A Feasible Strategy for Fabricating Surface Porous Network in Fe-Si Ribbons
Abstract
:1. Introduction
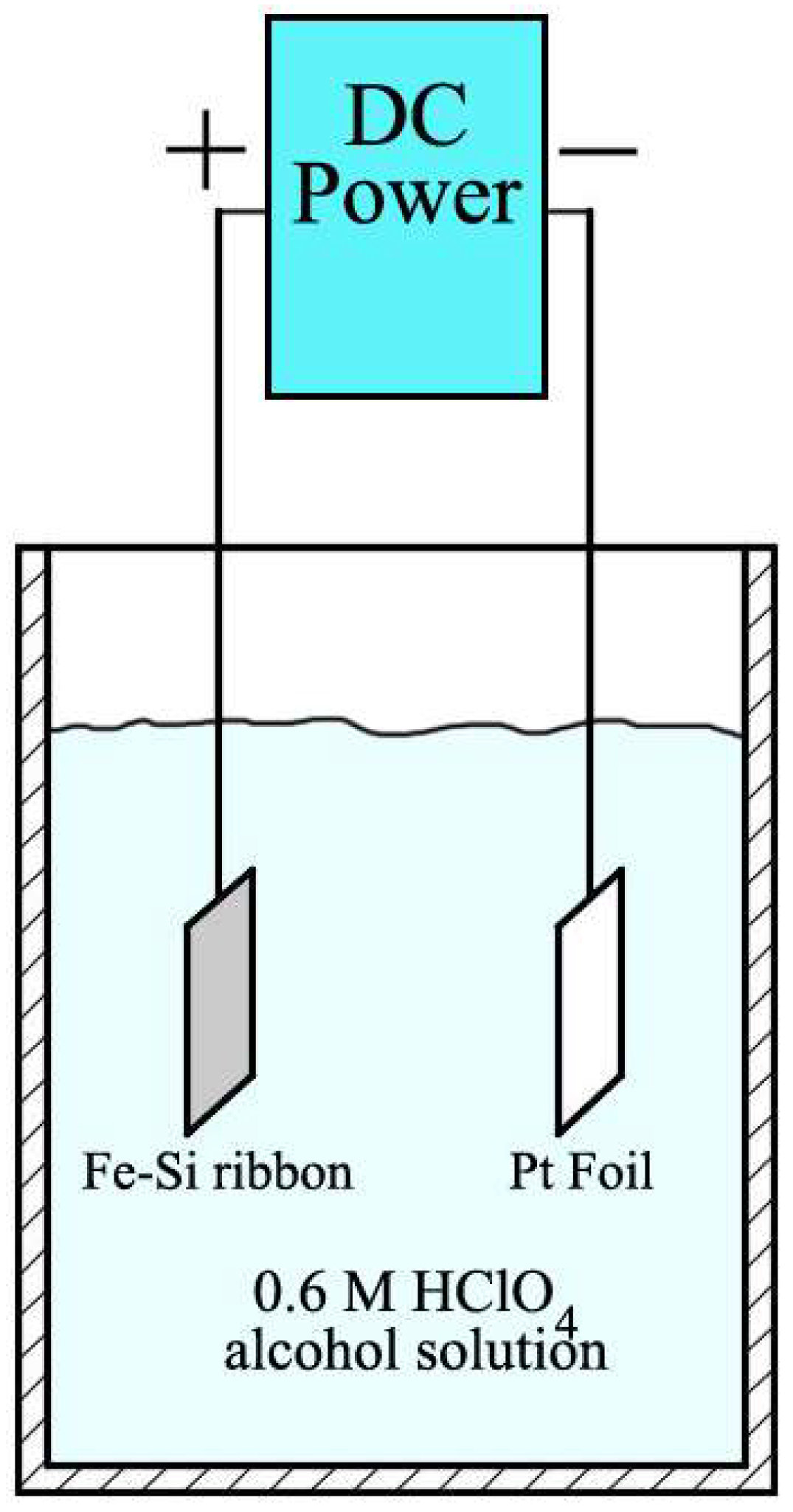
2. Materials and Methods
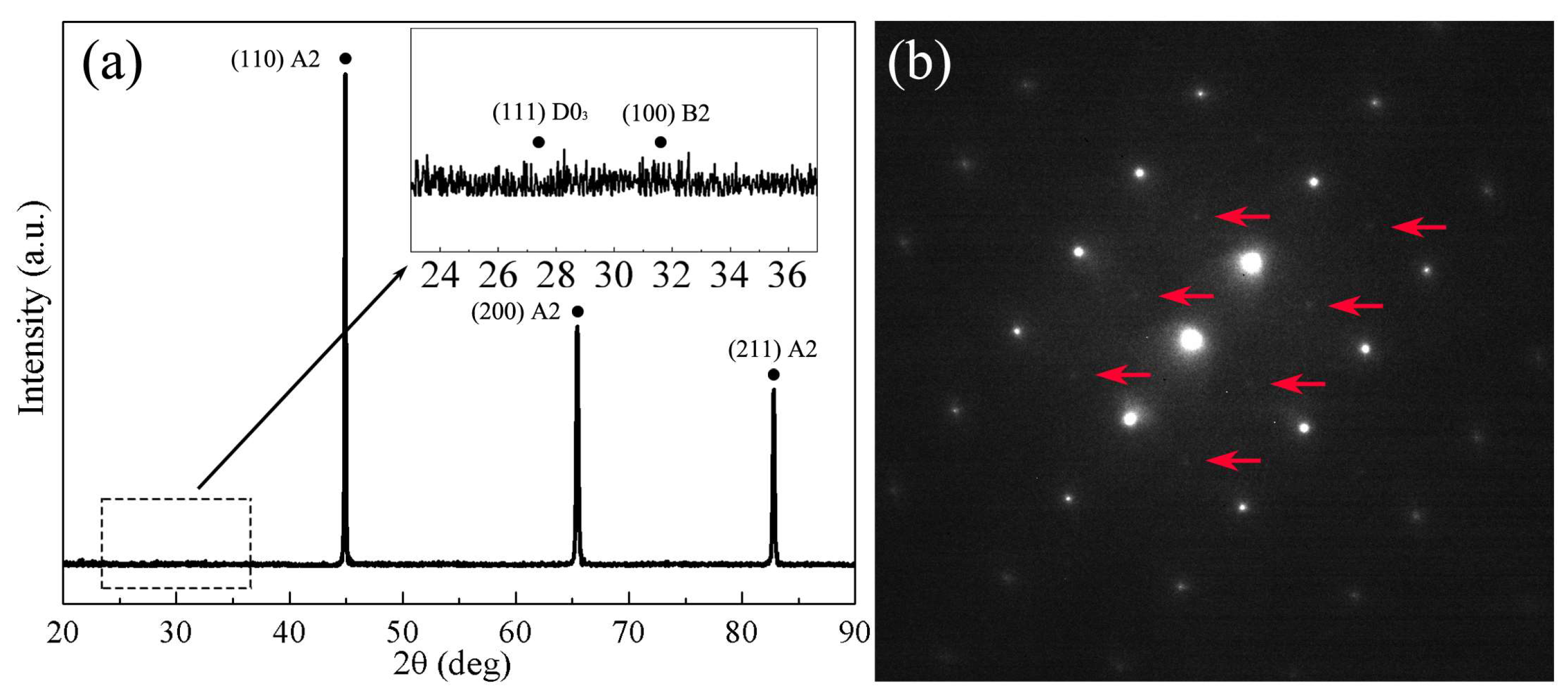
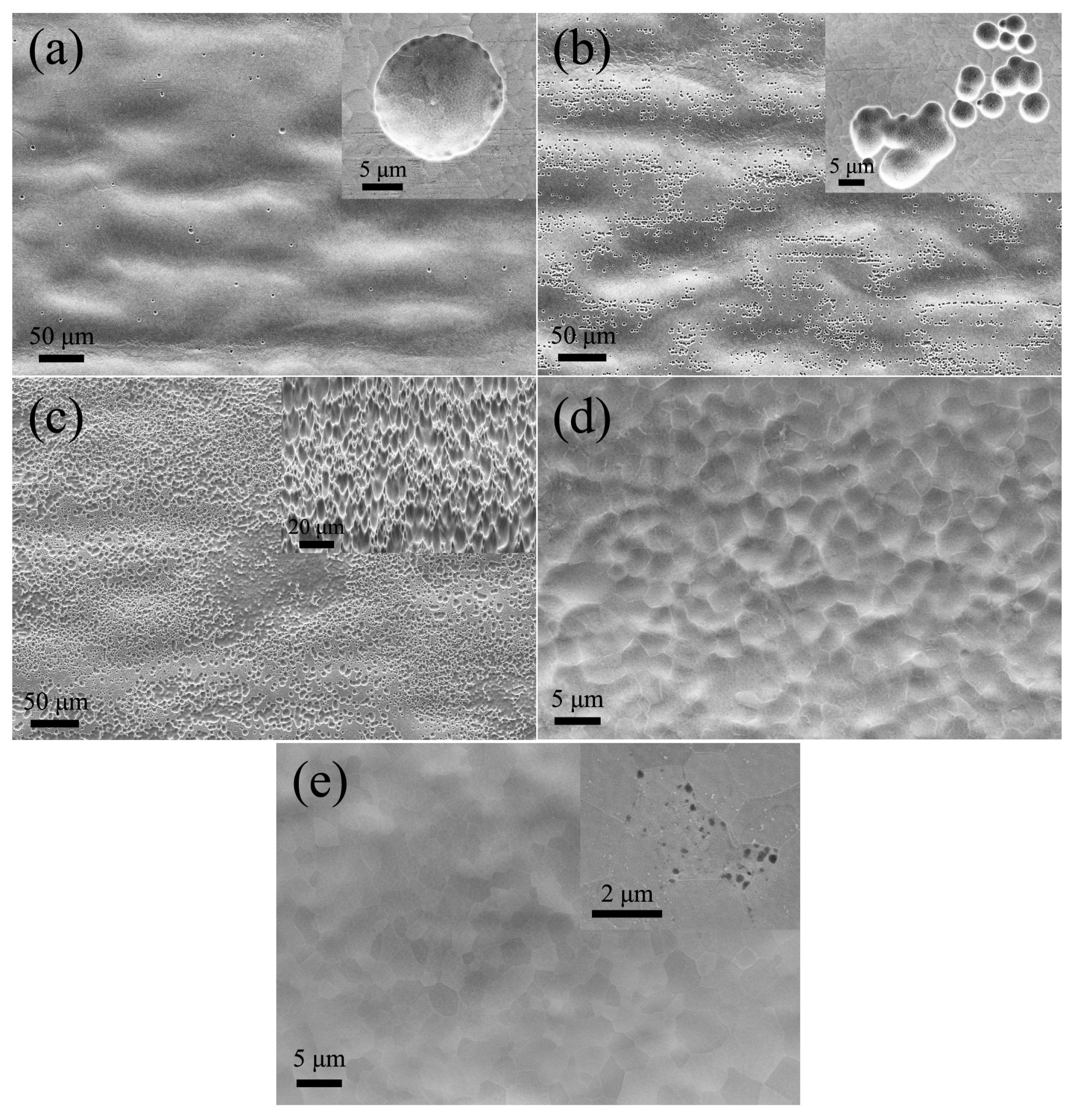
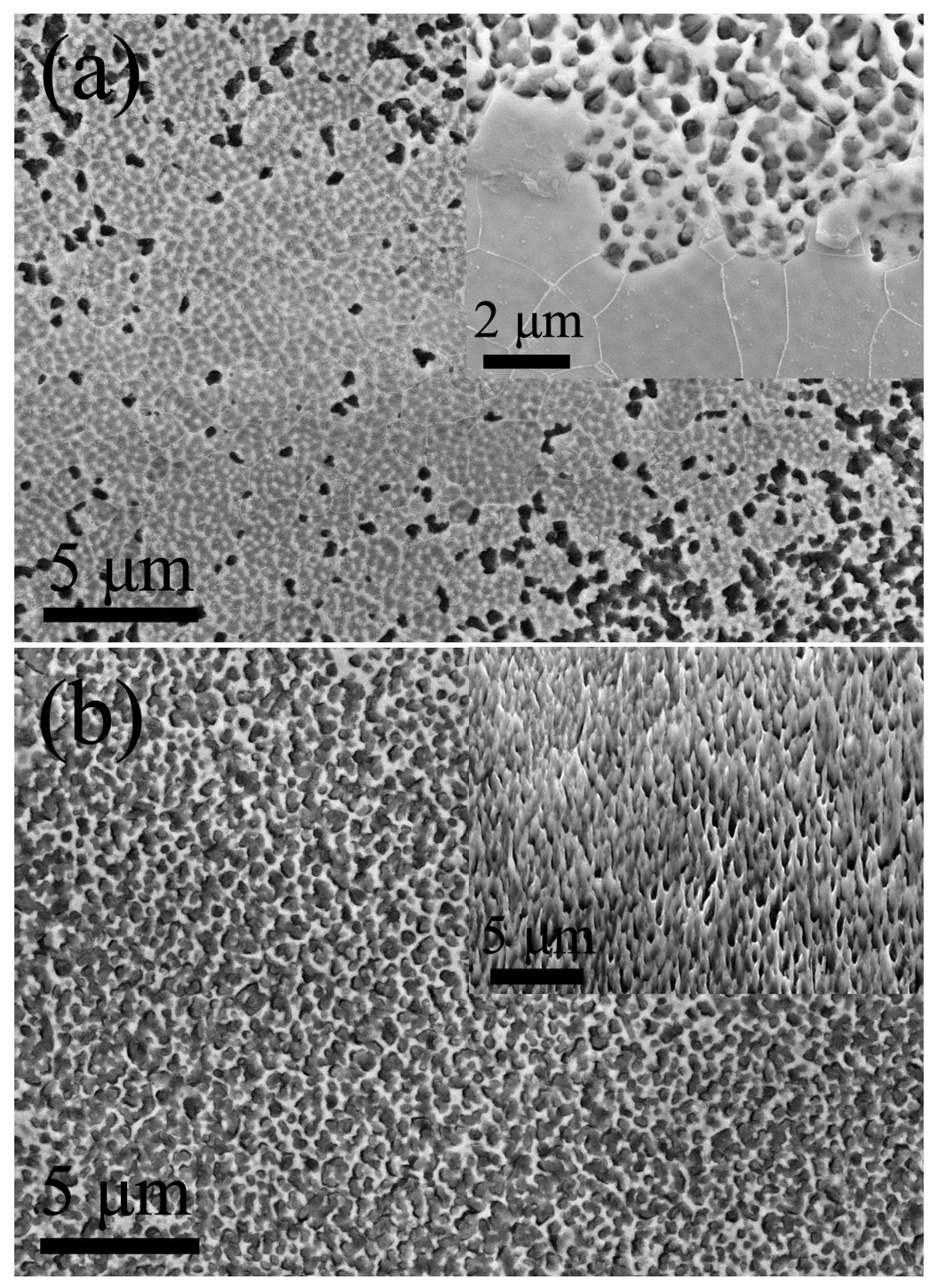
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Biener, J.; Nyce, G.W.; Hodge, A.M.; Biener, M.M.; Hamza, A.V.; Maier, S.A. Nanoporous plasmonic metamaterials. Adv. Mater. 2008, 20, 1211–1217. [Google Scholar] [CrossRef]
- Wittstock, A.; Biener, J.; Bäumer, M. Nanoporous gold: A new material for catalytic and sensor applications. Phys. Chem. Chem. Phys. 2010, 12, 12919–12930. [Google Scholar] [CrossRef] [PubMed]
- Minakshi, M.; Barmi, M.J.; Jones, R.T. Rescaling metal molybdate nanostructures with biopolymer for energy storage having high capacitance with robust cycle stability. Dalton Trans. 2017, 46, 3588–3600. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Chen, M.; Erlebacher, J. Metallic mesoporous nanocomposites for electrocatalysis. J. Am. Chem. Soc. 2004, 126, 6876–6877. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhao, P.; Li, Y. General preparation for Pt-based alloy nanoporous nanoparticles as potential nanocatalysts. Sci. Rep. 2011, 1, 37. [Google Scholar] [CrossRef] [PubMed]
