Microstructure Evolution of AlSi10Mg(Cu) Alloy Related to Isothermal Exposure
Abstract
1. Introduction
2. Materials and Experiments
3. Results
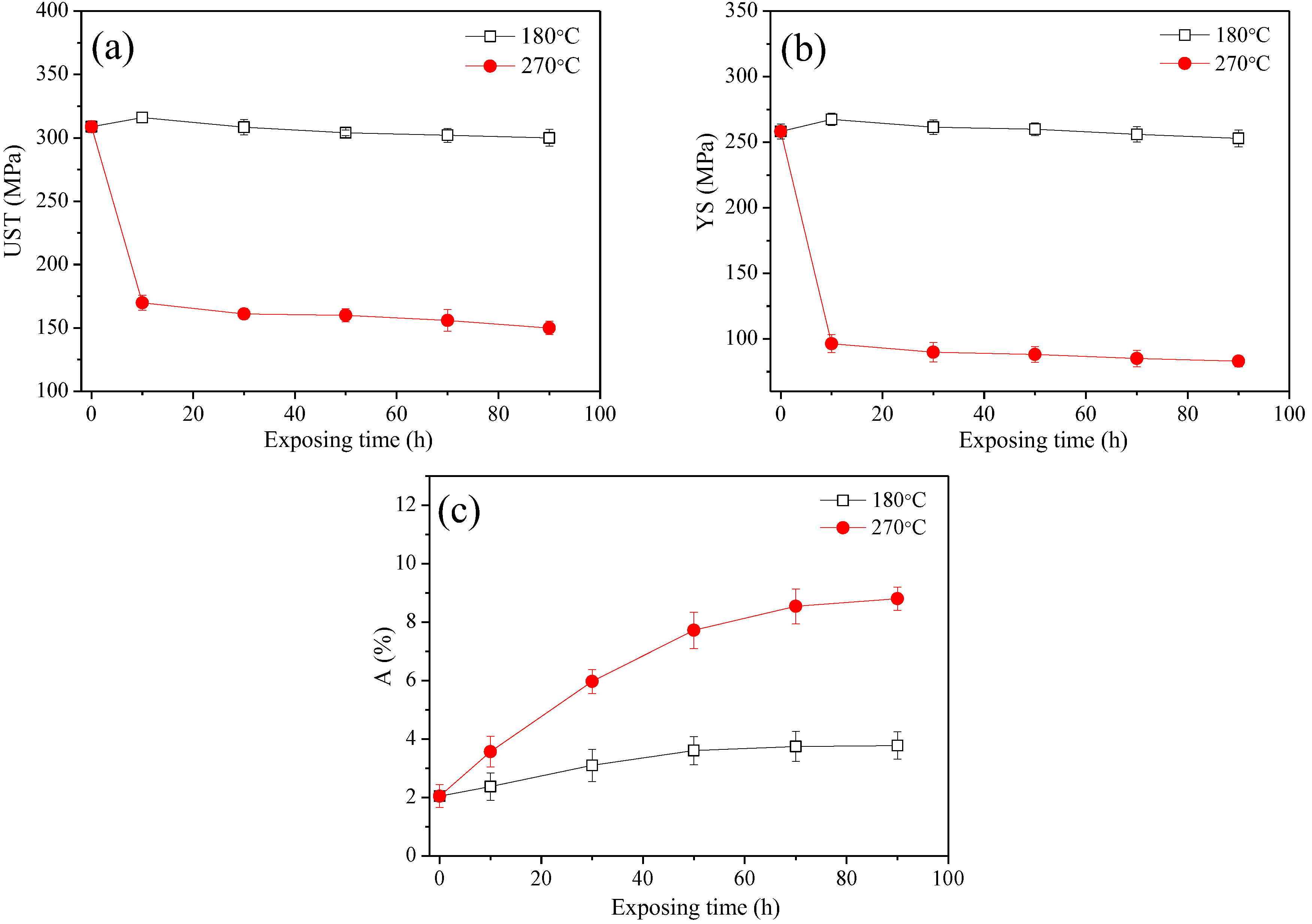
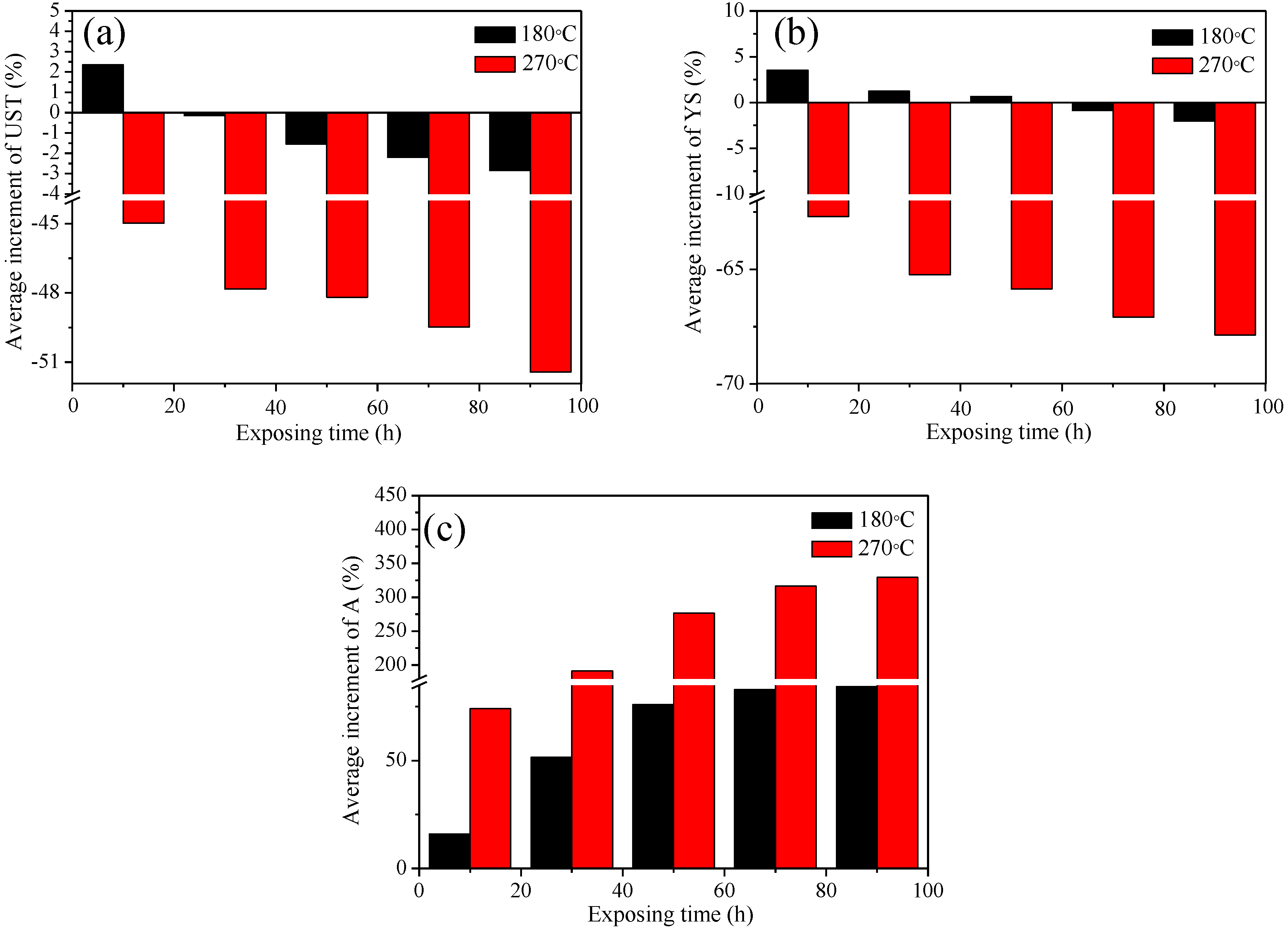
3.1. The Evaluation of Mechanical Properties
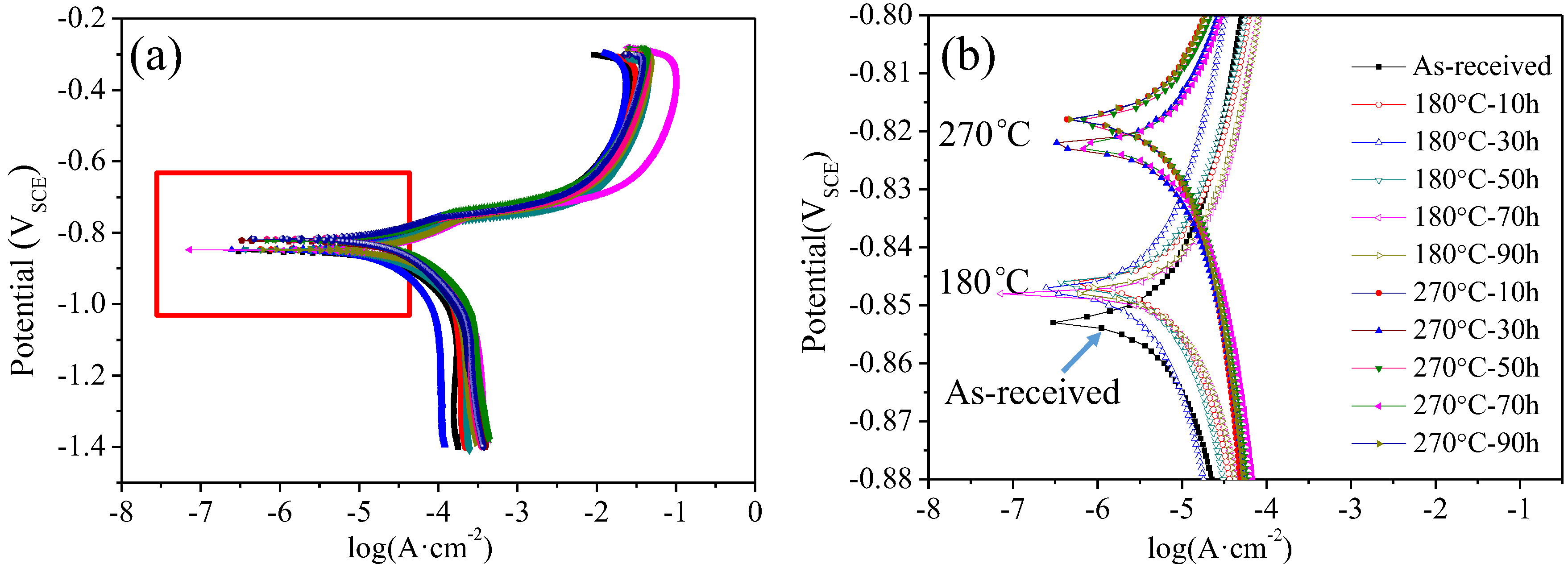
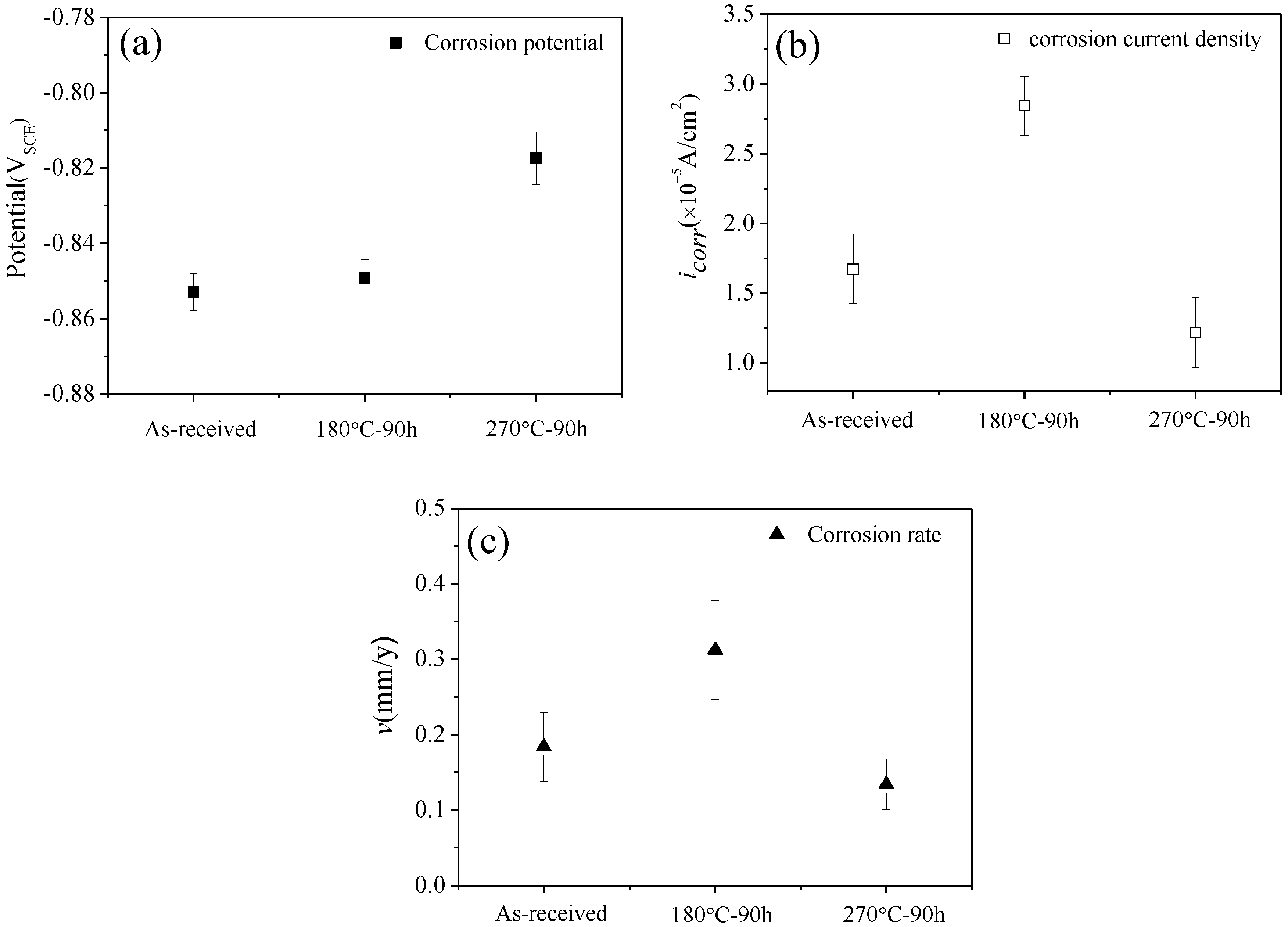
3.2. The Evaluation of Corrosion Resistance
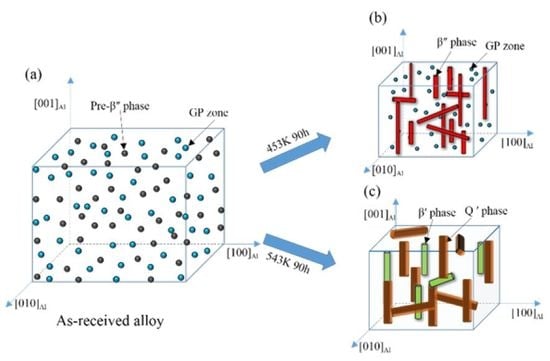
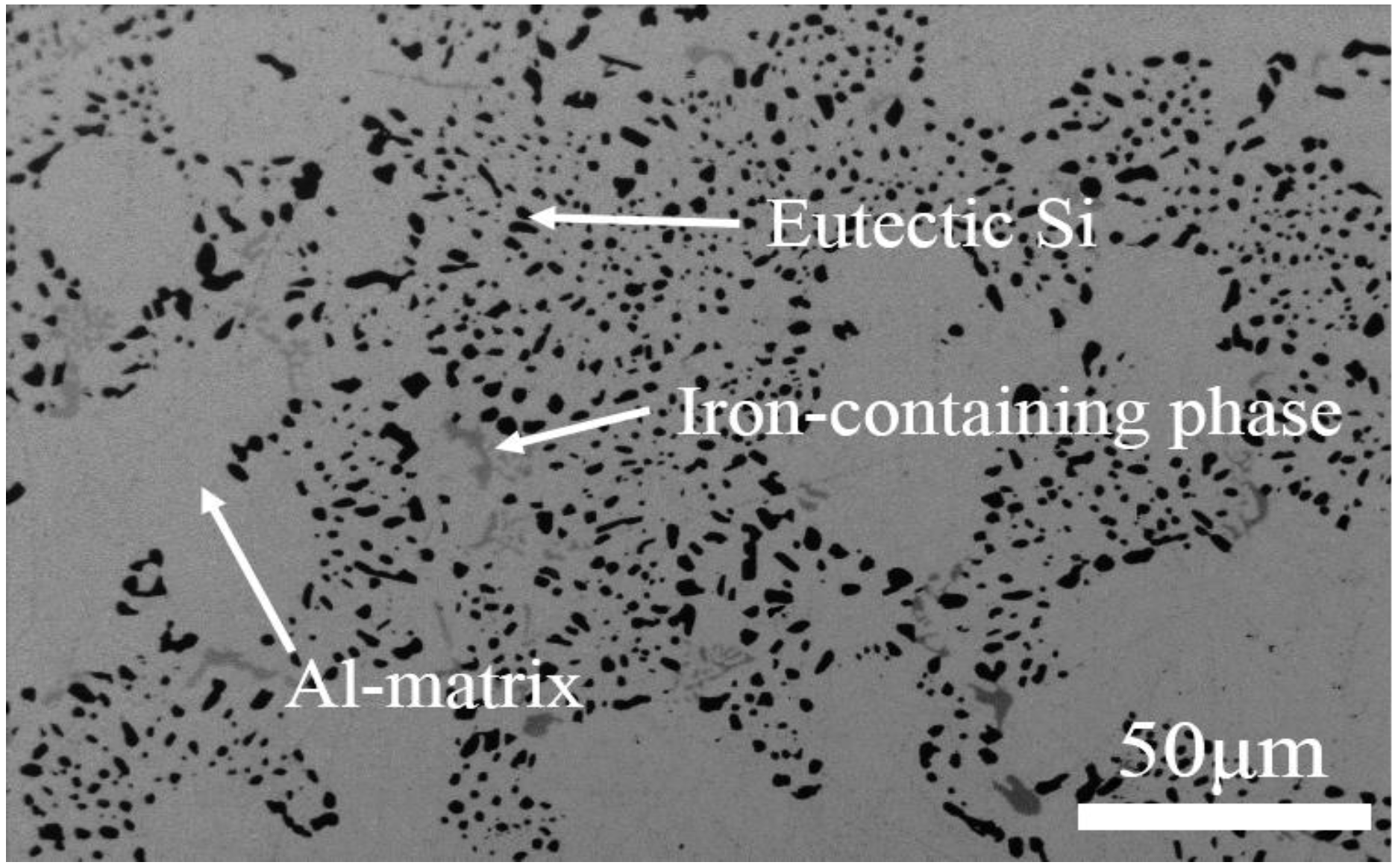
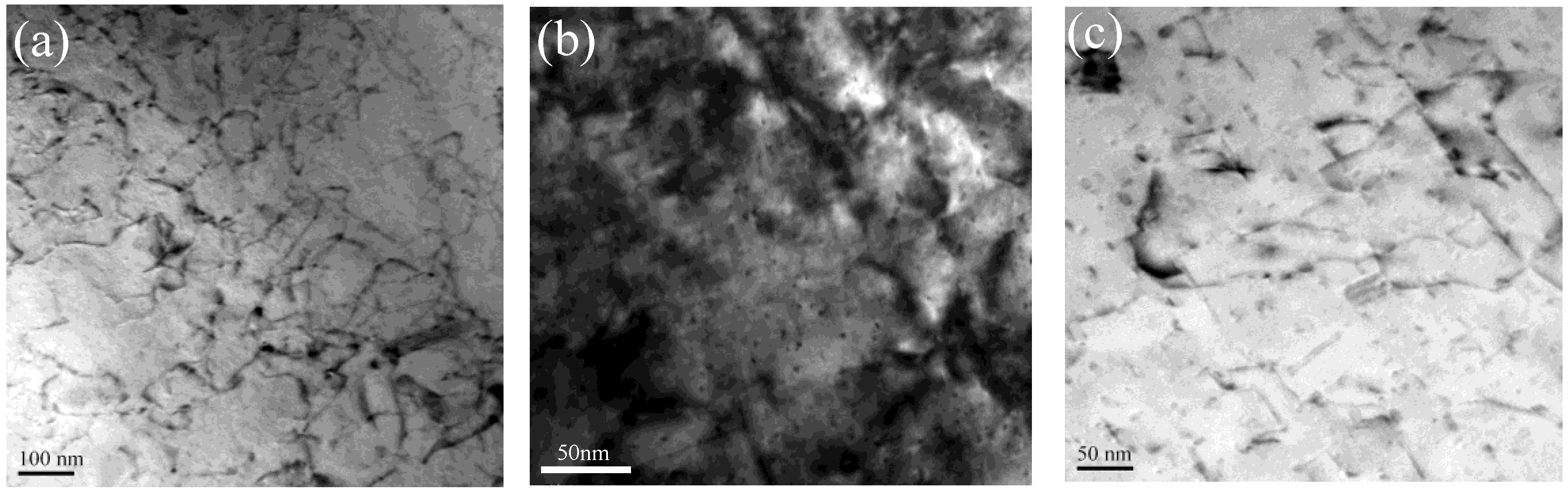
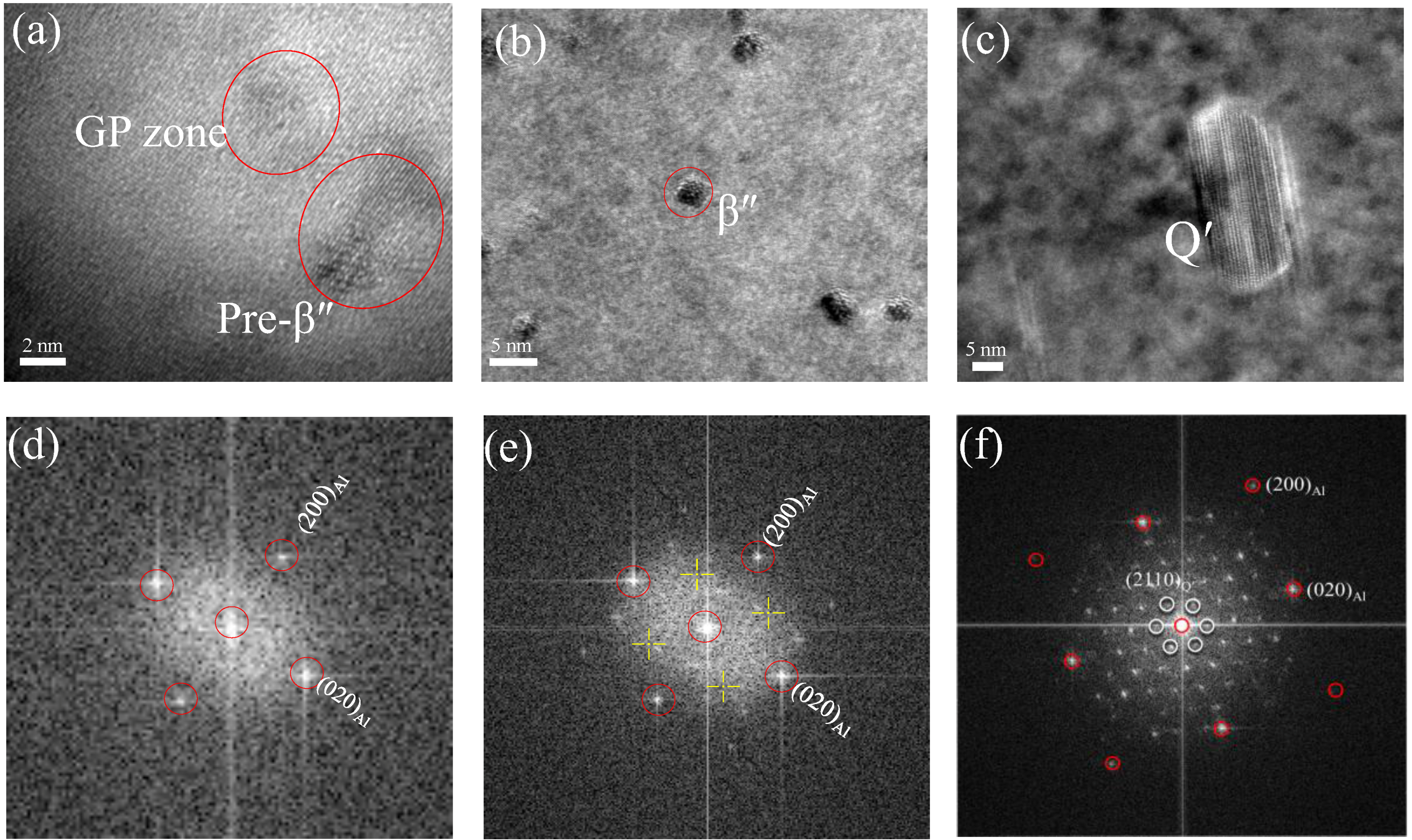
3.3. Microstructures Observation
4. Discussion
4.1. Evolution Mechanism of Mechanical Properties
4.2. Evolution Mechanism of Corrosion Resistance
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Javidani, M.; Larouche, D. Application of cast Al-Si alloys in internal combustion engine components. Int. Mater. Rev. 2014, 59, 132–158. [Google Scholar] [CrossRef]
- Kores, S.; Zak, H.; Tonn, B. Aluminium alloys for cylinder heads. RMZ-Mater. Geoenviron. 2008, 55, 307–317. [Google Scholar]
