Fabrication of Metal-Substituted Polyoxometalates for Colorimetric Detection of Dopamine and Ractopamine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Catalysts
2.2. Procedure for the Detection of RAC
2.3. Procedure for the Detection of DA
2.4. Other Analytical Measurements
3. Results
3.1. Characterization of Catalysts
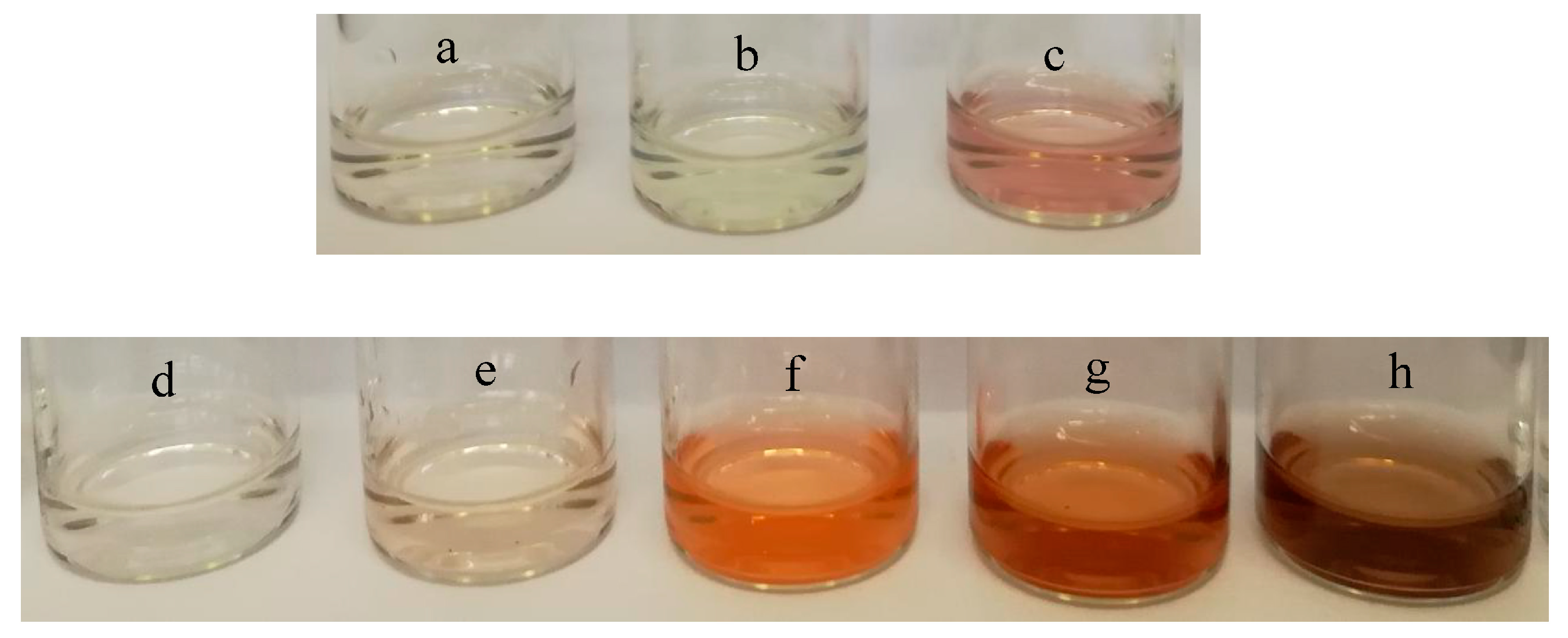
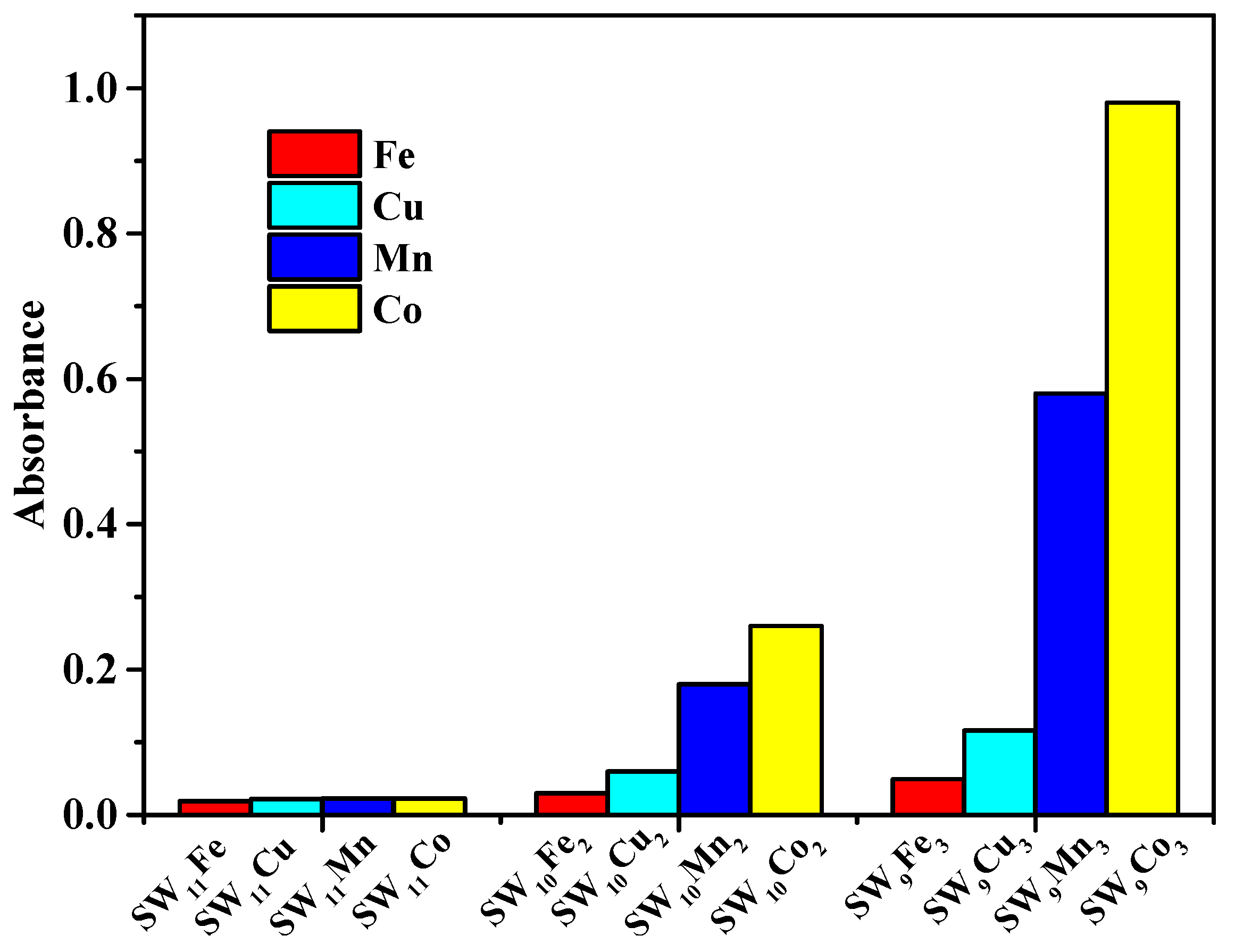
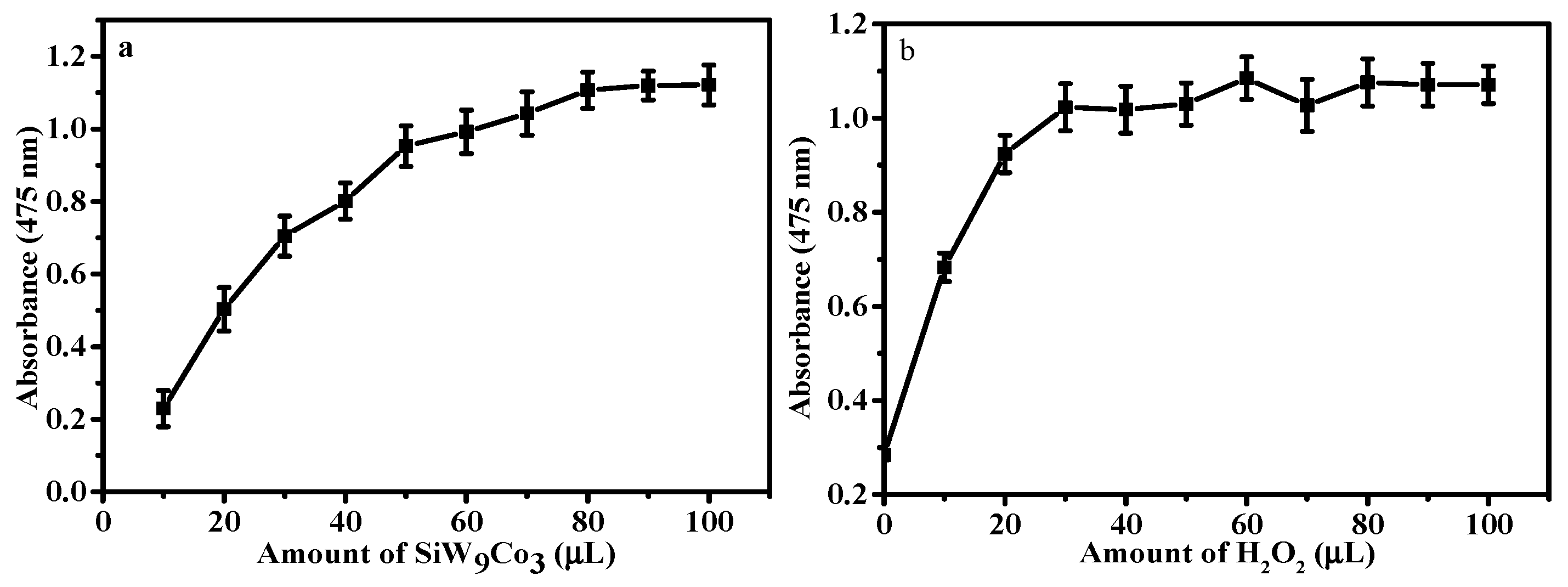
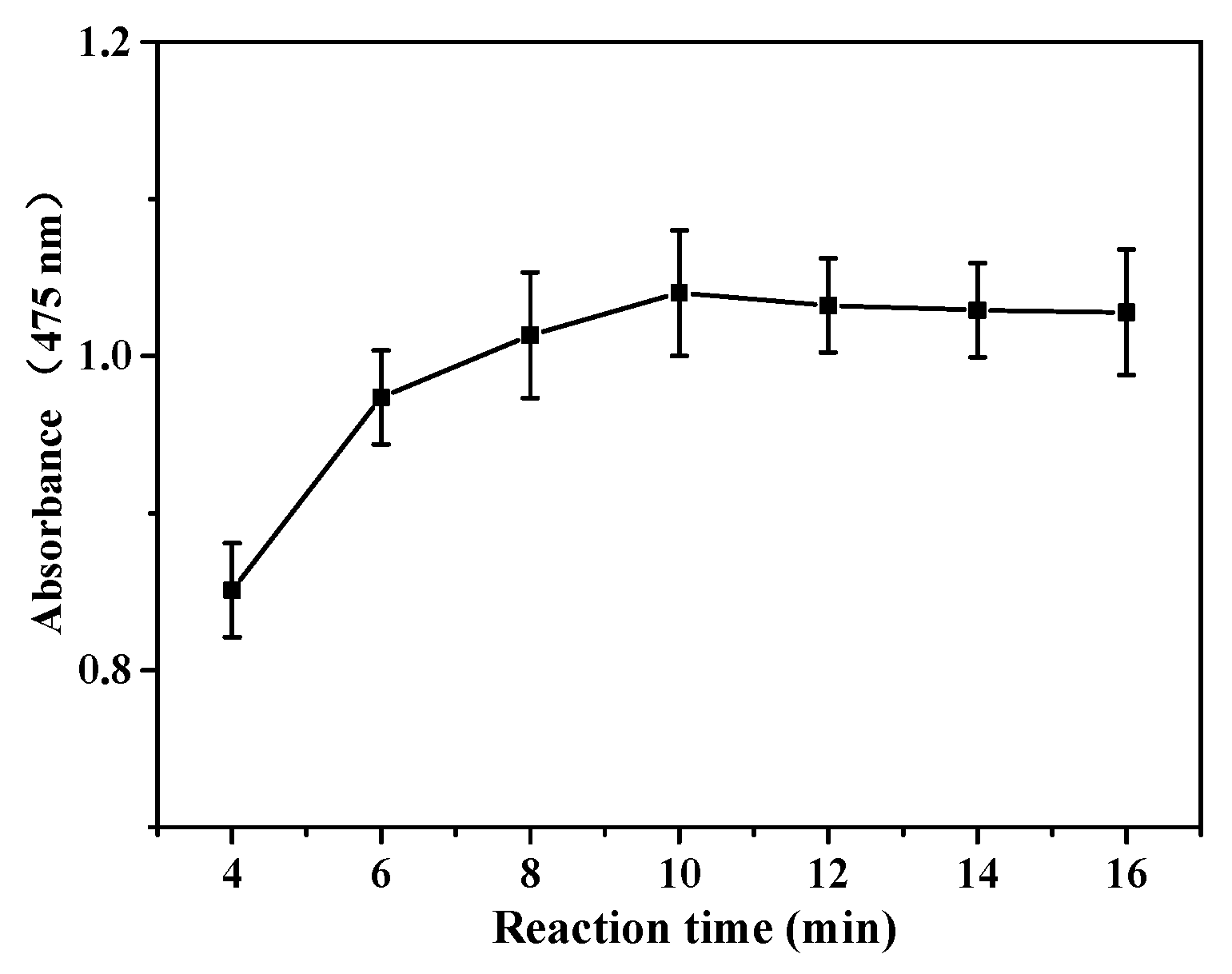
3.2. Catalytic Activity of POM Catalysts
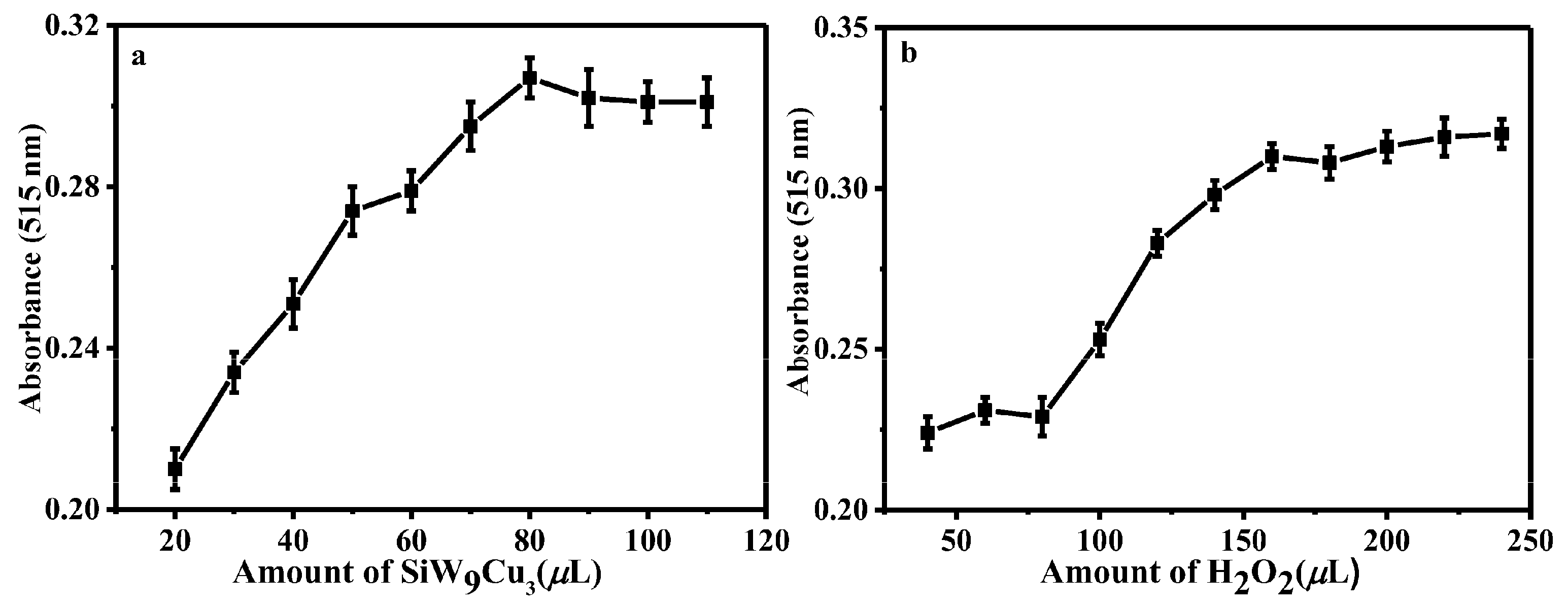
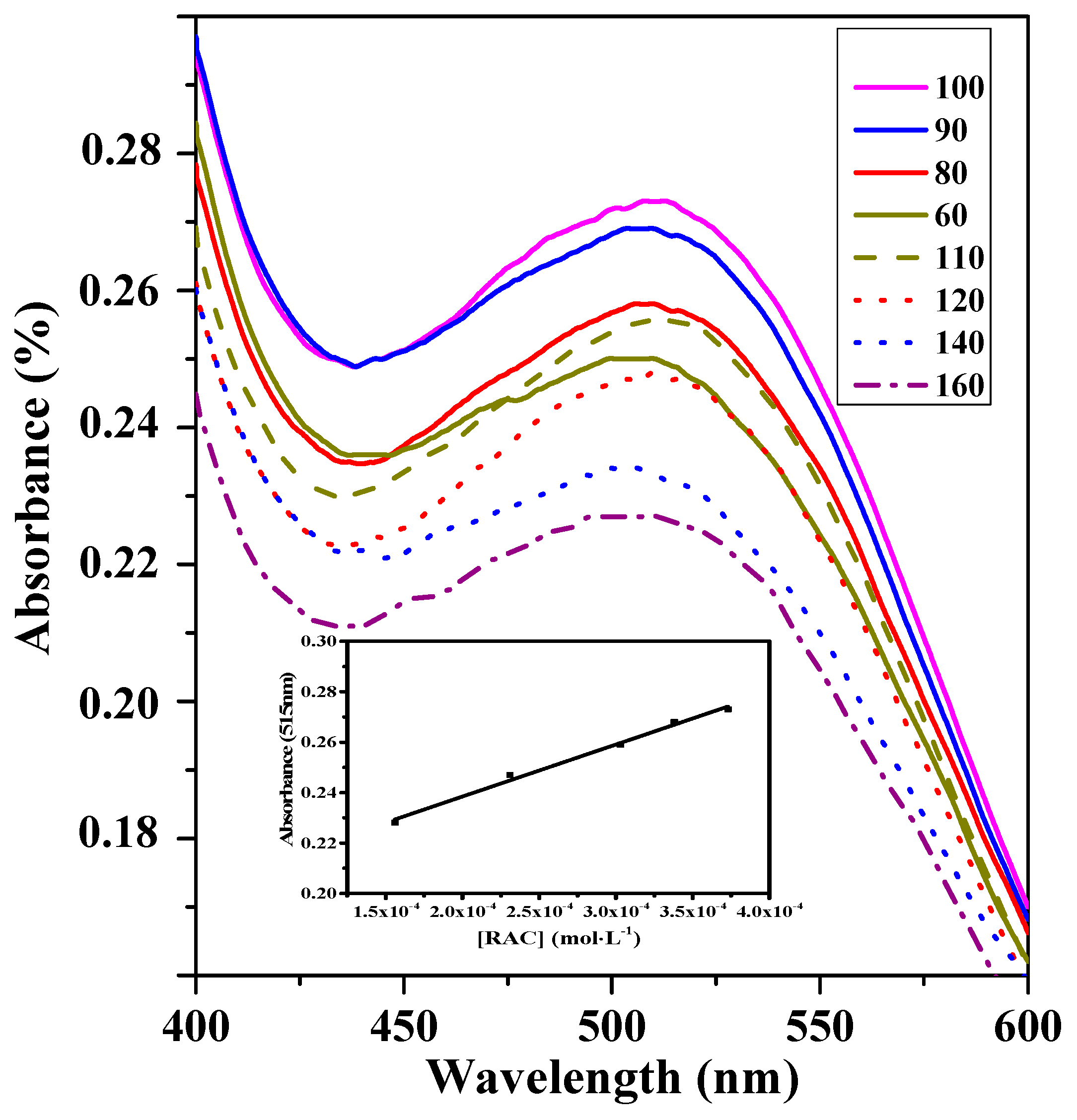
3.3. Detection of RAC
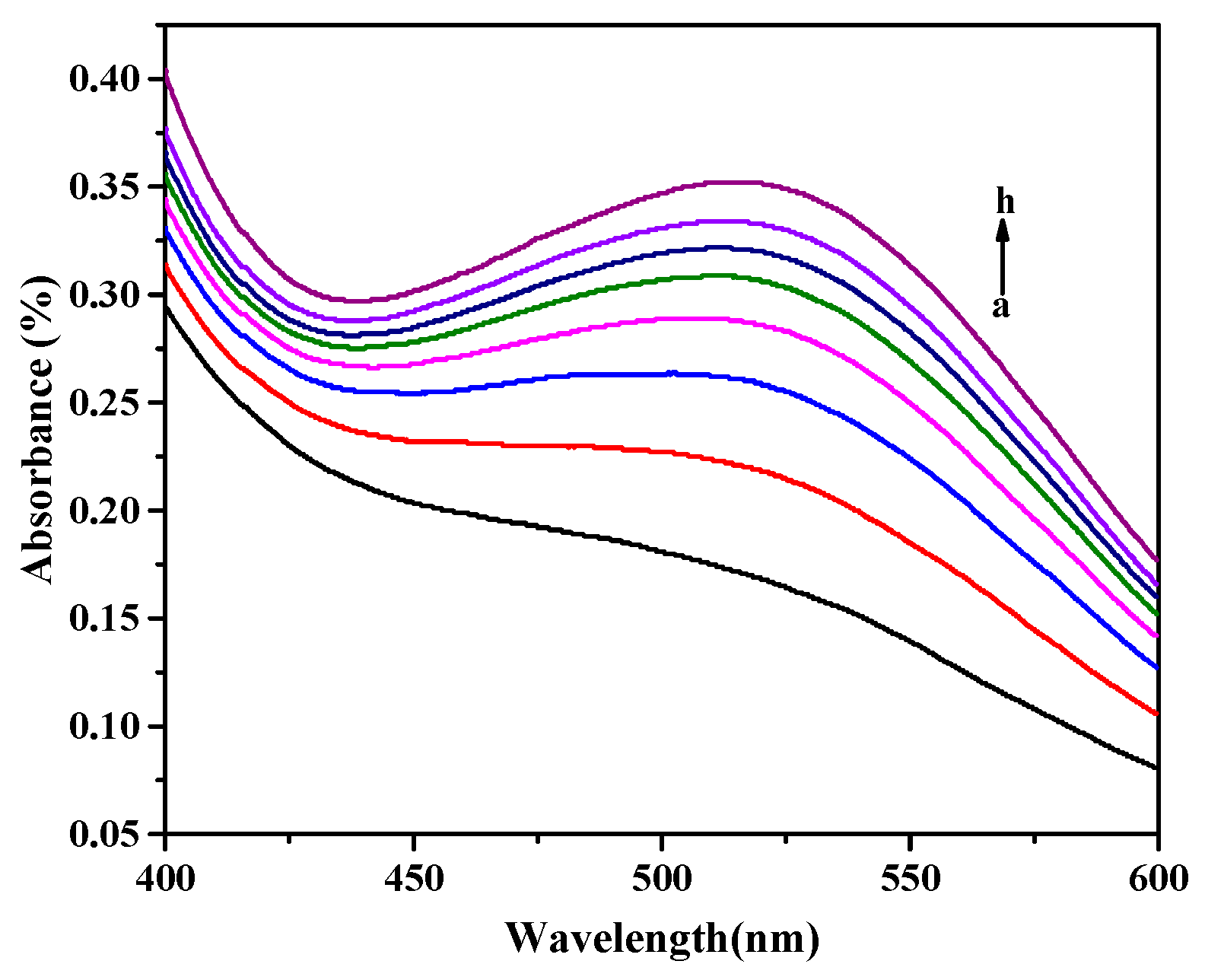
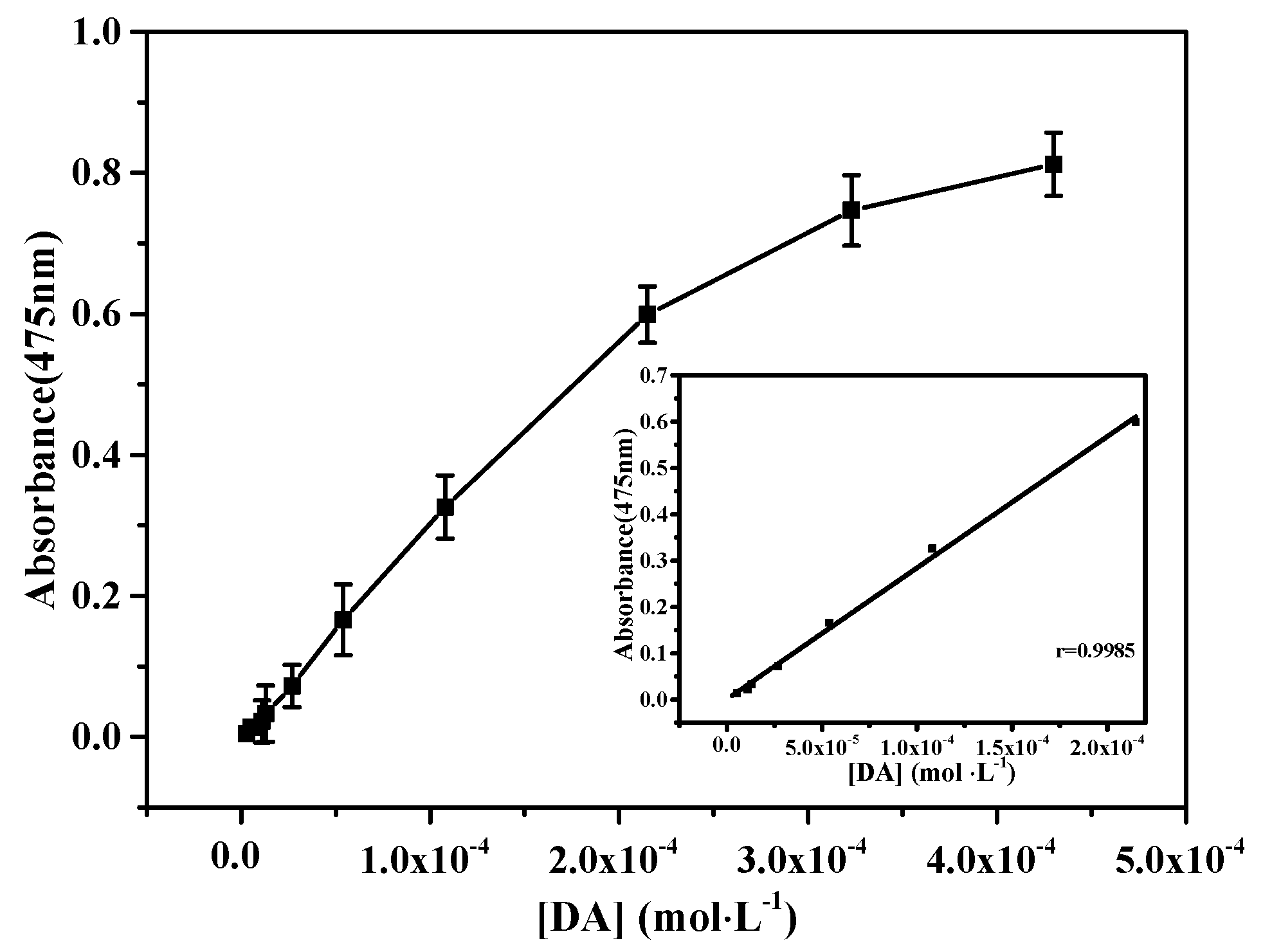
3.4. DA Detection
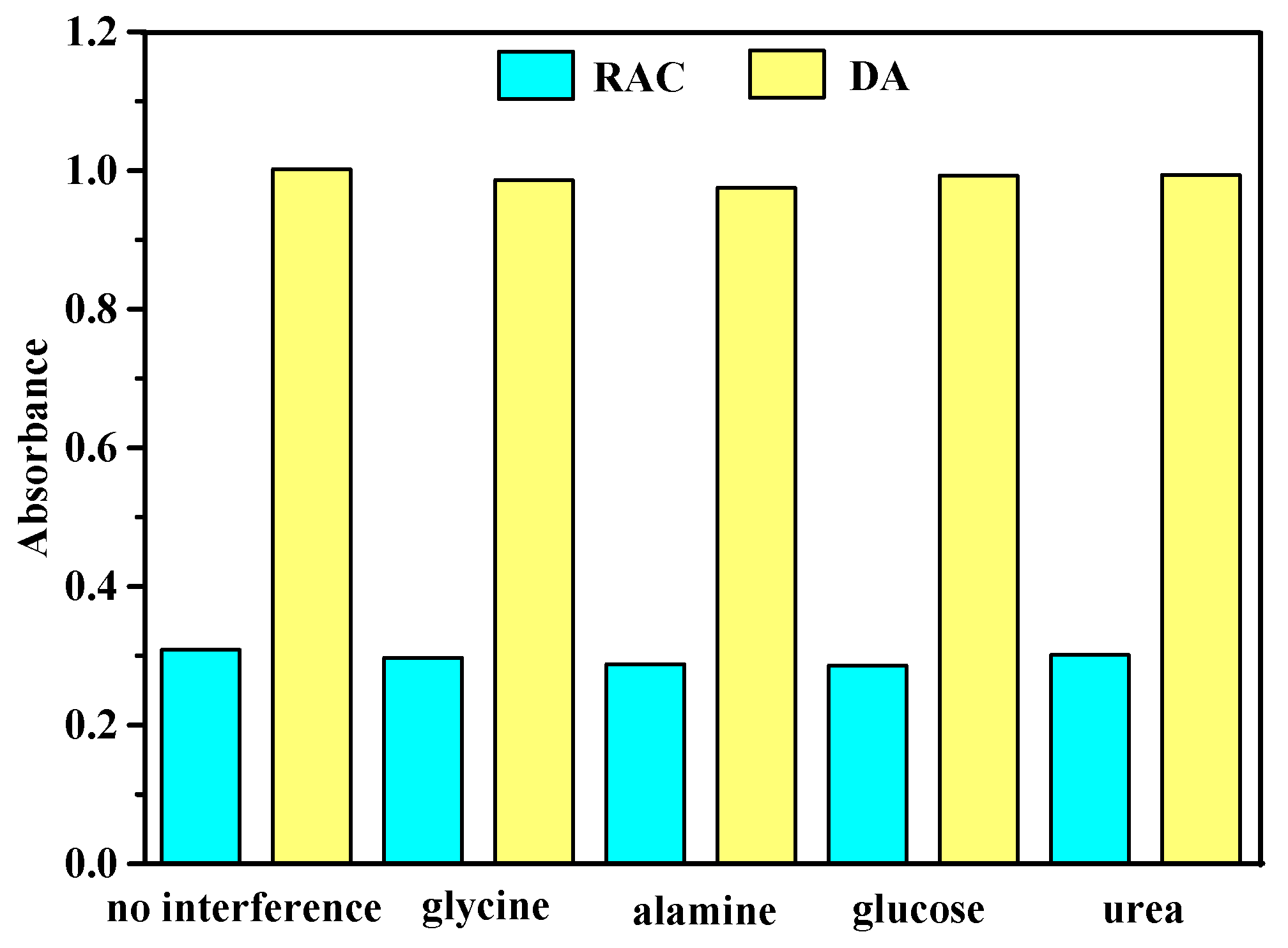
3.5. Interference Detection
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Baldrich, E.; Rodrigo, G.; Gemma, G.X.; Francesc, M. Magnetic entrapment for fast, simple and reversible electrode modification with carbon nanotubes: Application to dopamine detection. Biosens. Bioelect. 2011, 26, 1876–1882. [Google Scholar] [CrossRef] [PubMed]
- Syslova, K.; Rambousek, L.; Kuzma, M.; Najmanova, V.; Bubenikova-Valesova, V.; Slamberova, R.; Kacer, P. Monitoring of dopamine and its metabolites in brain microdialysates: Method combining freeze-drying with liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 3382–3391. [Google Scholar] [CrossRef] [PubMed]
