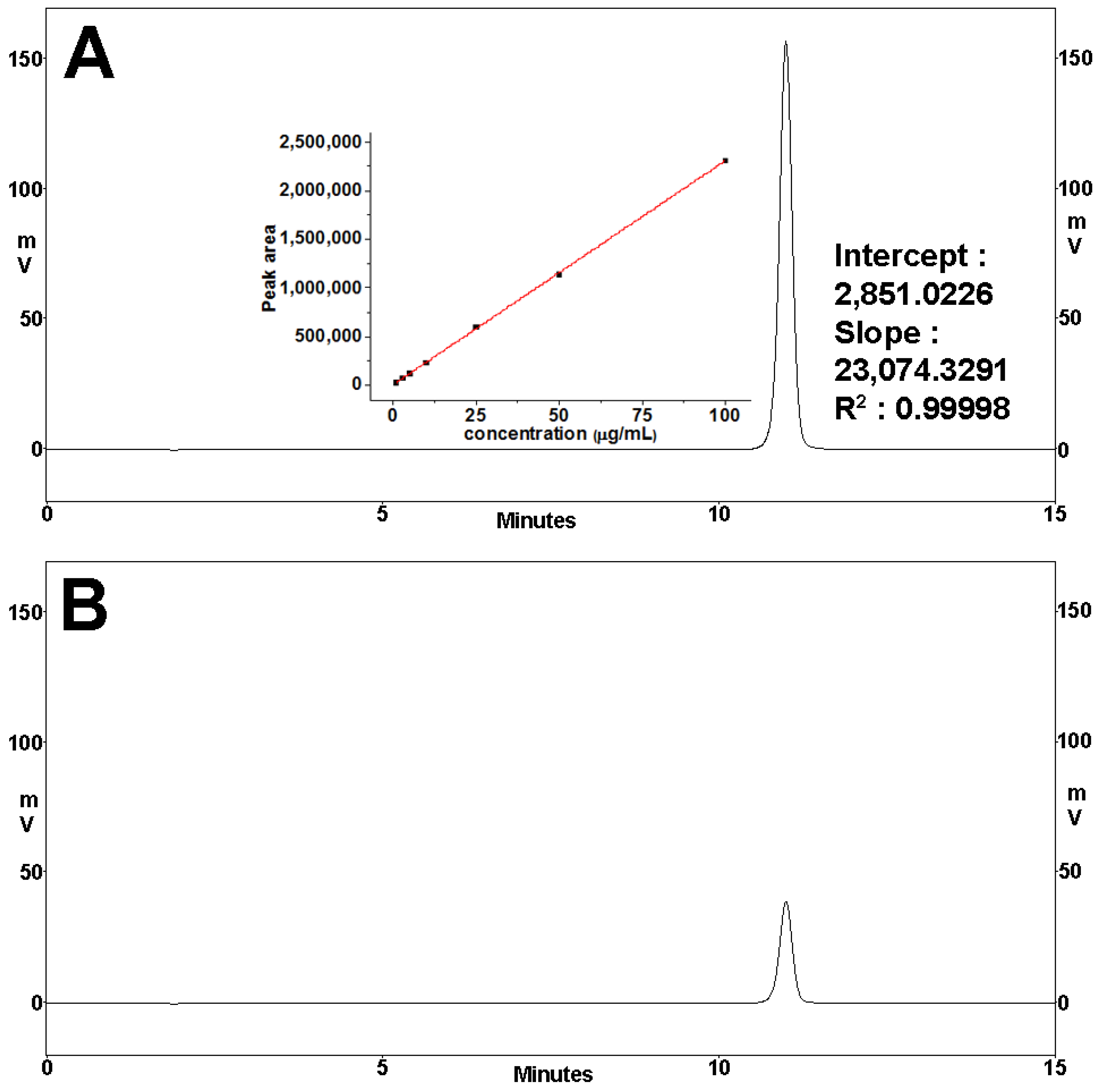
2.8. Quantification of DNa
2.9. Statistical Analysis
3. Results and Discussion
3.1. Solubility Studies
3.2. Viscosity and Surface Tension of the Liquid Ink
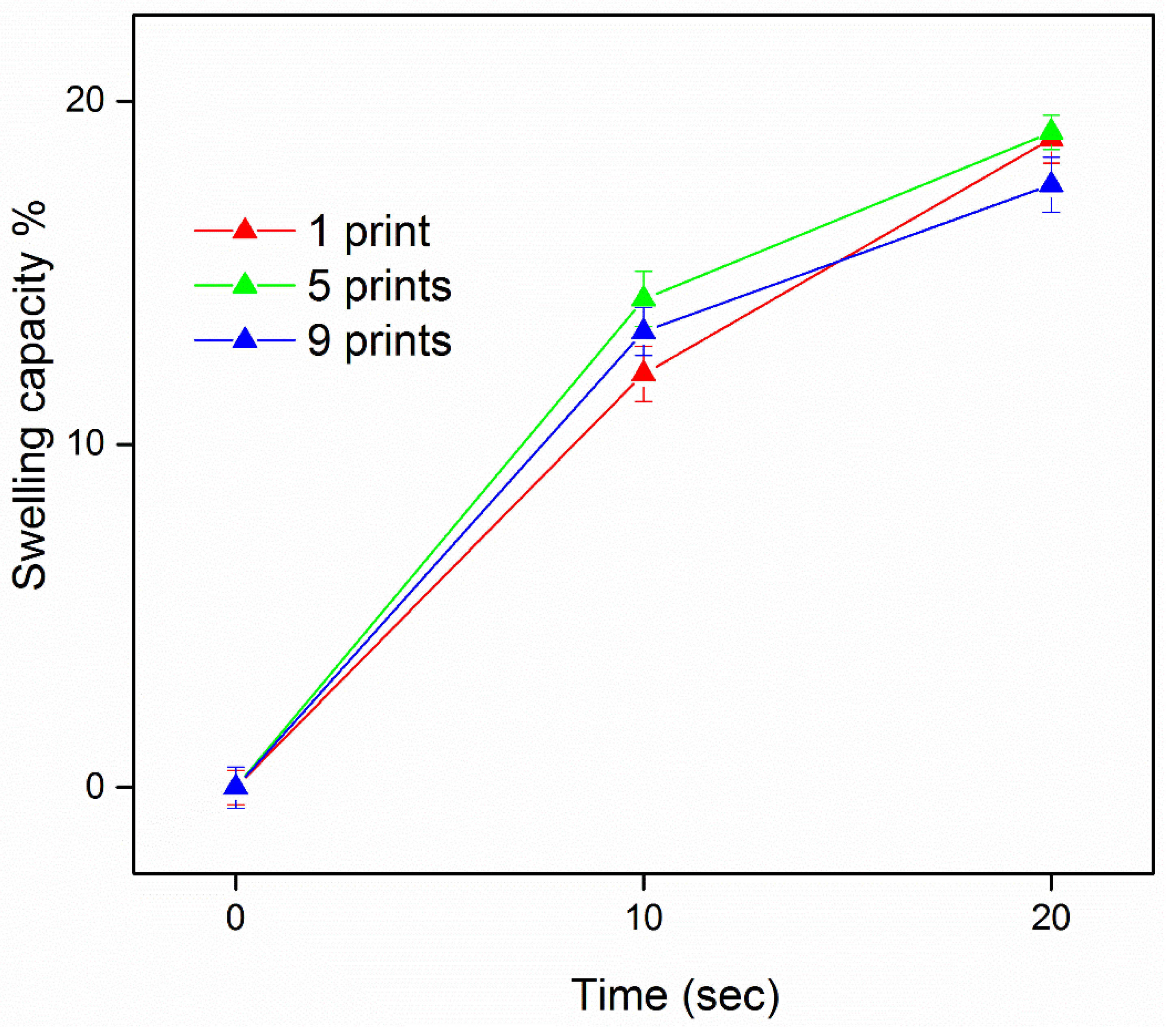
3.3. Drug Content and Water Uptake of the Developed Films
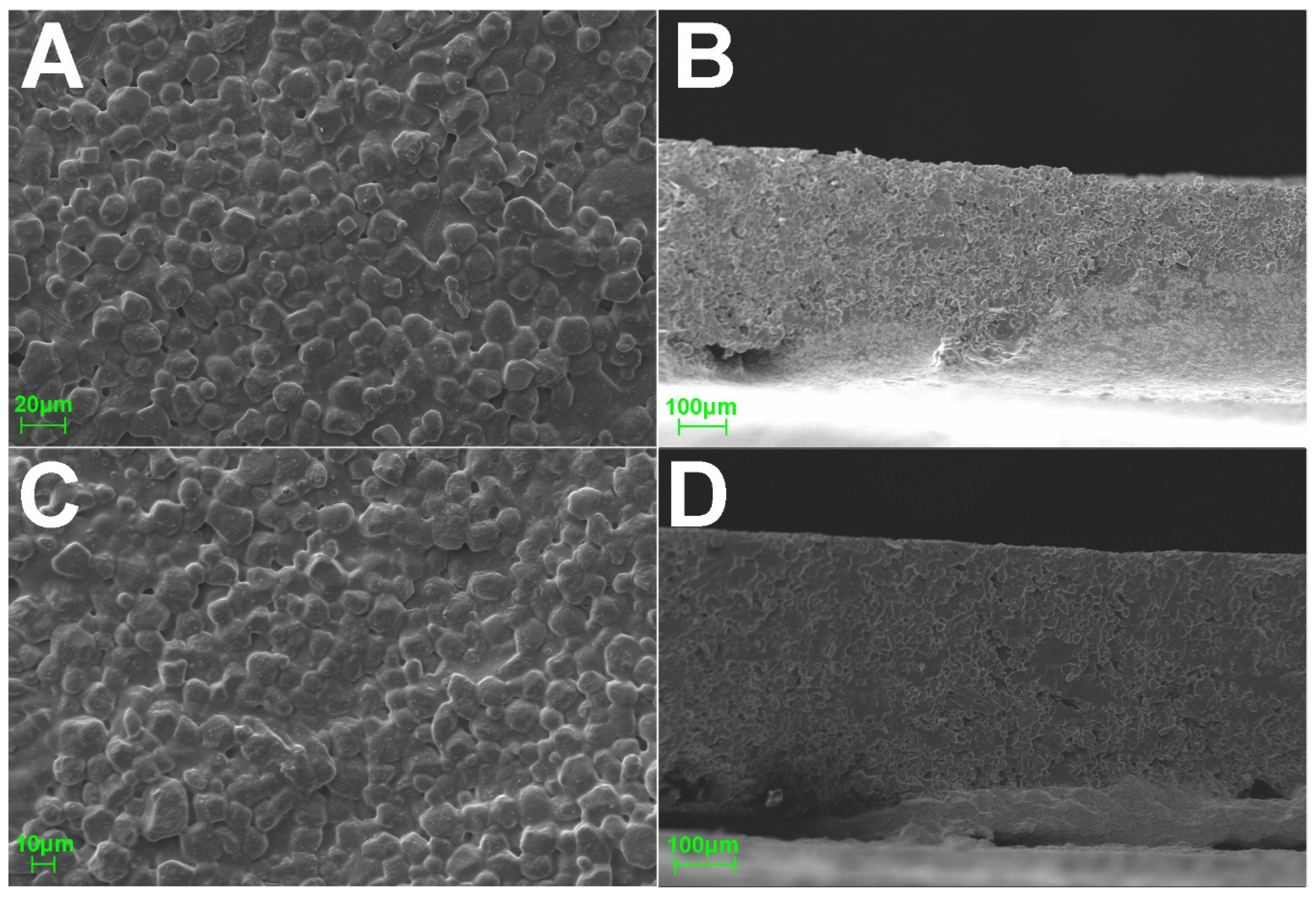
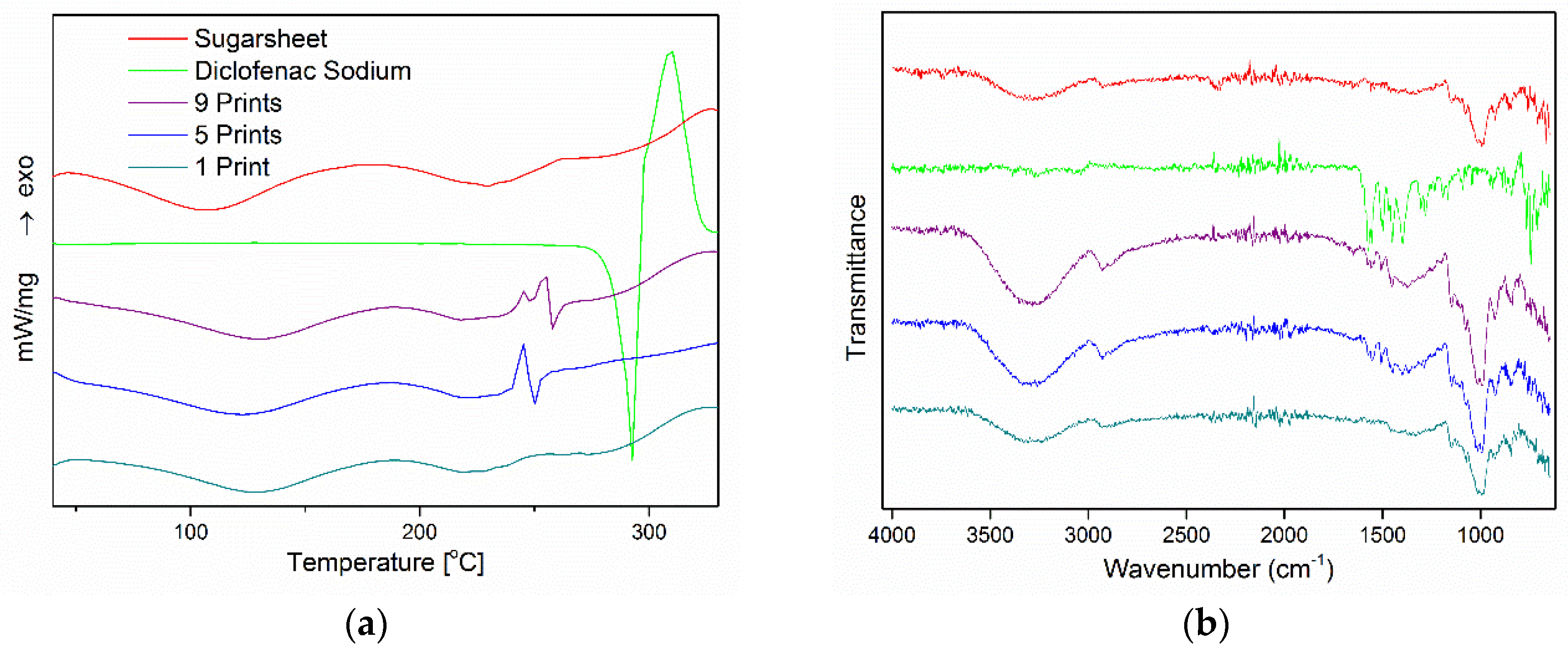
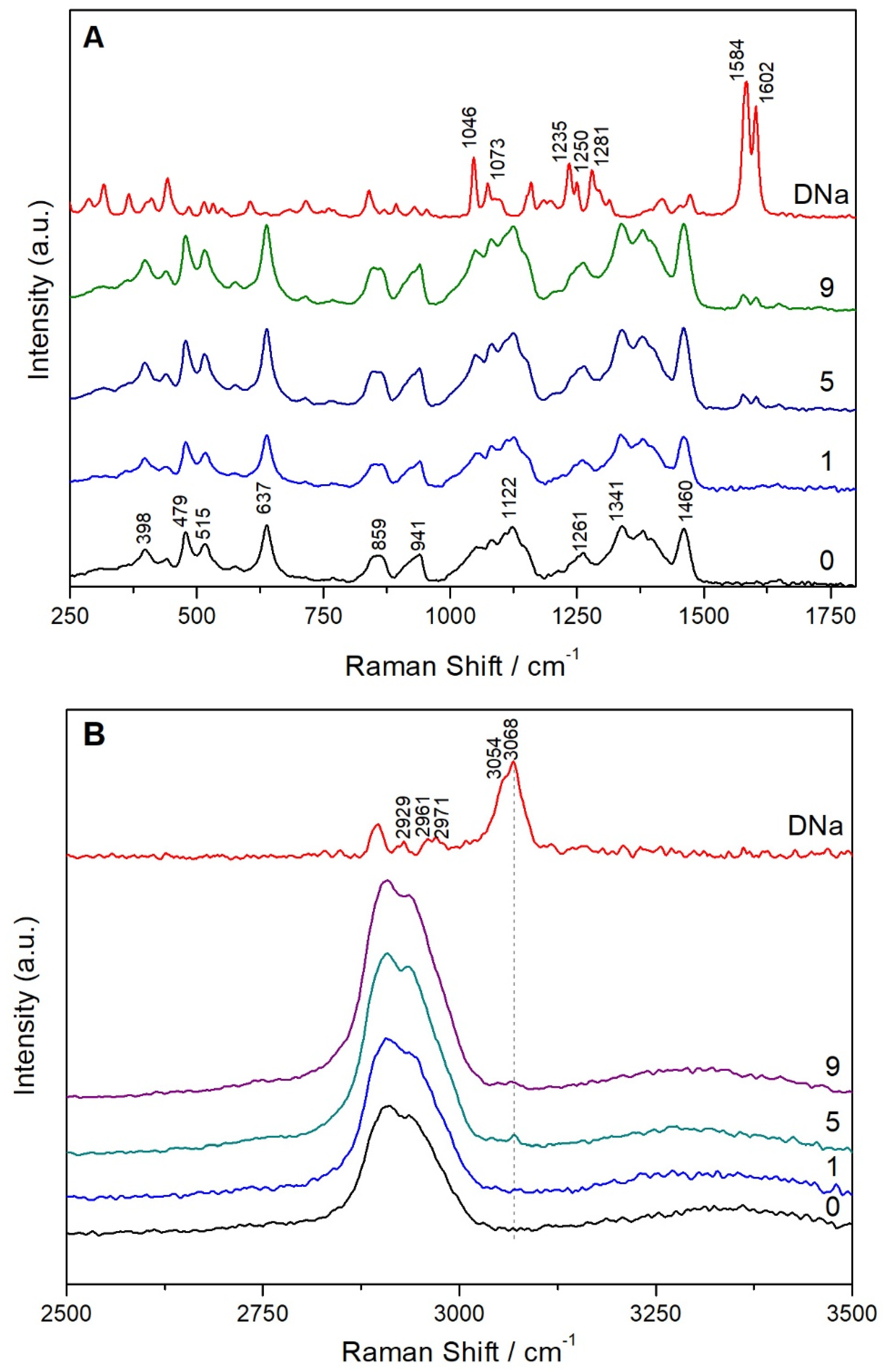
3.4. Physicochemical Characterization
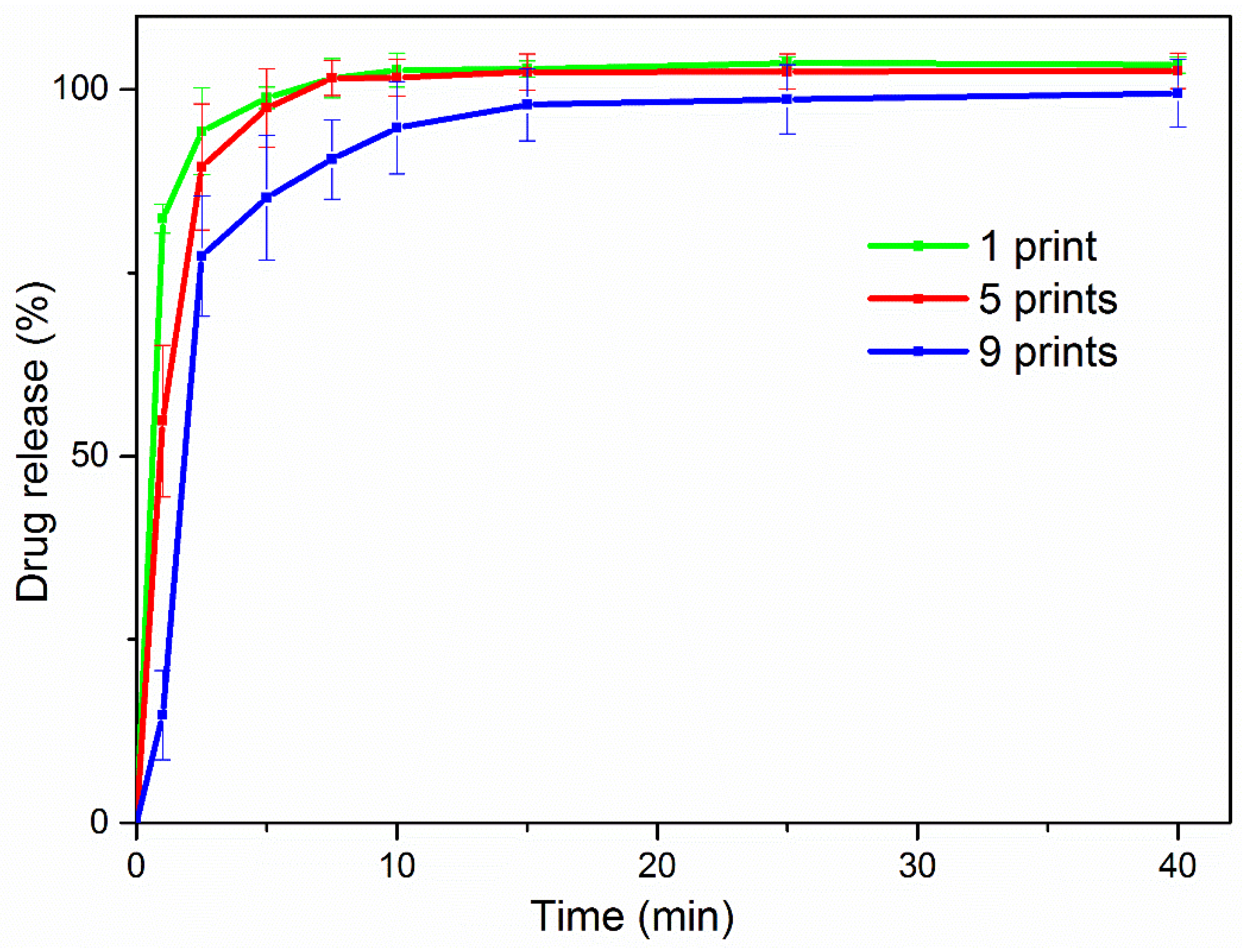
3.5. In Vitro Release
3.6. Drug Permeation
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Alomari, M.; Mohamed, F.H.; Basit, A.W.; Gaisford, S. Personalised dosing: Printing a dose of one’s own medicine. Int. J. Pharm. 2015, 494, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Magdassi, S. The Chemistry of Inkjet Inks; World Scientific Publishers: Singapore, 2010; Volume 16, ISBN 9789812818218. [Google Scholar]
- Dudley, J.T.; Listgarten, J.; Stegle, O.; Brenner, S.E.; Parts, L. Personalized medicine: From genotypes, molecular phenotypes and the quantified self, towrds improved medicine. In Biocomputing 2015; World Scientific: Singapore, 2014; pp. 342–346. [Google Scholar]