- Zolotorevsky, V.S.; Belov, N.A.; Glazoff, M.V. Casting Aluminum Alloys; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Moustafa, M.A.; Samuel, F.H.; Doty, H.W.; Valtierra, S. Effect of Mg and Cu additions on the microstructural characteristics and tensile properties of Sr-modified Al-Si eutectic alloys. Int. J. Cast Met. Res. 2002, 14, 235–253. [Google Scholar] [CrossRef]
- Sjölander, E.; Seifeddine, S. The heat treatment of Al-Si-Cu-Mg casting alloys. J. Mater. Process. Technol. 2010, 210, 1249–1259. [Google Scholar] [CrossRef]
- Arrabal, R.; Mingo, B.; Pardo, A.; Mohedano, M.; Matykina, E.; Rodríguez, I. Pitting corrosion of rheocast A356 aluminium alloy in 3.5 wt. % NaCl solution. Corros. Sci. 2013, 73, 342–355. [Google Scholar] [CrossRef]
- Jamaati, R.; Amirkhanlou, S.; Toroghinejad, M.R.; Niroumand, B. Significant improvement of semi-solid microstructure and mechanical properties of A356 alloy by ARB process. Mater. Sci. Eng. A 2011, 528, 2495–2501. [Google Scholar] [CrossRef]
- Suárez-Peña, B.; Asensio-Lozano, J. Influence of Sr modification and Ti grain refinement on the morphology of Fe-rich precipitates in eutectic Al-Si die cast alloys. Scr. Mater. 2006, 54, 1543–1548. [Google Scholar] [CrossRef]