- Li, L.L.; Liu, H.Y.; Shen, Y.Y.; Zhang, J.R.; Zhu, J.J. Electrogenerated chemiluminescence of Au nanoclusters for the detection of dopamine. Anal. Chem. 2011, 83, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, Y.; Wang, J.; Shi, X.; Ye, J. Determination of beta-agonists in pig feed, pig urine and pig liver using capillary electrophoresis with electrochemical detection. Meat Sci. 2010, 85, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Zhao, G.; Fu, Q.; Sun, M.; Zhou, H.; Chang, C. Combined microextraction by packed sorbent and high-performance liquid chromatography-ultraviolet detection for rapid analysis of ractopamine in porcine muscle and urine samples. Food Chem. 2014, 145, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Pleadin, J.; Persi, N.; Vulic, A.; Milic, D.; Vahcic, N. Determination of residual ractopamine concentrations by enzyme immunoassay in treated pig’s tissues on days after withdrawal. Meat Sci. 2012, 90, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yang, M.; Zheng, N.J.; Xie, N.; Liu, D.L.; Xie, C.F.; Yao, D.S. A novel aptasensor for electrochemical detection of ractopamine, clenbuterol, salbutamol, phenylethanolamine and procaterol. Biosens. Bioelectron. 2016, 80, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Z.; Wang, M.Z.; Chen, Z.L.; Fang, J.H.; Fang, M.M.; Liu, J.; Yu, X.P. Development of a colloidal gold-based lateral-flow immunoassay for the rapid simultaneous detection of clenbuterol and ractopamine in swine urine. Anal. Bioanal. Chem. 2009, 395, 2591–2599. [Google Scholar] [CrossRef] [PubMed]
- Jv, Y.; Li, B.; Cao, R. Positively-charged gold nanoparticles as peroxidiase mimic and their application in hydrogen peroxide and glucose detection. Chem. Commun. 2010, 46, 8017–8019. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Brook, M.A.; Li, Y.F. Design of gold nanoparticle-based colorimetric biosensing assays. ChemBioChem 2008, 9, 2363–2371. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Mi, X.G.; Guan, H.Y.; Wang, X.H.; Wu, Y. Assembly of folate-polyoxometalate hybrid spheres for colorimetric immunoassay like oxidase. Chem. Commun. 2011, 47, 2940–2942. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Bie, H.Z.; Wei, M.J.; Wang, J.J.; Mi, X.G.; Wang, X.H.; Wu, Y. Fabrication of inorganic—Organic hybrid based on polyoxometalate SiW10Fe2 and folate as peroxidases for colorimetric immunoassay of cancer cells. Chin. Chem. Lett. 2013, 24, 76–78. [Google Scholar] [CrossRef]
- Sun, C.L.; Chen, X.L.; Xu, J.; Wei, M.J.; Wang, J.J.; Mi, X.G.; Wang, X.H.; Wu, Y.; Liu, Y. Fabrication of an inorganic–organic hybrid based on an iron-substituted polyoxotungstate as a peroxidase for colorimetric immunoassays of H2O2 and cancer cells. J. Mater. Chem. A 2013, 1, 4699–4705. [Google Scholar] [CrossRef]