- Preis, M.; Breitkreutz, J.; Sandler, N. Perspective: Concepts of printing technologies for oral film formulations. Int. J. Pharm. 2015, 494, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Kolakovic, R.; Viitala, T.; Ihalainen, P.; Genina, N.; Peltonen, J.; Sandler, N. Printing technologies in fabrication of drug delivery systems. Expert Opin. Drug Deliv. 2013, 10, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Varan, C.; Wickström, H.; Sandler, N.; Aktaş, Y.; Bilensoy, E. Inkjet printing of antiviral PCL nanoparticles and anticancer cyclodextrin inclusion complexes on bioadhesive film for cervical administration. Int. J. Pharm. 2017, 531, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Daly, R.; Harrington, T.S.; Martin, G.D.; Hutchings, I.M. Inkjet printing for pharmaceutics—A review of research and manufacturing. Int. J. Pharm. 2015, 494, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Meléndez, P.A.; Kane, K.M.; Ashvar, C.S.; Albrecht, M.; Smith, P.A. Thermal Inkjet Application in the Preparation of Oral Dosage Forms: Dispensing of Prednisolone Solutions and Polymorphic Characterization by Solid-State Spectroscopic Techniques. J. Pharm. Sci. 2008, 97, 2619–2636. [Google Scholar] [CrossRef] [PubMed]
- Pardeike, J.; Strohmeier, D.M.; Schrödl, N.; Voura, C.; Gruber, M.; Khinast, J.G.; Zimmer, A. Nanosuspensions as advanced printing ink for accurate dosing of poorly soluble drugs in personalized medicines. Int. J. Pharm. 