- Jain, S. Corrosion and Protection of Heterogeneous Cast Al-Si (356) and Al-Si-Cu-Fe (380) Alloys by Chromate and Cerium Inhibitors. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2006. [Google Scholar]
- Yasakau, K.A.; Zheludkevich, M.L.; Lamaka, S.V.; Ferreira, M.G.S. Role of intermetallic phases in localized corrosion of AA5083. Electrochim. Acta 2007, 52, 7651–7659. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, P.; Li, X.; Wang, L.; Liao, W.; Huang, L.; Xia, X. Microstructure and thermal stability evolution behavior of Sc-containing A356.2 aluminum alloy under cyclic thermal exposure conditions. Mater. Sci. Eng. A 2018, 723, 165–173. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Z. Evolution of silicon particle damage on fatigue crack initiation and early propagation in an aluminum alloy. Rare Met. 2017, 1–7. [Google Scholar] [CrossRef]
- Taylor, J.A. Iron-containing intermetallic phases in Al-Si based casting alloys. Procedia Mater. Sci. 2012, 1, 19–33. [Google Scholar] [CrossRef]
- Wang, G.; Sun, Q.; Feng, L.; Hui, L.; Jing, C. Influence of Cu content on ageing behavior of AlSiMgCu cast alloys. Mater. Des. 2007, 28, 1001–1005. [Google Scholar] [CrossRef]