- Wang, J.J.; Han, D.X.; Wang, X.H.; Qi, B.; Zhao, M.S. Polyoxometalates as peroxidase mimetics and their applications in H2O2 and glucose detection. Biosens. Bioelectron. 2012, 36, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.L.; Li, S.; Zhu, W.; Pang, H.J.; Ma, H.Y. A sensor of a polyoxometalate and Au–Pd alloy for simultaneously detection of dopamine and ascorbic acid. Electrochim. Acta 2013, 113, 454–463. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, L.Y.; Xie, Z.C.; Xin, X.; Sun, D.; Yuan, S.L. Tunable aggregation-induced emission of polyoxometalates via amino acid-directed self-assembly and their application in detecting dopamine. Langmuir 2016, 32, 13736–13745. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ning, L.; Li, S.B.; Pang, H.J.; Zhang, Z.F.; Ma, H.Y.; Yan, H. Selective electrochemical detection of dopamine in the presence of uric acid and ascorbic acid based on a composite film modified electrode. RSC Adv. 2016, 6, 66468–66476. [Google Scholar] [CrossRef]
- Ginsberg, A.P. Inorganic Synthesis; John Wiley & Sons Inc.: New York, NY, USA, 1990; pp. 87–88. [Google Scholar]
- Xu, Q.Z.; Wang, X.F.; Zhu, Z.H.; Yu, D.J.; Chen, W.; Hua, Y.J.; Wang, C.T. Synthesis, characterization and electrochemical properties of Keggin-type Co-substituted heteropolyanion SiW11O39Co(II)(H2O)6−. J. Hainan Normal Univ. (Nat. Sci.) 2010, 23, 278–299. [Google Scholar]
- Ronny, N.; Mohammad, G. Highly active manganese-containing polyoxometalate as catalyst for epoxidation of akanes with hydrogen peroxide. J. Am. Chem. Soc. 1994, 116, 5509–5510. [Google Scholar]
- Liu, J.F.; Ortega, F.; Sethuraman, P.; Katsoulis, D.E.; Costello, C.E.; Pope, M.T. Trimetallo derivatives of lacunary 9-tungstosilicate heteropolyanions. Part 1, Synthesis and characterization. J. Chem. Soc. Dalton Trans. 1992, 12, 1901–1906. [Google Scholar] [CrossRef]
- Ilgen, F.; Ott, D.; Kralisch, D.; Reil, C.; Palmbergera, A.; König, B. Conversion of carbohydrates into 5-hydroxymethylfurfural in highly concentrated low melting mixtures. Green Chem. 2009, 11, 1948–1954. [Google Scholar] [CrossRef]
- Weakly, T.J.R. Some aspects of the heteropolymolybdates and heteropolytungstates. Struct. Bonding (Berlin) 1974, 18, 131–176. [Google Scholar]
- Newman, A.D.; Lee, A.F.; Wilson, K.; Young, N.A. On the active site in H3PW12O40/SiO2 catalysts for fine chemical synthesis. Catal. Lett. 2005, 102, 45–50. [Google Scholar] [CrossRef]
- Pawelec, B.; Mariscal, R.; Fierro, J.L.G.; Greenwood, A.; Vasudevan, P.T. Carbon-supported tungsten and nickel catalysts for hydrodesulfurization and hydrogenation reactions. Appl. Catal. A 2001, 206, 295–307. [Google Scholar] [CrossRef]