- Petegem, S.V.; Brandstetter, S.; Maass, R.; Hodge, A.M.; El-Dasher, B.S.; Biener, J. r.; Schmitt, B.; Borca, C.; Swygenhoven, H.V. On the microstructure of nanoporous gold: An X-ray diffraction study. Nano Lett. 2009, 9, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, X.; Wang, H.; Wu, Y.; Chu, X.; Lu, Z. Nanoporous silver with tunable pore characteristics and superior surface enhanced Raman scattering. Corros. Sci. 2014, 84, 159–164. [Google Scholar] [CrossRef]
- Chen, L.Y.; Yu, J.S.; Fujita, T.; Chen, M.W. Nanoporous copper with tunable nanoporosity for SERS applications. Adv. Funct. Mater. 2009, 19, 1221–1226. [Google Scholar] [CrossRef]
- Huber, D.L. Synthesis, properties, and applications of iron nanoparticles. Small 2005, 1, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Li, R.; Xu, H.; Chen, X.-B.; Zhang, T. A new strategy to fabricate nanoporous iron-based metallic glasses: Selective phase tailoring of amorphous-nanocrystalline composite alloys through electrochemical dissolution. Scr. Mater. 2017, 133, 14–18. [Google Scholar] [CrossRef]
- Liu, S.; Bian, X.; Liu, J.; Wang, J.; Yu, M.; Yang, Y.; Fan, R. A new preparation method of Fe2B: Generating rodlike/scale-like Fe2B by dealloying Fe78Si9B13 atomized particle. Intermetallics 2018, 94, 186–191. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, S.; Li, H.; Jiang, Y.; Ye, F.; Lin, J. Fabrication of Fe-6.5 wt% Si ribbons by melt spinning method on large scale. Adv. Mater. Sci. Eng. 2015, 2015, 296797. [Google Scholar] [CrossRef]
- Trivedi, R.; Kurz, W. Solidification microstructures: A conceptual approach. Acta Metall. Mater. 1994, 42, 15–23. [Google Scholar] [CrossRef]
- Ludwig, A.; Kurz, W. Direct observation of solidification microstructures around absolute stability. Acta Mater. 1996, 44, 3643–3654. [Google Scholar] [CrossRef]
- Wittig, J.; Frommeyer, G. Deformation and fracture behavior of rapidly solidified and annealed iron-silicon alloys. Metall. Mater. Trans. A 2008, 39, 252–265. [Google Scholar] [CrossRef]
- Chen, L.; Hou, Y.; Kang, J.; Hirata, A.; Fujita, T.; Chen, M. Toward the theoretical capacitance of RuO2 reinforced by highly conductive nanoporous gold. Adv. Energy Mater. 2013, 3, 851–856. [Google Scholar] [CrossRef]