- Izcara, X.L.; Blank, A.G.; Pyczak, F.; Staron, P.; Schumann, S.; Huber, N. Characterization and modeling of the influence of artificial aging on the microstructural evolution of age-hardenable AlSi10Mg(Cu) aluminum alloys. Mater. Sci. Eng. A 2014, 610, 46–53. [Google Scholar] [CrossRef]
- Sjölander, E.; Seifeddine, S. Artificial ageing of Al-Si-Cu-Mg casting alloys. Mater. Sci. Eng. A 2011, 528, 7402–7409. [Google Scholar] [CrossRef]
- Ibrahim, M.F.; Samuel, A.M.; Doty, H.W.; Samuel, F.H. Effect of aging conditions on precipitation hardening in Al-Si-Mg and Al-Si-Cu-Mg alloys. Int. J. Met. 2017, 11, 274–286. [Google Scholar] [CrossRef]
- Ramgopal, T.; Gouma, P.I.; Frankel, G.S. Role of grain-boundary precipitates and solute-depleted zone on the intergranular corrosion of aluminum alloy 7150. Corrosion 2002, 58, 687–697. [Google Scholar] [CrossRef]
- ASTM International. Standard Test Methods for Tension Testing of Metallic Materials; ASTM E8/E8M-13; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Zhang, F.; Huang, M.; Shi, D. The relationship between the strain-hardening exponent n and the microstructure of metals. Mater. Sci. Eng. A 1989, 122, 211–213. [Google Scholar]