2011, 420, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Scoutaris, N.; Alexander, M.R.; Gellert, P.R.; Roberts, C.J. Inkjet printing as a novel medicine formulation technique. J. Control. Release 2011, 156, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Buanz, A.B.M.; Saunders, M.H.; Basit, A.W.; Gaisford, S. Preparation of Personalized-dose Salbutamol Sulphate Oral Films with Thermal Ink-Jet Printing. Pharm. Res. 2011, 28, 2386–2392. [Google Scholar] [CrossRef] [PubMed]
- Genina, N.; Fors, D.; Palo, M.; Peltonen, J.; Sandler, N. Behavior of printable formulations of loperamide and caffeine on different substrates—Effect of print density in inkjet printing. Int. J. Pharm. 2013, 453, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Genina, N.; Janßen, E.M.; Breitenbach, A.; Breitkreutz, J.; Sandler, N. Evaluation of different substrates for inkjet printing of rasagiline mesylate. Eur. J. Pharm. Biopharm. 2013, 85, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Vakili, H.; Wickström, H.; Desai, D.; Preis, M.; Sandler, N. Application of a handheld NIR spectrometer in prediction of drug content in inkjet printed orodispersible formulations containing prednisolone and levothyroxine. Int. J. Pharm. 2017, 524, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Wickström, H.; Broos, A.; Nyman, J.O.; Kortesmäki, E.; Eklund, P.; de Beer, T.; Preis, M.; Sandler, N. Handheld colorimeter as quality control tool for inkjet printed flexible levothyroxine doses for pediatric use. Int. J. Pharm. 2018, 536, 508–509. [Google Scholar] [CrossRef]
