Microstructure Evolution and the Resulted Influence on Localized Corrosion in Al-Zn-Mg-Cu Alloy during Non-Isothermal Ageing
Abstract
:1. Introduction
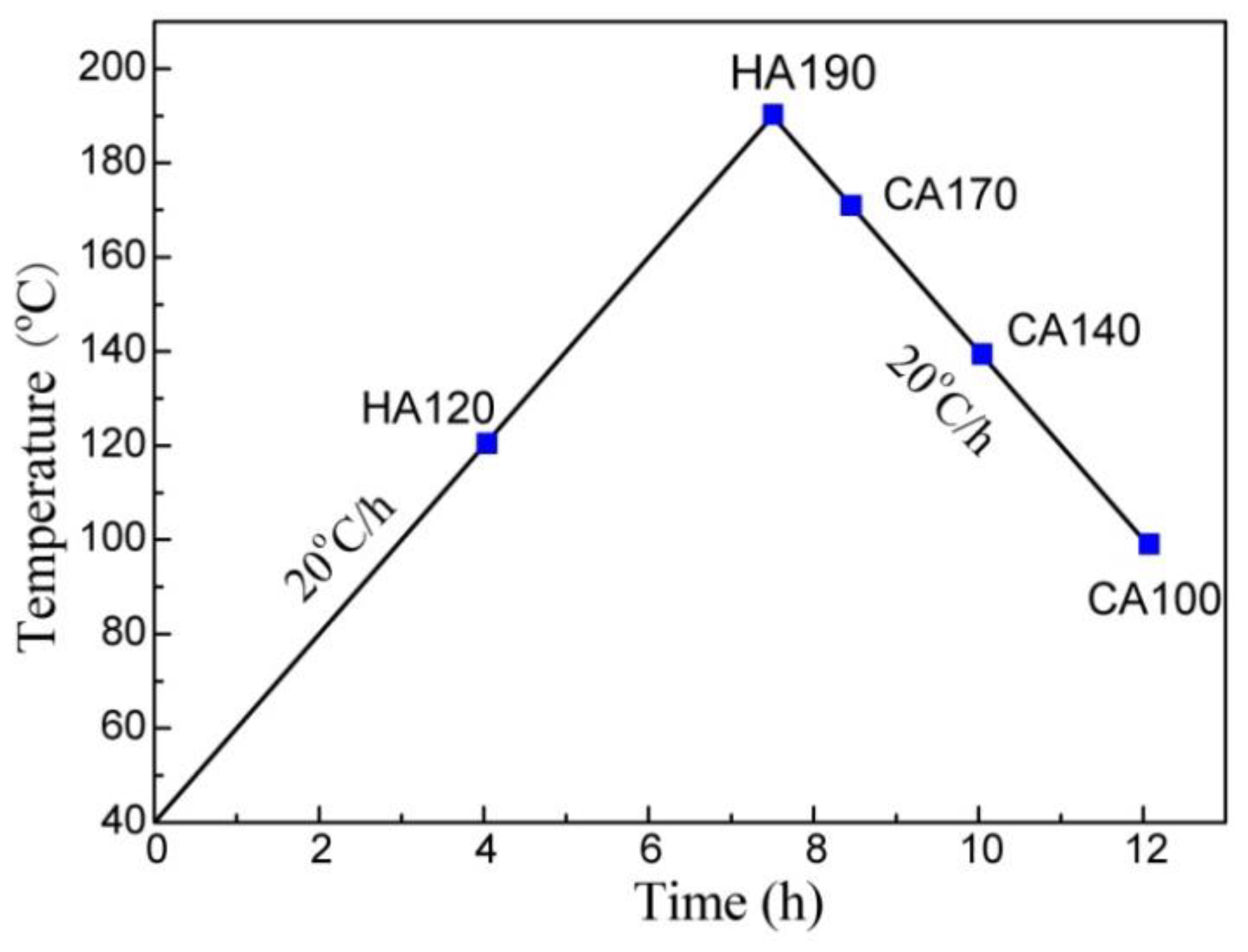
2. Experimental
3. Results and Discussion
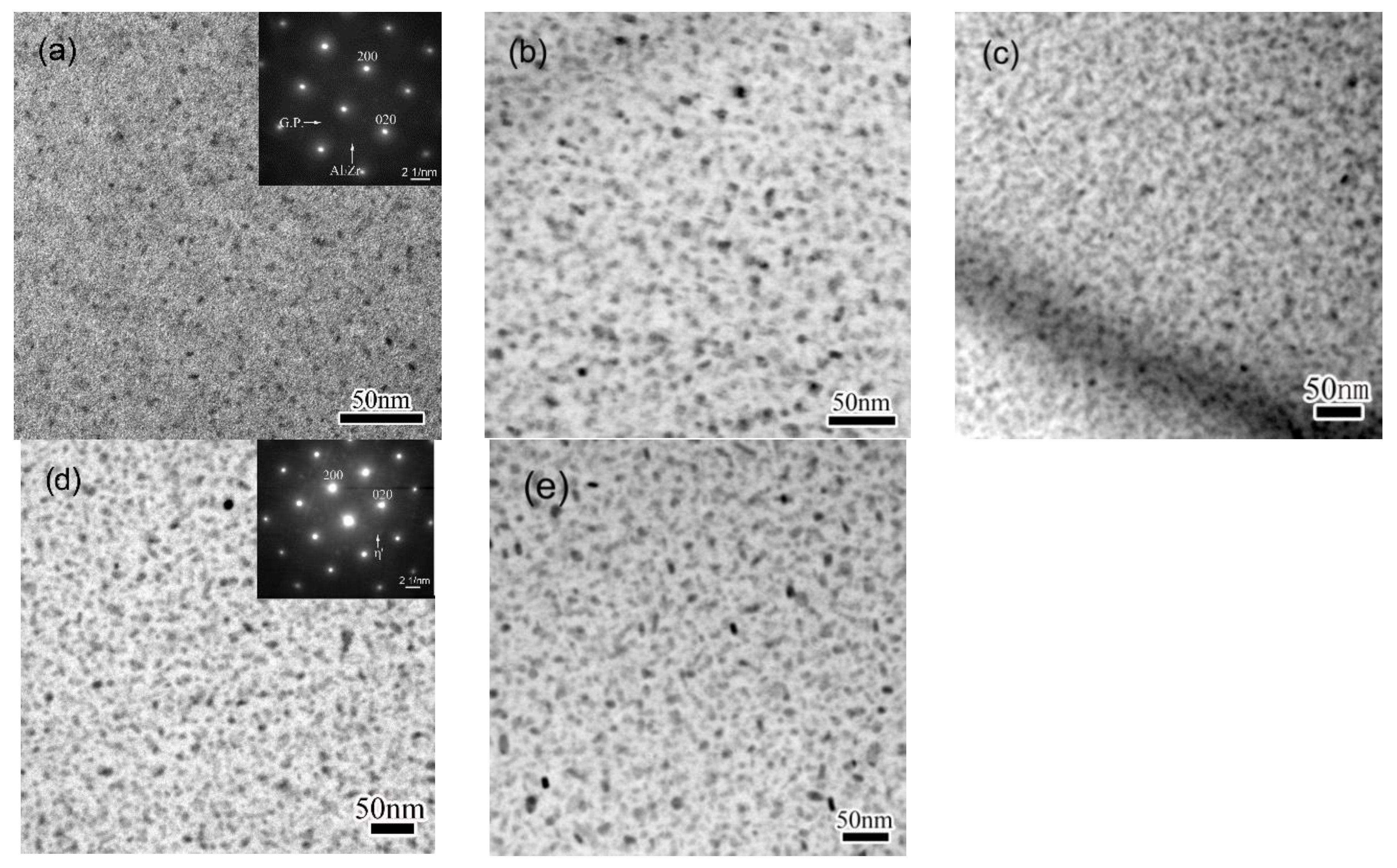
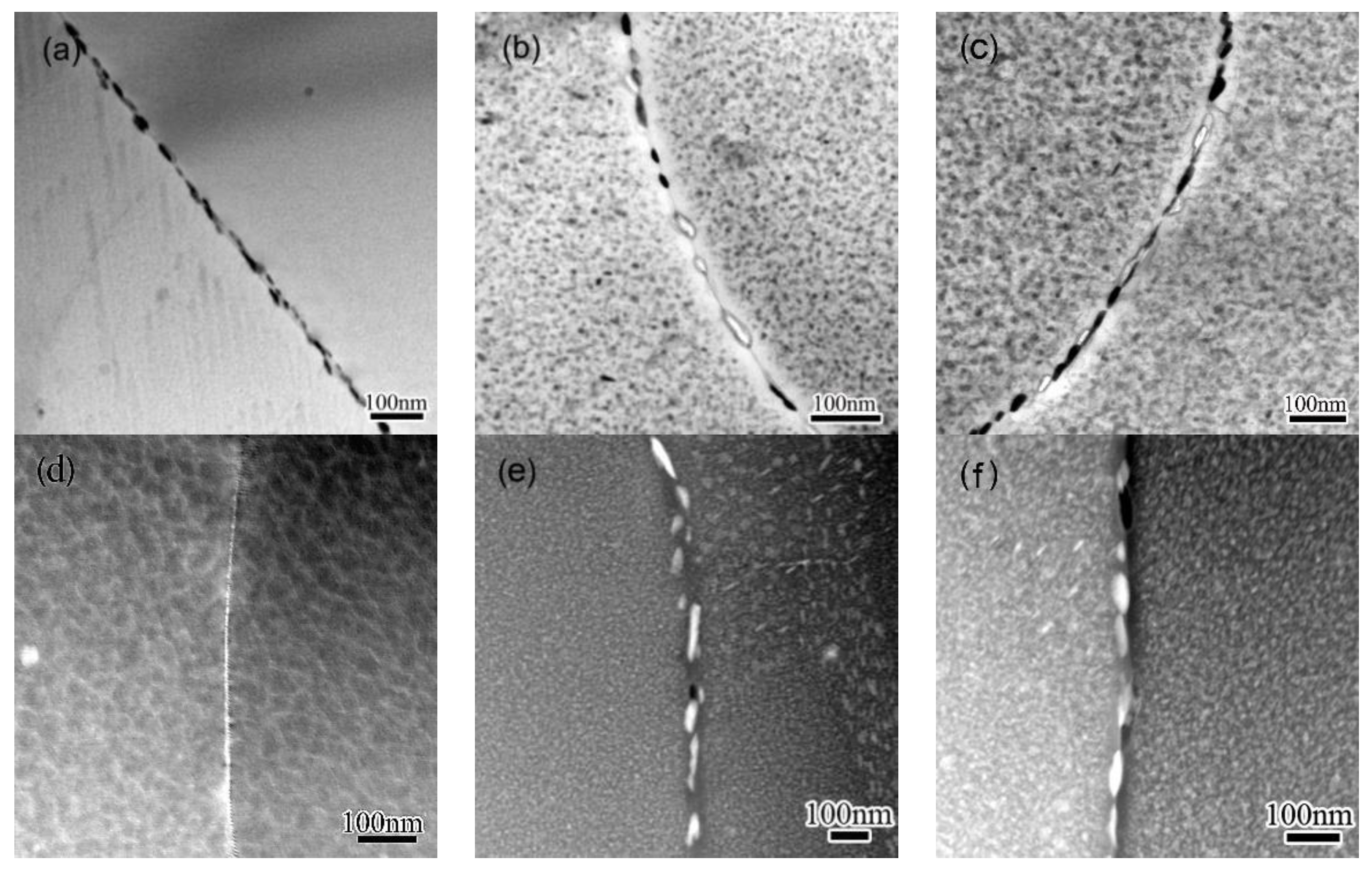