- Bisaglia, M.; Mammi, S.; Bubacco, L. Kinetic and structural analysis of the early oxidation products of dopamine. J. Biol. Chem. 2007, 282, 15597–15605. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; McCloskey, B.D.; Choi, T.H.; Lee, C.; Kim, M.J.; Freeman, B.D.; Park, H.B. Oxygen concentration control of dopamine-induced high uniformity surface coating chemistry. Appl. Mater. Interfaces 2013, 5, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Z.; Zhu, Y.; Chi, M.Q.; Wang, C.; Wei, Y.; Lu, X.F. Fabrication of cobalt ferrite/cobalt sulfide hybrid nanotubes with enhanced peroxidase-like activity for colorimetric detection of dopamine. J. Colloid Interface Sci. 2017, 511, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.M.; Xie, K.; Ye, L.Q.; Li, G.Q.; Lu, Z.W.; He, J.B. Gold-nanoparticle-based colorimetric array for detection of dopamine in urine and serum. Talanta 2015, 139, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, Y.; Yang, X.R. Aptamer-based colorimetric biosensing of dopamine using unmodified gold nanoparticles. Sens. Actuators B 2011, 156, 95–99. [Google Scholar] [CrossRef]
- Liu, J.M.; Wang, X.X.; Cui, M.L.; Lin, L.P.; Jiang, S.L.; Jiao, L.; Zhang, L.H. A promising non-aggregation colorimetric sensor of AuNRs–Ag+ for determination of dopamine. Sens. Actuators B 2013, 176, 97–102. [Google Scholar] [CrossRef]