- Kollamaram, G.; Hopkins, S.C.; Glowacki, B.A.; Croker, D.M.; Walker, G.M. Inkjet printing of paracetamol and indomethacin using electromagnetic technology: Rheological compatibility and polymorphic selectivity. Eur. J. Pharm. Sci. 2018, 115, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Vuddanda, P.R.; Alomari, M.; Dodoo, C.C.; Trenfield, S.J.; Velaga, S.; Basit, A.W.; Gaisford, S. Personalisation of warfarin therapy using thermal ink-jet printing. Eur. J. Pharm. Sci. 2018, 117, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Edinger, M.; Bar-Shalom, D.; Sandler, N.; Rantanen, J.; Genina, N. QR encoded smart oral dosage forms by inkjet printing. Int. J. Pharm. 2018, 536, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Chuasuwan, B.; Binjesoh, V.; Polli, J.E.; Zhang, H.; Amidon, G.L.; Junginger, H.E.; Midha, K.K.; Shah, V.P.; Stavchansky, S.; Dressman, J.B.; et al. Biowaiver Monographs for Immediate Release Solid Oral Dosage Forms: Diclofenac Sodium and Diclofenac Potassium. J. Pharm. Sci. 2009, 98, 1206–1219. [Google Scholar] [CrossRef] [PubMed]
- Calixto, G.; Garcia, M.; Cilli, E.; Chiavacci, L.; Chorilli, M. Design and Characterization of a Novel p1025 Peptide-Loaded Liquid Crystalline System for the Treatment of Dental Caries. Molecules 2016, 21, 158. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Boateng, J. Effects of Cyclodextrins (β and γ) and l-Arginine on Stability and Functional Properties of Mucoadhesive Buccal Films Loaded with Omeprazole for Pediatric Patients. Polymers 2018, 10, 157. [Google Scholar] [CrossRef]
- Marques, M.R.C.; Loebenberg, R.; Almukainzi, M. Simulated Biological Fluids with Possible Application in Dissolution Testing. Dissolut. Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Buanz, A.B.M.; Belaunde, C.C.; Soutari, N.; Tuleu, C.; Gul, M.O.; Gaisford, S. Ink-jet printing versus solvent casting to prepare oral films: Effect on mechanical properties and physical stability. Int. J. Pharm. 2015, 494, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, A.K.; Smyth, H.D.C. Hollow crystalline straws of diclofenac for high-dose and carrier-free dry powder inhaler formulations. Int. J. Pharm. 2016, 502, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Balogh, A.; Horváthová, T.; Fülöp, Z.; Loftsson, T.; Harasztos, A.H.; Marosi, G.; Nagy, Z.K. Electroblowing and electrospinning of fibrous diclofenac sodium-cyclodextrin complex-based reconstitution injection. J. Drug Deliv. Sci. Technol. 2015, 26, 28–34. [Google Scholar] [CrossRef]
- Elnaggar, Y.S.R.; El-Massik, M.A.; Abdallah, O.Y.; Ebian, A.E.R. Maltodextrin: A Novel Excipient Used in Sugar-Based Orally Disintegrating Tablets and Phase Transition Process. AAPS PharmSciTech 2010, 11, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Cabrera, M.A.; Schmidt, S.J. Determination of glass transition temperatures during cooling and heating of low-moisture amorphous sugar mixtures. J. Food Eng. 2015, 146, 36–43. [Google Scholar] [CrossRef]
- AL-Kahtani, A.A.; Sherigara, B.S. Controlled release of diclofenac sodium through acrylamide grafted hydroxyethyl cellulose and sodium alginate. Carbohydr. Polym. 2014, 104, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Barzegar-Jalali, M.; Alaei-Beirami, M.; Javadzadeh, Y.; Mohammadi, G.; Hamidi, A.; Andalib, S.; Adibkia, K. Comparison of physicochemical characteristics and drug release of diclofenac sodium-eudragit® RS100 nanoparticles and solid dispersions. Powder Technol. 2012, 219, 211–216. [Google Scholar] [CrossRef]
- Bukara, K.; Drvenica, I.; Ilić, V.; Stančić, A.; Mišić, D.; Vasić, B.; Gajić, R.; Vučetić, D.; Kiekens, F.; Bugarski, B. Comparative studies on osmosis based encapsulation of sodium diclofenac in porcine and outdated human erythrocyte ghosts. J. Biotechnol. 2016, 240, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Gaitano, R.O.; Calvo, N.L.; Narda, G.E.; Kaufman, T.S.; Maggio, R.M.; Brusau, E.V. Preparation and Physical Characterization of a Diclofenac-Ranitidine Co-precipitate for Improving the Dissolution of Diclofenac. J. Pharm. Sci. 2016, 105, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Iliescu, T.; Baia, M.; Miclăuş, V. A