3.1. Precipitation on the Matrix
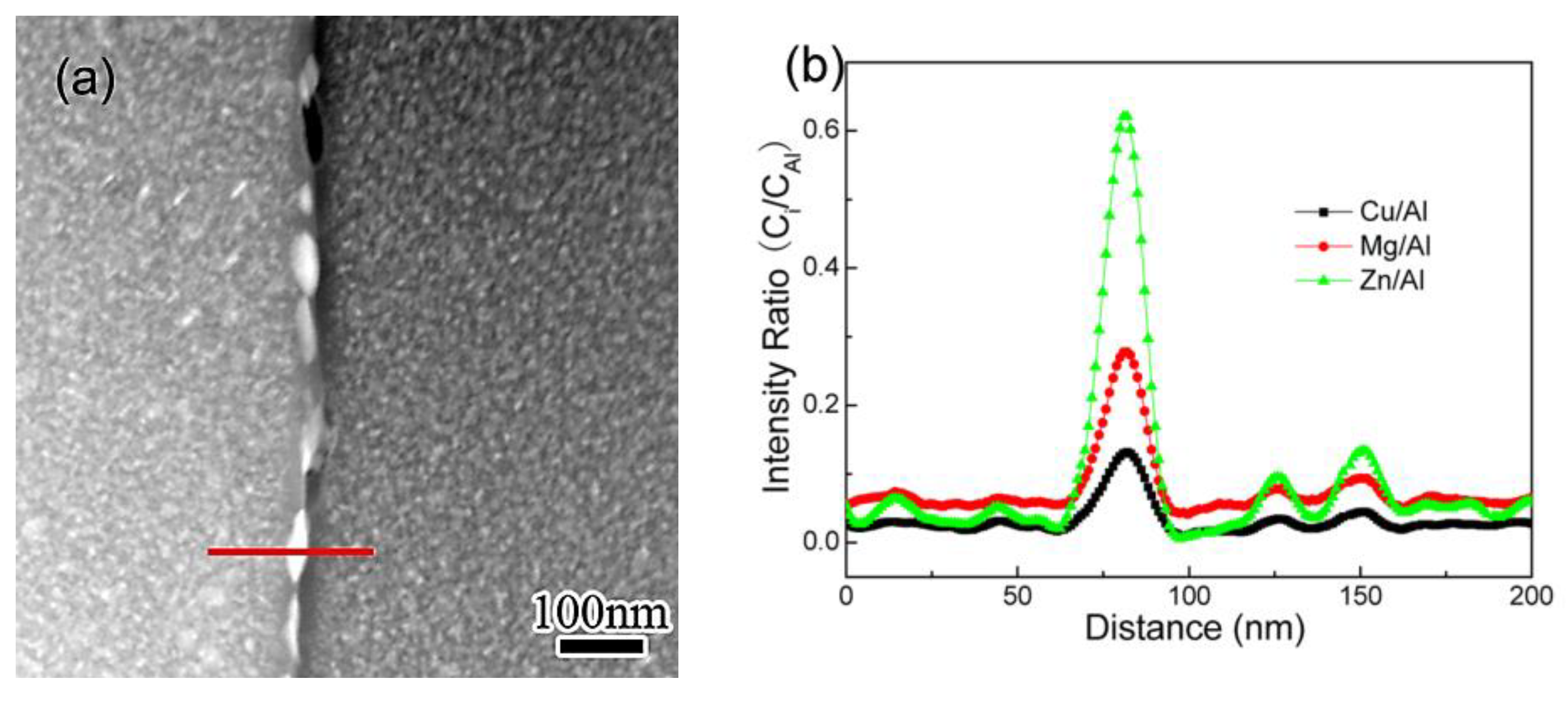
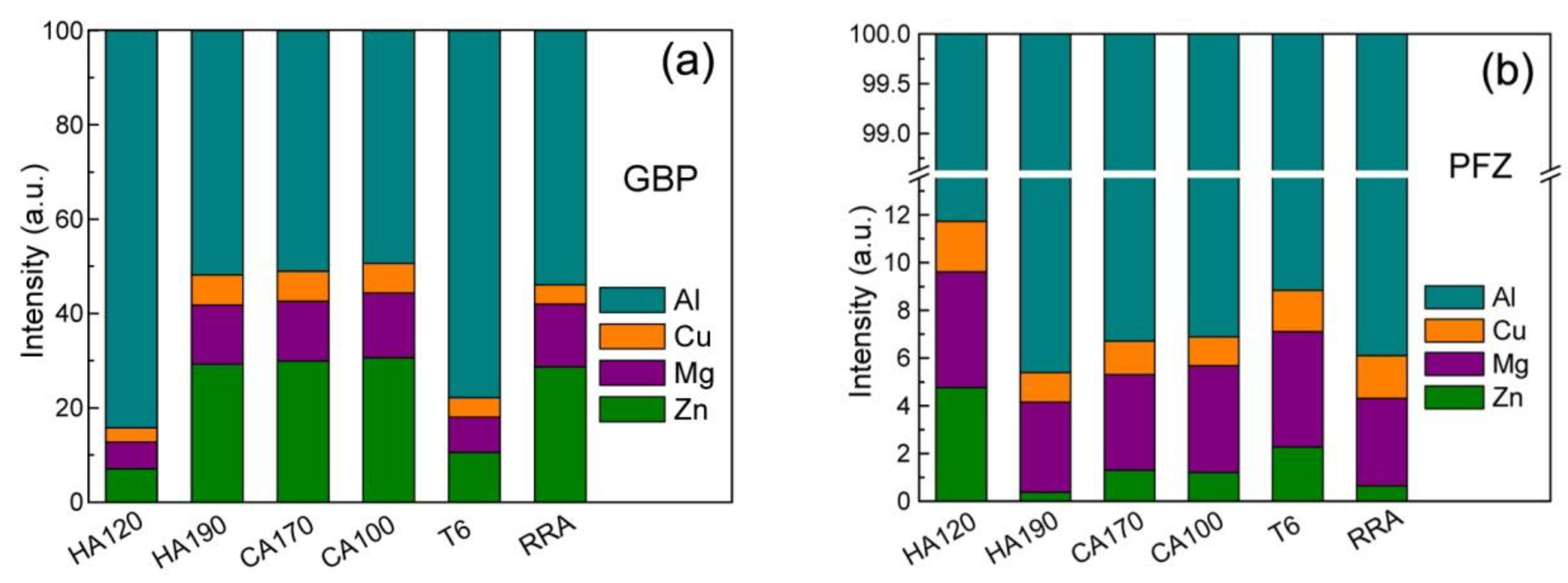
3.2. EDS Analysis
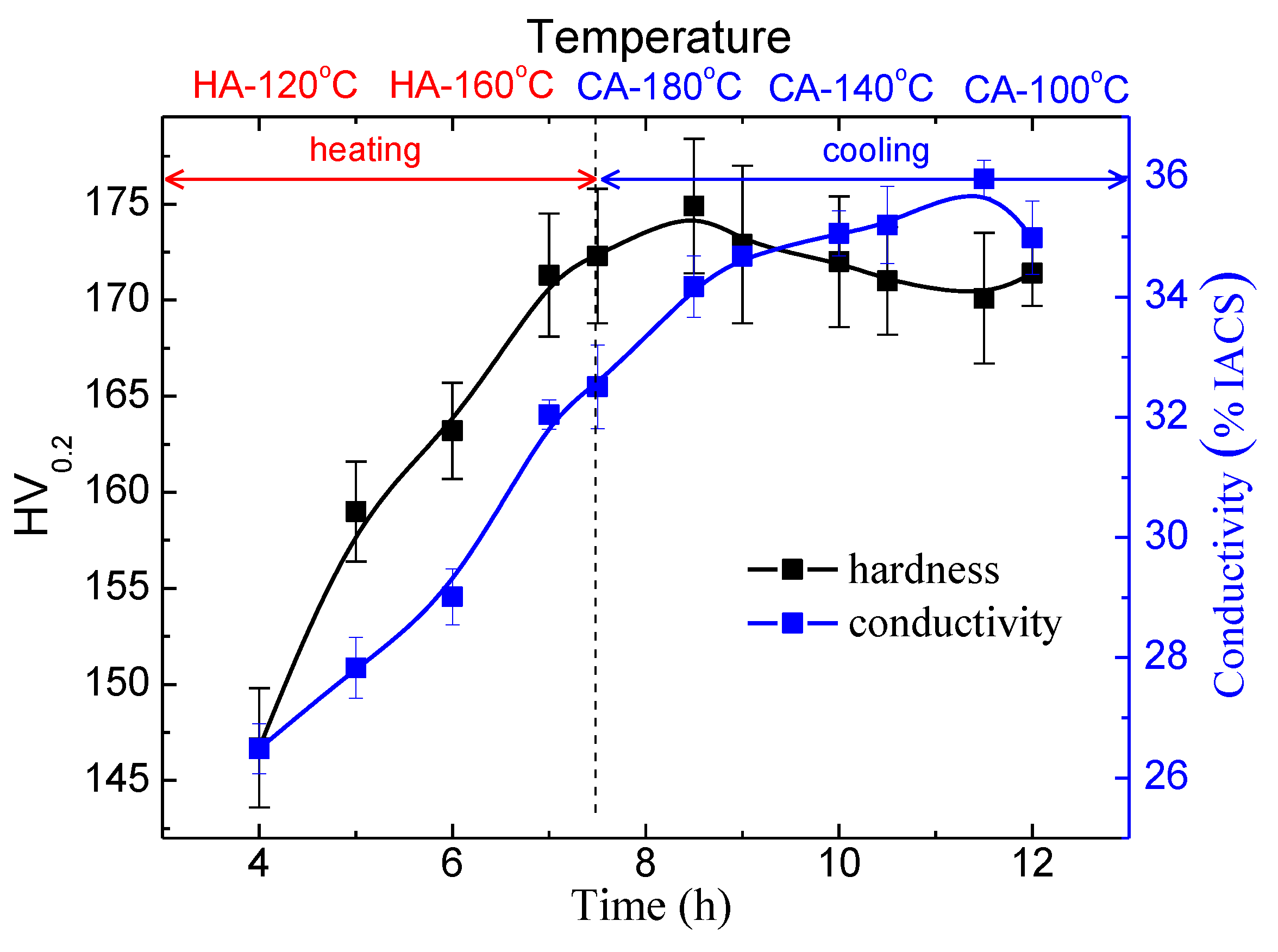
3.3. The Ageing Hardening
3.4. Localized Corrosion Behavior
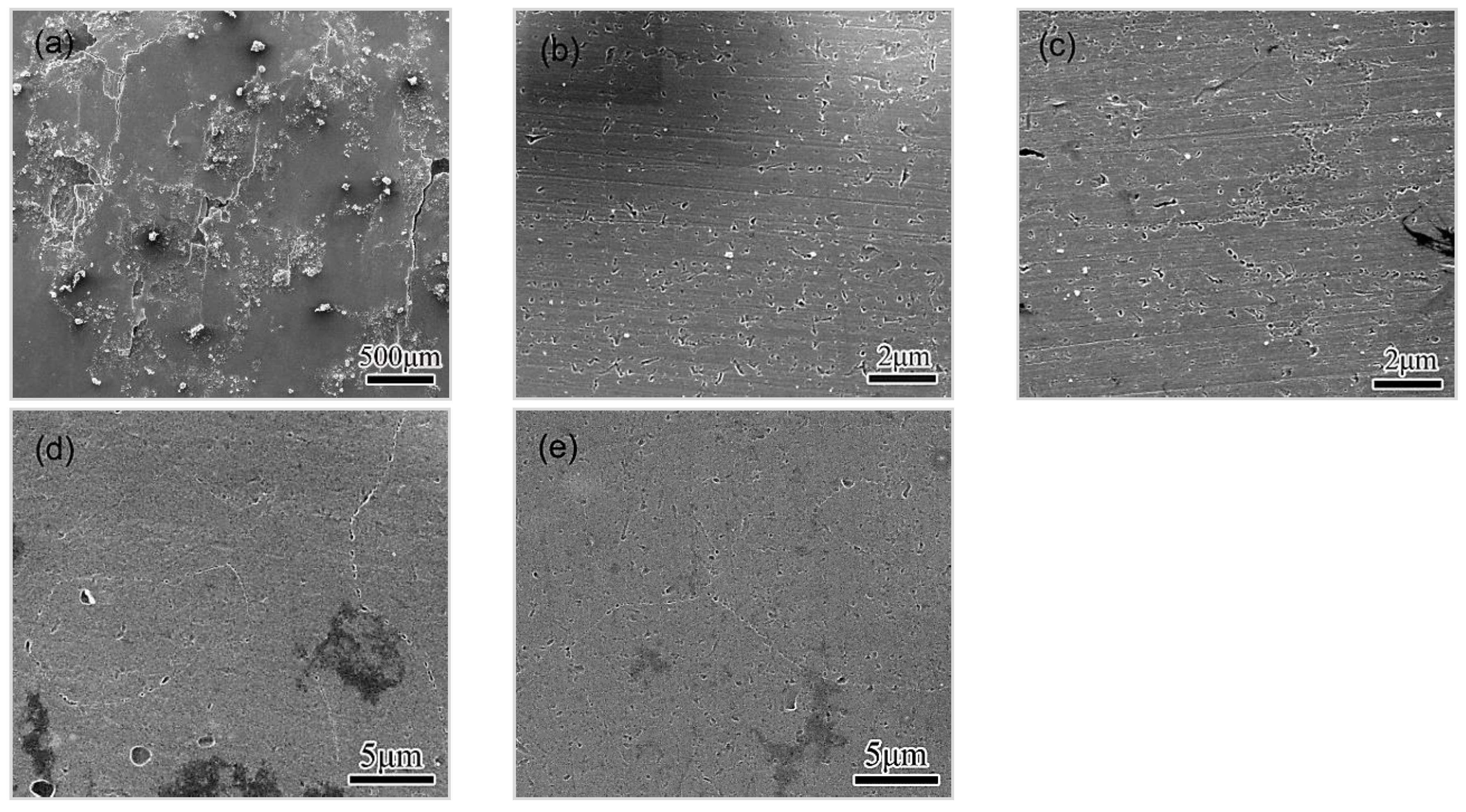
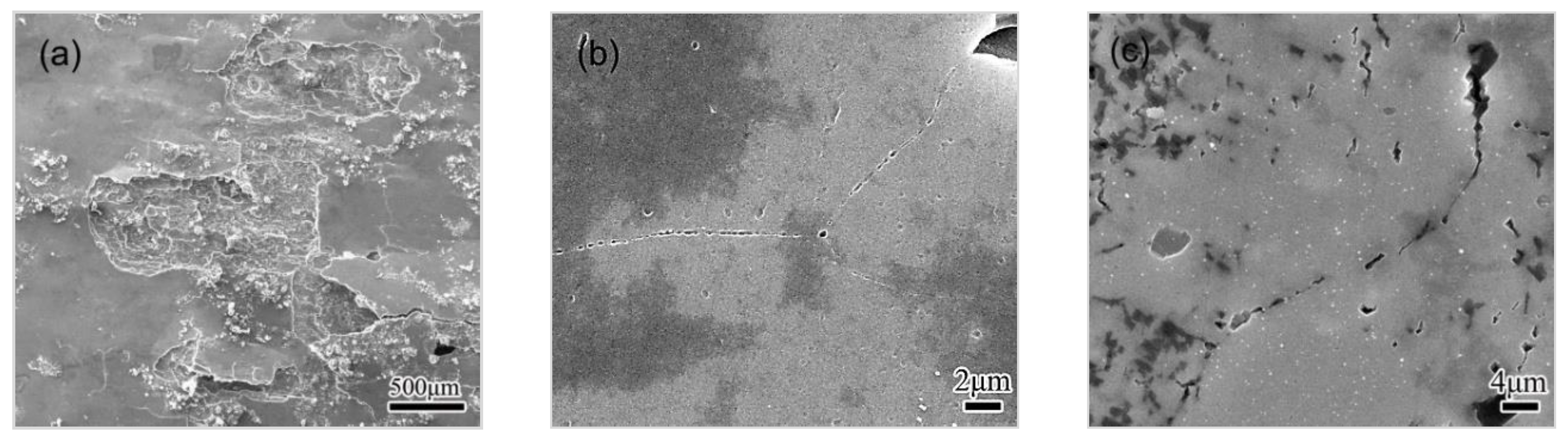