- Li, R.; Liu, X.; Wang, H.; Wu, Y.; Lu, Z. Development of electrochemical supercapacitors with uniform nanoporous silver network. Electrochim. Acta 2015, 182, 224–229. [Google Scholar] [CrossRef]
- Luo, S.; Wu, H.; Wu, Y.; Jiang, K.; Wang, J.; Fan, S. Mn3O4 nanoparticles anchored on continuous carbon nanotube network as superior anodes for lithium ion batteries. J. Power Sources 2014, 249, 463–469. [Google Scholar] [CrossRef]
- Chen, J.S.; Tan, Y.L.; Li, C.M.; Cheah, Y.L.; Luan, D.; Madhavi, S.; Boey, F.Y.C.; Archer, L.A.; Lou, X.W. Constructing hierarchical spheres from large ultrathin anatase TiO2 nanosheets with nearly 100% exposed (001) facets for fast reversible lithium storage. J. Am. Chem. Soc. 2010, 132, 6124–6130. [Google Scholar] [CrossRef] [PubMed]
- Zemtsova, E.G.; Arbenin, A.Y.; Valiev, R.Z.; Orekhov, E.V.; Semenov, V.G.; Smirnov, V.M. Two-level micro-to-nanoscale hierarchical TiO2 nanolayers on titanium surface. Materials 2016, 9, 1010. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Wang, Z.; Hao, Q.; Liu, B.; Xu, C. Facile fabrication of Fe3O4 octahedra/nanoporous copper network composite for high-performance anode in Li-Ion batteries. J. Colloid Interface Sci. 2017, 493, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Minakshi, M. Lithium intercalation into amorphous FePO4 cathode in aqueous solutions. Electrochim. Acta 2010, 55, 9174–9178. [Google Scholar] [CrossRef]