Raman spectroscopic study of the diclofenac sodium–β-cyclodextrin interaction. Eur. J. Pharm. Sci. 2004, 22, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Shayanfar, A.; Acree, W.E.; Jouyban, A. Solubility of Lamotrigine, Diazepam, Clonazepam, and Phenobarbital in Propylene Glycol + Water Mixtures at 298.15 K. J. Chem. Eng. Data 2009, 54, 1153–1157. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; Chorilli, M. An overview of polymeric dosage forms in buccal drug delivery: State of art, design of formulations and their in vivo performance evaluation. Mater. Sci. Eng. C 2018, 86, 129–143. [Google Scholar] [CrossRef] [PubMed]


| Solvent | Solubility (mg/mL) |
|---|---|
| Distilled Water | 33.7 ± 1.3 |
| EtOH | 74.3 ± 1.9 |
| PEG | 64.4 ± 1.8 |
| EtOH:PG Ratio (% v/v) | DNa (mg·mL−1) | Kinematic Viscosity (mm2·s−1) | Density (g·cm−3) | Dynamic Viscosity (mPa·s) | Surface Tension (mN·m−1) |
|---|---|---|---|---|---|
| 20:80 | - | 19.16 ± 0.03 | 0.923 ± 0.009 | 17.68 ± 0.04 | - |
| 40:60 | - | 9.08 ± 0.04 | 0.902 ± 0.010 | 8.19 ± 0.03 | - |
| 50:50 | - | 6.03 ± 0.07 | 0.868 ± 0.008 | 5.23 ± 0.03 | 25.7 ± 0.4 |
| 50:50 | 375.0 | 29.20 ± 0.05 | 0.982 ± 0.008 | 28.67 ± 0.04 | - |
| 50:50 | 227.3 | 18.29 ± 0.04 | 0.965 ± 0.007 | 17.64 ± 0.06 | 27.9 ± 0.5 |
| Formulation | First Order Model | Korsmeyer–Peppas Model | |||
|---|---|---|---|---|---|