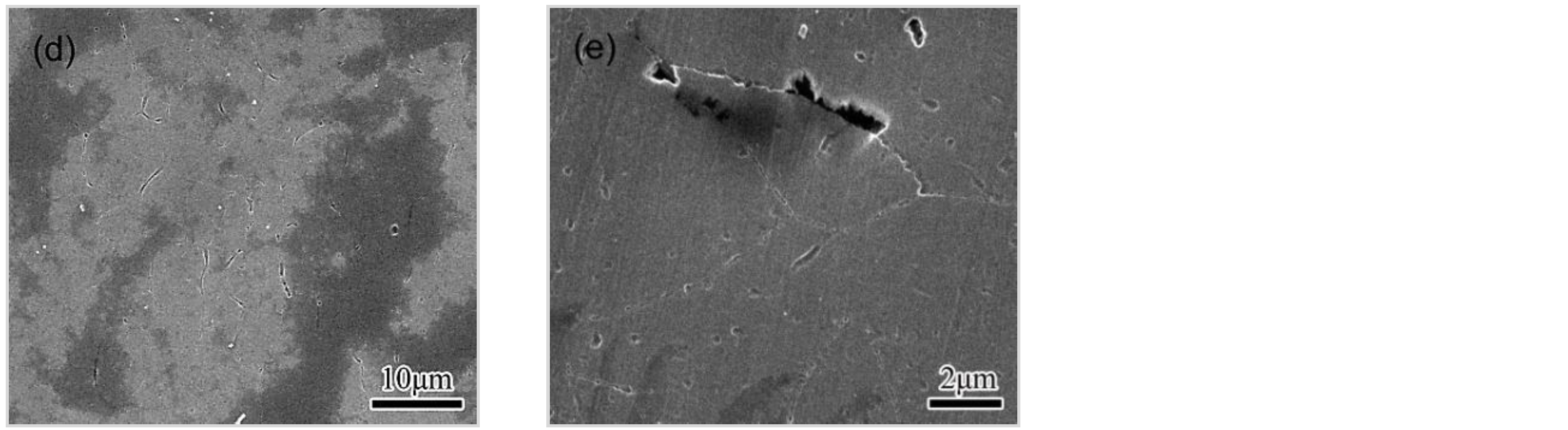
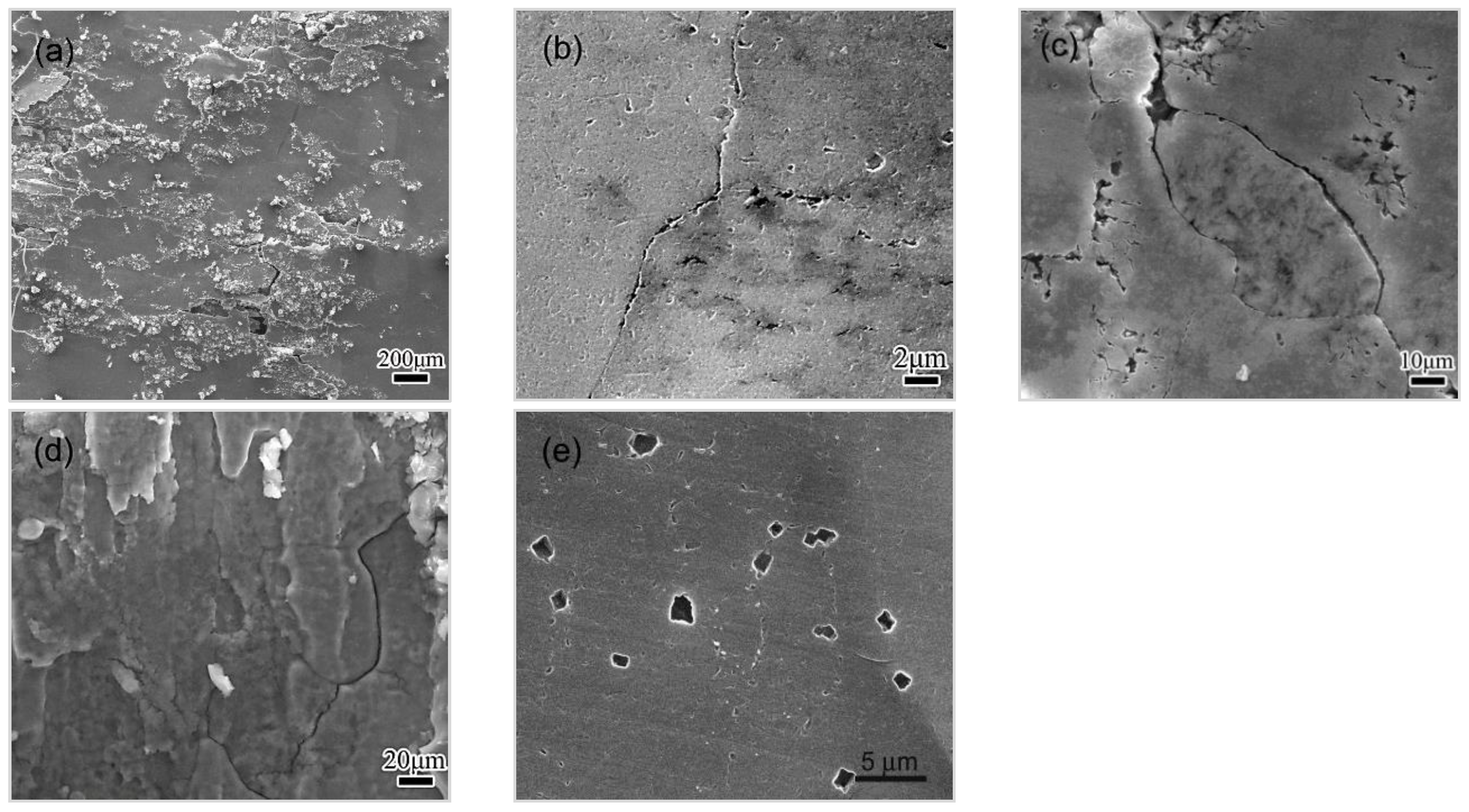
3.4.1. Observations of the LC Process
3.4.2. Effect of Microstructure and Microchemistry on Intergranular Corrosion
4. Conclusions
- (1)
- The precipitation as well as the coarsening develops very quickly when specimens are exposed to an increasing temperature. A secondary precipitation occurs when a cooling procedure is introduced, leading to increased number density of precipitates. The secondary precipitation on GBs contributes to the linking-up of primary GBPs.
- (2)
- A peaked hardness comparable to that of RRA condition is obtained when heated to 190 °C. The secondary precipitation contributes to the increase in hardness at the terminal stage, although a slight coarsening of primary precipitates occurs.
- (3)
- The solutes’ enrichment in GBPs, as well as the depletion in PFZs, develops as the NIA proceeds, and reaches a peak level when the heating procedure ends. The cooling does not influence the enrichment in GBPs evidently, but weakens the depletion of Zn in PFZ.
- (4)
- Enrichment of solutes in GBPs, as well as the depletion in PFZs, is believed decrease the pitting’s preference for GBs. GBPs that continuously distribute along GBs, on the other hand, accelerate the pitting–IGC transition.