- Tao, Y.; Lin, Y.H.; Ren, J.S.; Qu, X.G. A dual fluorometric and colorimetric sensor for dopamine based on BSA-stabilized Au nanoclusters. Biosens. Bioelectron. 2013, 42, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Ngomane, N.; Torto, N.; Krause, R.; Vilakazi, S. A colorimetric probe for dopamine based on gold nanoparticles electrospun nanofibre composite. Mater. Today Proc. 2015, 2, 4060–4069. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, P.L.; Su, X.O.; Zhao, H.; He, Y.J. Colorimetric detection of ractopamine and salbutamol using gold nanoparticles functionalized with melamine as a probe. Talanta 2013, 112, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Dessapt, R.; Collet, M.; Violaine, C.; Bujoli, D.M.; Jobic, S.; Lee, C.; Whangbo, M.H. Kinetics of coloration in photochromic organoammonium polyoxomolybdates. Inorg. Chem. 2009, 48, 574–580. [Google Scholar] [CrossRef] [PubMed]
| Catalyst | Detection | Time (min) | Linear Range (mol/L) | Detection Limit (mol/L) | Reference |
|---|---|---|---|---|---|
| CoFe2O4/CoS | DA (TMB + H2O2) | 10 | 0–5 × 10−5 | 5.8 × 10−7 | [28] |
| AuNPs | DA (NaOH + TGA) | 20 | 0–10−6 | 5.7 × 10−7 | [29] |
| AuNPs | DA (DBA + NaCl) | 20 | 5.4 × 10−7–5.4 × 10−6 | 3.6 × 10−7 | [30] |
| Au-AgNRs | DA | 25 (70 °C) | 0.20–12 | 0.047 | [31] |