| k | R2 | k | n | R2 | |
| 1-printed | 1.687 | 0.9952 | 88.20 | 0.0537 | 0.9909 |
| 5-printed | 0.826 | 0.9978 | 74.26 | 0.1112 | 0.9364 |
| 9-printed | 0.387 | 0.9714 | 52.08 | 0.2115 | 0.9108 |
| Printed Layers | Jss (μg·cm−2·h−1) | P·10−4 (cm·h−1) |
|---|---|---|
| 1 | 0.806 ± 0.014 | 6.672 ± 0.142 |
| 5 | 4.469 ± 0.193 | 12.386 ± 0.655 |
| 9 | 6.921 ± 0.248 | 14.371 ± 0.631 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eleftheriadis, G.K.; Monou, P.K.; Bouropoulos, N.; Fatouros, D.G. In Vitro Evaluation of 2D-Printed Edible Films for the Buccal Delivery of Diclofenac Sodium. Materials 2018, 11, 864. https://doi.org/10.3390/ma11050864
Eleftheriadis GK, Monou PK, Bouropoulos N, Fatouros DG. In Vitro Evaluation of 2D-Printed Edible Films for the Buccal Delivery of Diclofenac Sodium. Materials. 2018; 11(5):864. https://doi.org/10.3390/ma11050864
Chicago/Turabian StyleEleftheriadis, Georgios K., Paraskevi Kyriaki Monou, Nikolaos Bouropoulos, and Dimitrios G. Fatouros. 2018. "In Vitro Evaluation of 2D-Printed Edible Films for the Buccal Delivery of Diclofenac Sodium" Materials 11, no. 5: 864. https://doi.org/10.3390/ma11050864
APA StyleEleftheriadis, G. K., Monou, P. K., Bouropoulos, N., & Fatouros, D. G. (2018). In Vitro Evaluation of 2D-Printed Edible Films for the Buccal Delivery of Diclofenac Sodium. Materials, 11(5), 864. https://doi.org/10.3390/ma11050864