- Lin, Y.C.; Liu, G.; Chen, M.S.; Huang, Y.-C.; Chen, Z.-G.; Ma, X.; Jiang, Y.-Q.; Li, J. Corrosion resistance of a two-stage stress-aged Al-Cu-Mg alloy: Effects of stress-aging temperature. J. Alloys Compd. 2016, 657, 855–865. [Google Scholar] [CrossRef]
- McCafferty, E. Validation of corrosion rates measured by the Tafel extrapolation method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Li, R.X.; Li, R.D.; Zhao, Y.H.; He, L.Z.; Li, C.X.; Guan, H.R.; Hu, Z.Q. Age-hardening behavior of cast Al-Si base alloy. Mater. Lett. 2004, 58, 2096–2101. [Google Scholar] [CrossRef]
- Bergant, Z.; Trdan, U.; Grum, J. Effect of high-temperature furnace treatment on the microstructure and corrosion behavior of NiCrBSi flame-sprayed coatings. Corros. Sci. 2014, 88, 372–386. [Google Scholar] [CrossRef]
- Son, S.K.; Takeda, M.; Mitome, M.; Bando, Y.; Endo, T. Precipitation behavior of an Al-Cu alloy during isothermal aging at low temperatures. Mater. Lett. 2005, 59, 629–632. [Google Scholar] [CrossRef]
- Lin, Y.C.; Jiang, Y.Q.; Xia, Y.C.; Zhang, X.-C.; Zhou, H.-M.; Deng, J. Effects of creep-aging processing on the corrosion resistance and mechanical properties of an Al-Cu-Mg alloy. Mater. Sci. Eng. A 2014, 605, 192–202. [Google Scholar] [CrossRef]