| BSA-AuNCs | DA (TMB + H2O2) | 10 | 1 × 10−8–1 × 10−3 | 1 × 10−8 | [32] |
| UF-AuNPs | DA | 5 | 5 × 10−7–5 × 10−4 | 5 × 10−7 | [33] |
| SiW9Co3 | DA (H2O2) | 10 | 1.08 × 10−4–5.38 × 10−6 | 5.38 × 10−6 | This work |
| MA–AuNPs | RAC | 10 | 1 × 10−10–5 × 10−7 | 1 × 10−11 | [34] |
| SiW9Cu3 | RAC (H2O2) | 25 | 1.56 × 10−4–3.73 × 10−4 | 7.94 × 10−5 | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, X.; Bai, Z.; Shao, X.; Xu, J.; Yan, N.; Shi, J.; Wang, X. Fabrication of Metal-Substituted Polyoxometalates for Colorimetric Detection of Dopamine and Ractopamine. Materials 2018, 11, 674. https://doi.org/10.3390/ma11050674
Duan X, Bai Z, Shao X, Xu J, Yan N, Shi J, Wang X. Fabrication of Metal-Substituted Polyoxometalates for Colorimetric Detection of Dopamine and Ractopamine. Materials. 2018; 11(5):674. https://doi.org/10.3390/ma11050674
Chicago/Turabian StyleDuan, Xixin, Zhixian Bai, Xueting Shao, Jian Xu, Ning Yan, Junyou Shi, and Xiaohong Wang. 2018. "Fabrication of Metal-Substituted Polyoxometalates for Colorimetric Detection of Dopamine and Ractopamine" Materials 11, no. 5: 674. https://doi.org/10.3390/ma11050674
APA StyleDuan, X., Bai, Z., Shao, X., Xu, J., Yan, N., Shi, J., & Wang, X. (2018). Fabrication of Metal-Substituted Polyoxometalates for Colorimetric Detection of Dopamine and Ractopamine. Materials, 11(5), 674. https://doi.org/10.3390/ma11050674