- (5)
- The NIA process endues strength and LC resistance comparable to that of RRA condition, and simultaneously, accommodates non-isothermal procedures. It is then a promising method to age large components of aluminum alloys properly.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Starke Jr, E.A.; Staley, J.T. Application of modern aluminum alloys to aircraft. Prog. Aerosp. Sci. 1996, 2, 131–172. [Google Scholar] [CrossRef]
- Staley, J.T.; Liu, J.; Warren, H. Aluminum alloys for aerostructures. Adv. Mater. Processes 1997, 152, 17–20. [Google Scholar]
- Williams, J.C.; Starke, E.A., Jr. Progress in structural materials for aerospace systems. Acta Mater. 2003, 51, 5775–5799. [Google Scholar] [CrossRef]
- Woodward, R. Developments in Aluminum Alloys. Mater. Des. 1989, 10, 248–254. [Google Scholar] [CrossRef]
- Liu, J.; Kulak, M. A new paradigm in the design of aluminum alloys for aerospace applications. Mater. Sci. Forum 2000, 331–337, 127–140. [Google Scholar] [CrossRef]
- Warner, T. Recently-developed aluminum solutions for aerospace applications. Mater. Sci. Forum 2006, 519–521, 1271–1278. [Google Scholar] [CrossRef]
- Heinz, A.; Haszler, A.; Keidel, C.; Moldenhauer, S.; Benedictus, R.; Miller, W.S. Recent development in aluminum alloys for aerospace applications. Mater. Sci. Eng. A 2000, 280, 102–107. [Google Scholar] [CrossRef]
- Marlaud, T.; Malki, B.; Henon, C.; Deschamps, A.; Baroux, B. Relationship between alloy composition, microstructure and exfoliation corrosion in Al-Zn-Mg-Cu alloys. Corros. Sci. 2011, 53, 3139–3149. [Google Scholar] [CrossRef]
- Xiao, Y.P.; Pan, Q.L.; Li, W.B.; Liu, X.Y.; He, Y.B. Exfoliation corrosion of Al-Zn-Mg-Cu-Zr alloy containing Sc examined by electrochemical impedance spectroscopy. Mater. Corros. 2012, 63, 421–430. [Google Scholar] [CrossRef]
- Andreatta, F.; Terryn, H.; de Wit, J.H.W. Effect of solution heat treatment on galvanic coupling between intermetallics and matrix in AA7075-T6. Corros. Sci. 2003, 45, 1733–1746. [Google Scholar] [CrossRef]
- Andreatta, F.; Terryn, H.; de Wit, J.H.W. Corrosion behaviour of different tempers of AA7075 aluminum alloy. Electrochim. Acta 2004, 49, 2851–2862. [Google Scholar] [CrossRef]
- Meng, Q.; Frankel, G.S. Effect of Cu content on corrosion behavior of 7xxx series aluminum alloys. J. Electrochem. Soc. 2004, 151, B271–B283. [Google Scholar] [CrossRef]
- Ramgopal, T.; Gouma, P.I.; Frankel, G.S. Role of grain-boundary precipitates and solute-depleted zone on the intergranular corrosion of aluminum alloy 7150. Corrosion 2002, 58, 687–697. [Google Scholar] [CrossRef]
- Harrison, T.J.; Crawford, B.R.; Loader, C.; Clark, G.; Brandt, M. Predicting the likely causes of early crack initiation for extruded aircraft components containing intergranular corrosion. Int. J. Fatigue 2016, 82, 700–707. [Google Scholar] [CrossRef]
- Liao, M.; Renaud, G.; Bellinger, N. Fatigue modeling for aircraft structures containing natural exfoliation corrosion. Int. J. Fatigue 2007, 29, 677–686. [Google Scholar] [CrossRef]
- Burns, J.T.; Kim, S.; Gangloff, R.P. Effect of corrosion severity on fatigue evolution in Al-Zn-Mg-Cu. Corros. Sci. 2010, 52, 498–508. [Google Scholar] [CrossRef]
- Deng, Y.; Yin, Z.M.; Zhao, K.; Duan, J.Q.; Hu, J.; He, Z.B. Effects of Sc and Zr microalloying additions and aging time at 120 degrees C on the corrosion behaviour of an Al-Zn-Mg alloy. Corros. Sci. 2012, 65, 288–298. [Google Scholar] [CrossRef]
- Meng, C.Y.; Zhang, D.; Zhuang, L.Z.; Zhang, J.S. Correlations between stress corrosion cracking, grain boundary precipitates and Zn content of Al-Mg-Zn alloys’. J. Alloys Compd. 2016, 655, 178–187. [Google Scholar] [CrossRef]
- Fang, H.C.; Chao, H.; Chen, K.H. Effect of recrystallization on intergranular fracture and corrosion of Al-Zn-Mg-Cu-Zr alloy. J. Alloys Compd. 2015, 622, 166–173. [Google Scholar] [CrossRef]
- Liu, X.Y.; Li, M.J.; Gao, F.; Liang, S.X.; Zhang, X.L.; Cui, H.X. Effects of aging treatment on the intergranular corrosion behavior of Al-Cu-Mg-Ag alloy. J. Alloys Compd. 2015, 639, 263–267. [Google Scholar] [CrossRef]
- Baydogan, M.; Cimenoglu, H.; Kayali, E.S.; Rasty, J. Improved resistance to stress-corrosion-cracking failures via optimized retrogression and reaging of 7075-T6 aluminum sheets. Metall. Trans. A 2008, 39, 2470–2476. [Google Scholar] [CrossRef]
- Knight, S.P.; Birbilis, N.; Muddle, B.C.; Trueman, A.R.; Lynch, S.P. Correlations between intergranular stress corrosion cracking, grain-boundary microchemistry, and grain-boundary electrochemistry for Al-Zn-Mg-Cu alloys. Corros. Sci. 2010, 52, 4073–4080. [Google Scholar] [CrossRef]
- Oliveira, A.F., Jr.; de Barros, M.C.; Cardoso, K.R.; Travessa, D.N. The effect of RRA on the strength and SCC resistance on AA7050 and AA7150 aluminium alloys. Mater. Sci. Eng. A 2004, 379, 321–326. [Google Scholar]
- Chen, J.F.; Zhang, X.F.; Zou, L.C.; Yu, Y.; Li, Q. Effect of precipitate state on the stress corrosion behavior of 7050 aluminum alloy. Mater. Charact. 2016, 114, 1–8. [Google Scholar] [CrossRef]