- Marioara, C.D.; Andersen, S.J.; Jansen, J.; Zandbergen, H.W. The influence of temperature and storage time at RT on nucleation of the β″phase in a 6082 Al-Mg-Si alloy. Acta Mater. 2003, 51, 789–796. [Google Scholar] [CrossRef]
- Huanga, W.; Liua, Z.; Lina, M.; Zhou, X.; Zhao, L.; Ning, A.; Zeng, S. Reprecipitation behavior in Al-Cu binary alloy after severe plastic deformation-induced dissolution of particles. Mater. Sci. Eng. A 2012, 546, 26–33. [Google Scholar] [CrossRef]
- Eskin, D.G. Decomposition of supersaturated solid solutions in Al-Cu-Mg-Si alloys. J. Mater. Sci. 2003, 38, 279–290. [Google Scholar] [CrossRef]
- Yao, J.Y.; Graham, D.A.; Rinderer, B.; Couper, M.J. A TEM study of precipitation in Al-Mg-Si alloys. Micron 2001, 32, 865–870. [Google Scholar] [CrossRef]
- Sjölander, E.; Seifeddine, S. Optimisation of solution treatment of cast Al-Si-Cu alloys. Mater. Des. 2010, 31, S44–S49. [Google Scholar] [CrossRef]
- Xu, P.; Luo, H. Improving the ductility of nanostructured Al alloy using strongly textured nano-laminated structure combined with nano-precipitates. Mater. Sci. Eng. A 2016, 675, 323–337. [Google Scholar] [CrossRef]