- Yu, B.; He, L.; Wang, Y.; Cong, H. Multifunctional PMMA@ Fe3O4@ DR magnetic materials for efficient adsorption of dyes. Materials 2017, 10, 1239. [Google Scholar] [CrossRef] [PubMed]

| Site | Weight (%) | |
|---|---|---|
| Fe | Si | |
| A1 | 93.27 ± 1.15 | 6.72 ± 0.31 |
| B1 | 93.17 ± 1.28 | 6.82 ± 0.36 |
| A2 | 94.71 ± 2.02 | 5.28 ± 0.44 |
| B2 | 93.87 ± 1.38 | 6.12 ± 0.31 |
| A3 | 94.32 ± 1.22 | 5.67 ± 0.31 |
| B3 | 93.81 ± 1.25 | 6.18 ± 0.34 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Chen, B.; Liang, Y.; Ye, F.; Lin, J. A Feasible Strategy for Fabricating Surface Porous Network in Fe-Si Ribbons. Materials 2018, 11, 701. https://doi.org/10.3390/ma11050701
Wang S, Chen B, Liang Y, Ye F, Lin J. A Feasible Strategy for Fabricating Surface Porous Network in Fe-Si Ribbons. Materials. 2018; 11(5):701. https://doi.org/10.3390/ma11050701
Chicago/Turabian StyleWang, Shuai, Biao Chen, Yongfeng Liang, Feng Ye, and Junpin Lin. 2018. "A Feasible Strategy for Fabricating Surface Porous Network in Fe-Si Ribbons" Materials 11, no. 5: 701. https://doi.org/10.3390/ma11050701