- Peng, G.S.; Chen, K.H.; Chen, S.Y.; Fang, H.C. Influence of dual-RRA temper on the exfoliation corrosion and electrochemical behavior of Al-Zn-Mg-Cu alloy. Mater. Corros. 2013, 64, 284–289. [Google Scholar] [CrossRef]
- Buha, J.; Lumley, R.N.; Crosky, A.G.; Hono, K. Secondary precipitation in an Al-Mg-Si-Cu alloy. Acta Mater. 2007, 55, 3015–3024. [Google Scholar] [CrossRef]
- Buha, J.; Lumley, R.N.; Crosky, A.G. Secondary ageing in an aluminium alloy 7050. Mater. Sci. Eng. A 2008, 492, 1–10. [Google Scholar] [CrossRef]
- Guyot, P.; Cottignies, L. Precipitation kinetics, mechanical strength and electrical conductivity of AlZnMgCu alloys. Acta Mater. 1996, 44, 4161–4167. [Google Scholar] [CrossRef]
- Li, J.F.; Jia, Z.Q.; Li, C.X.; Birbilis, N.; Cai, C. Exfoliation corrosion of 7150 Al alloy with various tempers and its electrochemical impedance spectroscopy in EXCO solution. Mater. Corros. 2009, 60, 407–414. [Google Scholar] [CrossRef]
- Polmear, I.J. A century of age hardening. Mater. Forum 2004, 28, 1–14. [Google Scholar]
- Nicolas, M.N.; Deschamps, A. Characterisation and modelling of precipitate evolution in an Al-Zn-Mg alloy during non-isothermal heat treatments. Acta Mater. 2003, 51, 6077–6094. [Google Scholar] [CrossRef]
- Hutchinson, C.R.; Gouné, M.G.; Redjaimia, A. Selecting non-isothermal heat treatment schedules for precipitation hardening systems: An example of coupled process-property optimization. Acta Mater. 2007, 55, 213–223. [Google Scholar] [CrossRef]
- Staley, J.T. Precipitation Hardenable Alloy, e.g., Seven Thousand Series Aluminum Alloy, Aging Involves Heating Alloy at Continuous Rate of Increasing Temperatures Time to Age the Alloy. U.S. Patent 2007267113-A1, 22 November 2007. [Google Scholar]
- Jiang, J.T.; Tang, Q.J.; Yang, L.; Zhang, K.; Yuan, S.J.; Zhen, L. Non-isothermal ageing of an Al-8Zn-2Mg-2Cu alloy for enhanced properties. J. Mater. Process. Technol. 2016, 225, 110–116. [Google Scholar] [CrossRef]
- Jiang, J.T.; Xiao, W.Q.; Yang, L.; Shao, W.Z.; Yuan, S.J.; Zhen, L. Ageing behavior and stress corrosion cracking resistance of a non-isothermally aged Al-Zn-Mg-Cu alloy. Mater. Sci. Eng. A 2014, 605, 167–175. [Google Scholar] [CrossRef]
- Li, J.F.; Zheng, Z.Q.; Li, S.C.; Chen, W.J.; Ren, W.D.; Zhao, X.S. Simulation study on function mechanism of some precipitates in localized corrosion of Al alloys. Corros. Sci. 2007, 49, 2436–2449. [Google Scholar] [CrossRef]
- Li, H.Z.; Yao, S.C.; Liang, X.P.; Chen, Y.H.; Liu, C.; Huang, L. Grain boundary pre-precipitation and its contribution to enhancement of corrosion resistance of Al-Zn-Mg alloy. Trans. Nonferrous Met. Soc. China 2016, 26, 2523–2531. [Google Scholar] [CrossRef]
- Wang, Z.T.; Tian, R.Z. Handbook of Aluminum Alloy and Its Working; Central South University Press: Changsha, China, 2000. (In Chinese) [Google Scholar]
- Maitra, S.; English, G. Environmental factors affecting localized corrosion of 7075-T7351 aluminum alloy plate. Metall. Trans. A 1982, 13, 161–166. [Google Scholar] [CrossRef]
- Chen, S.Y.; Chen, K.H.; Peng, G.S.; Jia, L.; Dong, P.X. Effect of heat treatment on strength, exfoliation corrosion and electrochemical behavior of 7085 aluminum alloy. Mater. Des. 2012, 35, 93–98. [Google Scholar] [CrossRef]
- Marlaud, T.; Deschamps, A.; Bley, F.; Lefebvre, W.; Baroux, B. Influence of alloy composition and heat treatment on precipitate composition in Al-Zn-Mg-Cu alloys. Acta Mater. 2010, 58, 248–260. [Google Scholar] [CrossRef]
- Kannan, M.B.; Raja, V. Influence of heat treatment and scandium addition on the electrochemical polarization behavior of Al-Zn-Mg-Cu-Zr alloy. Metall. Trans. A 2007, 38, 2843–2852. [Google Scholar] [CrossRef]
- ASTM G34-01 (Reapproved 2013). Standard Test Method for Exfoliation Corrosion Susceptibility in 2XXX and 7XXX Series Aluminum Alloys (EXCO Test); ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-Z.; Li, G.-A.; Cai, X.; Jiang, J.-T.; Shao, W.-Z.; Yang, L.; Zhen, L. Microstructure Evolution and the Resulted Influence on Localized Corrosion in Al-Zn-Mg-Cu Alloy during Non-Isothermal Ageing. Materials 2018, 11, 720. https://doi.org/10.3390/ma11050720
Chen J-Z, Li G-A, Cai X, Jiang J-T, Shao W-Z, Yang L, Zhen L. Microstructure Evolution and the Resulted Influence on Localized Corrosion in Al-Zn-Mg-Cu Alloy during Non-Isothermal Ageing. Materials. 2018; 11(5):720. https://doi.org/10.3390/ma11050720
Chicago/Turabian StyleChen, Jun-Zhou, Guo-Ai Li, Xin Cai, Jian-Tang Jiang, Wen-Zhu Shao, Li Yang, and Liang Zhen. 2018. "Microstructure Evolution and the Resulted Influence on Localized Corrosion in Al-Zn-Mg-Cu Alloy during Non-Isothermal Ageing" Materials 11, no. 5: 720. https://doi.org/10.3390/ma11050720
APA StyleChen, J.-Z., Li, G.-A., Cai, X., Jiang, J.-T., Shao, W.-Z., Yang, L., & Zhen, L. (2018). Microstructure Evolution and the Resulted Influence on Localized Corrosion in Al-Zn-Mg-Cu Alloy during Non-Isothermal Ageing. Materials, 11(5), 720. https://doi.org/10.3390/ma11050720