- Vieira, A.C.; Pinto, A.M.; Rocha, L.A.; Mischler, S. Effect of Al2Cu precipitates size and mass transport on the polarization behavior of age-hardened Al-Si-Cu-Mg alloys in 0.05 M NaCl. Electrochem. Acta 2011, 56, 3821–3828. [Google Scholar] [CrossRef]
- Wang, Y.; Liao, H.; Wu, Y.; Yang, J. Effect of Si content on microstructure and mechanical properties of Al-Si-Mg alloys. Mater. Des. 2014, 53, 634–638. [Google Scholar] [CrossRef]
- Osório, W.R.; Garcia, L.R.; Goulart, P.R.; Garcia, A. Effects of eutectic modification and T4 heat treatment on mechanical properties and corrosion resistance of an Al-9 wt. % Si casting alloy. Mater. Chem. Phys. 2007, 106, 343–349. [Google Scholar] [CrossRef]
- Moustafa, M.A.; Samuel, F.H.; Doty, H.W. Effect of solution heat treatment and additives on the microstructure of Al-Si (A413. 1) automotive alloys. J. Mater. Sci. 2003, 38, 4507–4522. [Google Scholar] [CrossRef]
- Crowell, N.; Shivkumar, S. Solution Treatment Effects in Cast Al-Si-Cu Alloys (95-107). Trans. Am. Foundrym. Soc. 1995, 103, 721–726. [Google Scholar]
- Zor, S.; Zeren, M.; Ozkazanc, H.; Karakulak, E. Effect of Cu content on the corrosion of Al-Si eutectic alloys in acidic solutions. Anti-Corros. Methods Mater. 2010, 57, 185–191. [Google Scholar] [CrossRef]
- Tanem, B.S.; Svenningsen, G.; Mårdalen, J. Relations between sample preparation and SKPFM Volta potential maps on an EN AW-6005 aluminum alloy. Corros. Sci. 2005, 47, 1506–1515. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, C.; Geng, H.; Wang, S.; Gong, B.; Zhang, Z. Microstructure Evolution of AlSi10Mg(Cu) Alloy Related to Isothermal Exposure. Materials 2018, 11, 809. https://doi.org/10.3390/ma11050809
Cai C, Geng H, Wang S, Gong B, Zhang Z. Microstructure Evolution of AlSi10Mg(Cu) Alloy Related to Isothermal Exposure. Materials. 2018; 11(5):809. https://doi.org/10.3390/ma11050809
Chicago/Turabian StyleCai, Cheng, Huifang Geng, Shifu Wang, Boxue Gong, and Zheng Zhang. 2018. "Microstructure Evolution of AlSi10Mg(Cu) Alloy Related to Isothermal Exposure" Materials 11, no. 5: 809. https://doi.org/10.3390/ma11050809
APA StyleCai, C., Geng, H., Wang, S., Gong, B., & Zhang, Z. (2018). Microstructure Evolution of AlSi10Mg(Cu) Alloy Related to Isothermal Exposure. Materials, 11(5), 809. https://doi.org/10.3390/ma11050809