Synthesis of a Novel Electrospun Polycaprolactone Scaffold Functionalized with Ibuprofen for Periodontal Regeneration: An In Vitro andIn Vivo Study
Abstract
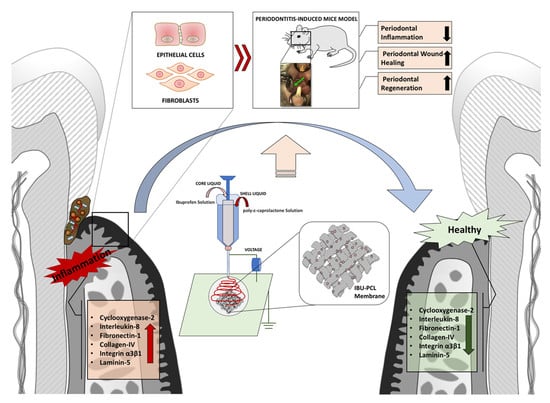
1. Introduction
2. Results
2.1. Release of IBU from IBU-PCL Membrane
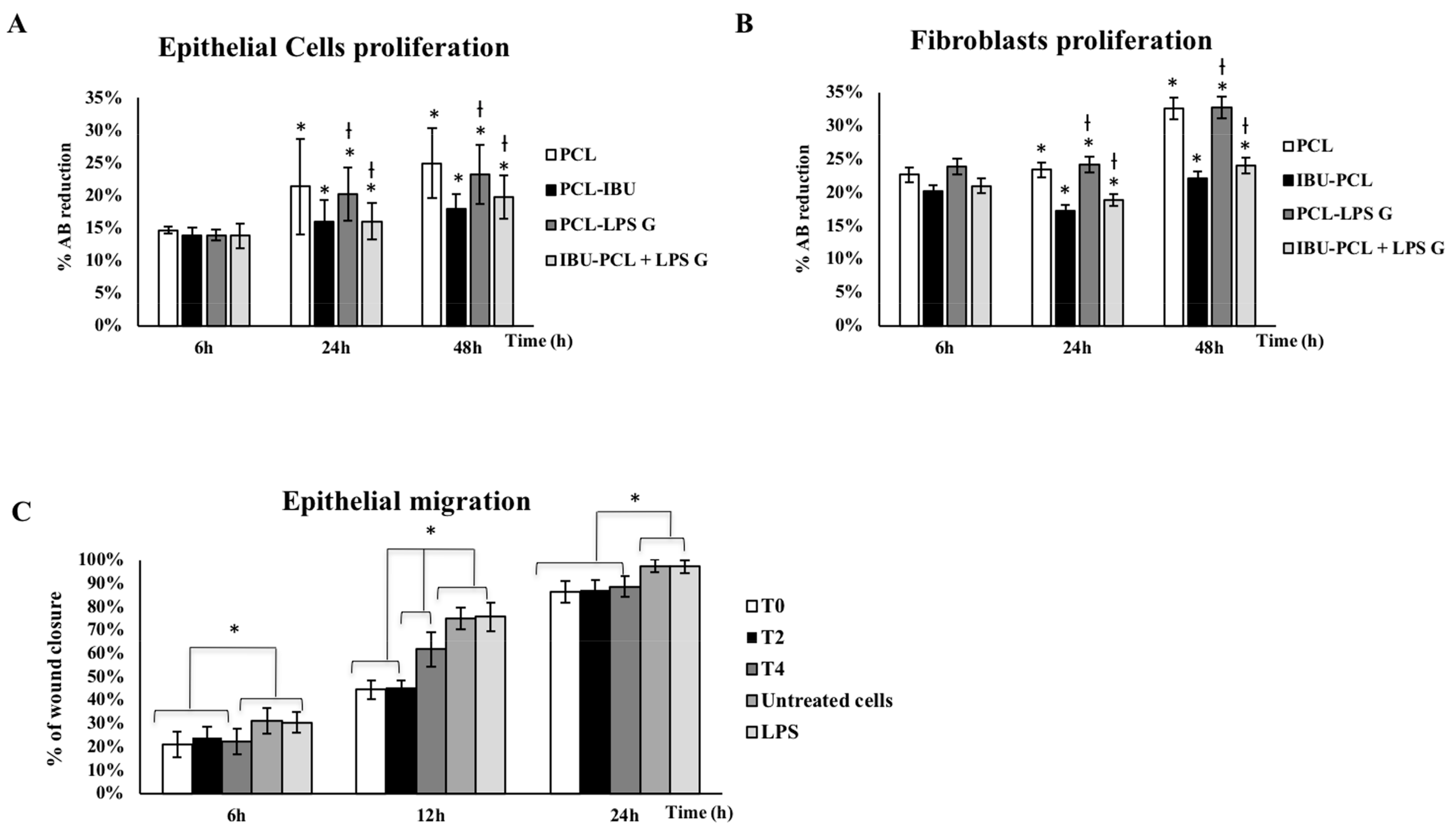
2.2. IBU-PCL Membrane Reduces Proliferation of Pg-LPS-Stimulated Cells
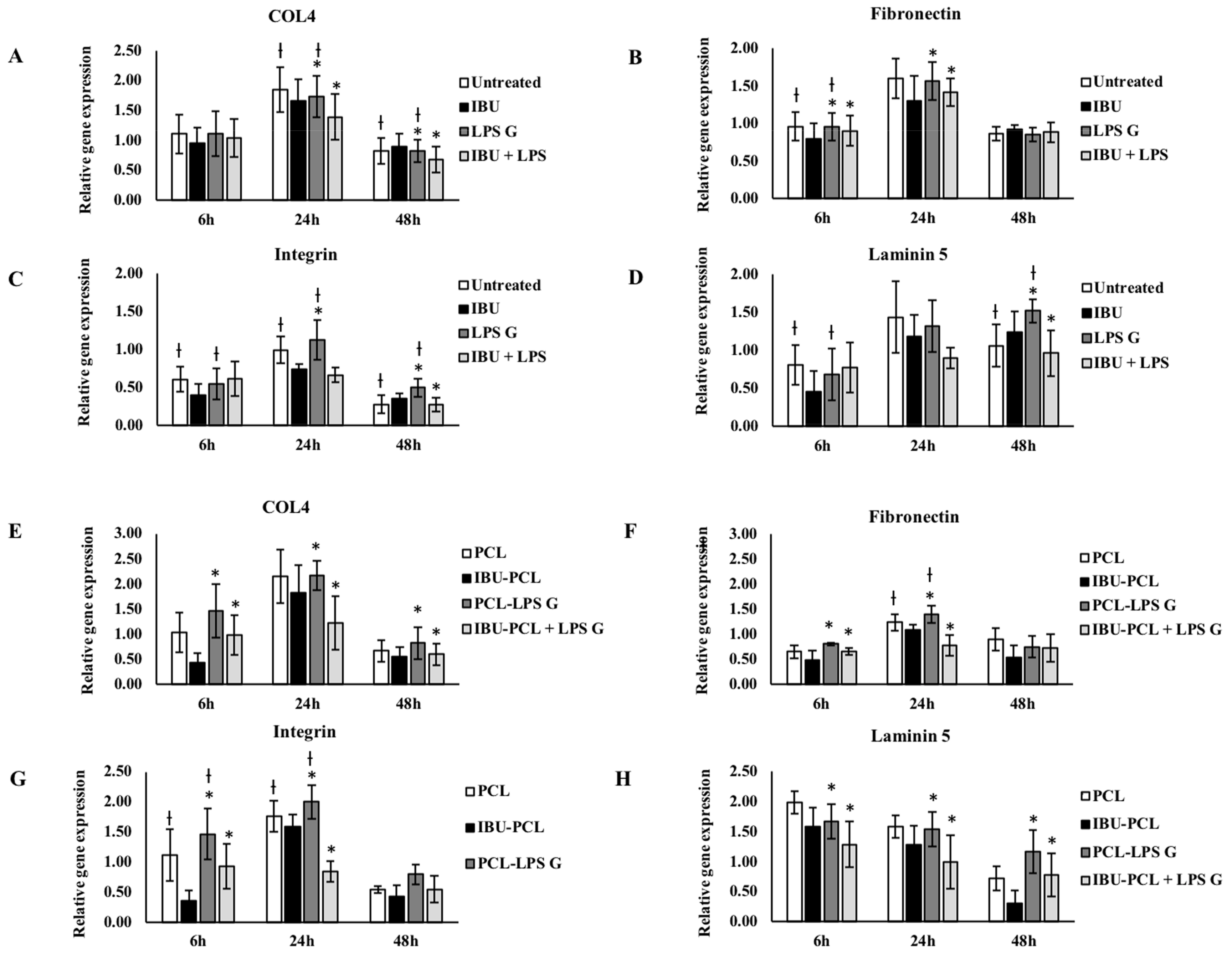
2.3. IBU-PCL Membrane Modulates mRNA Expression in Stimulated Cells
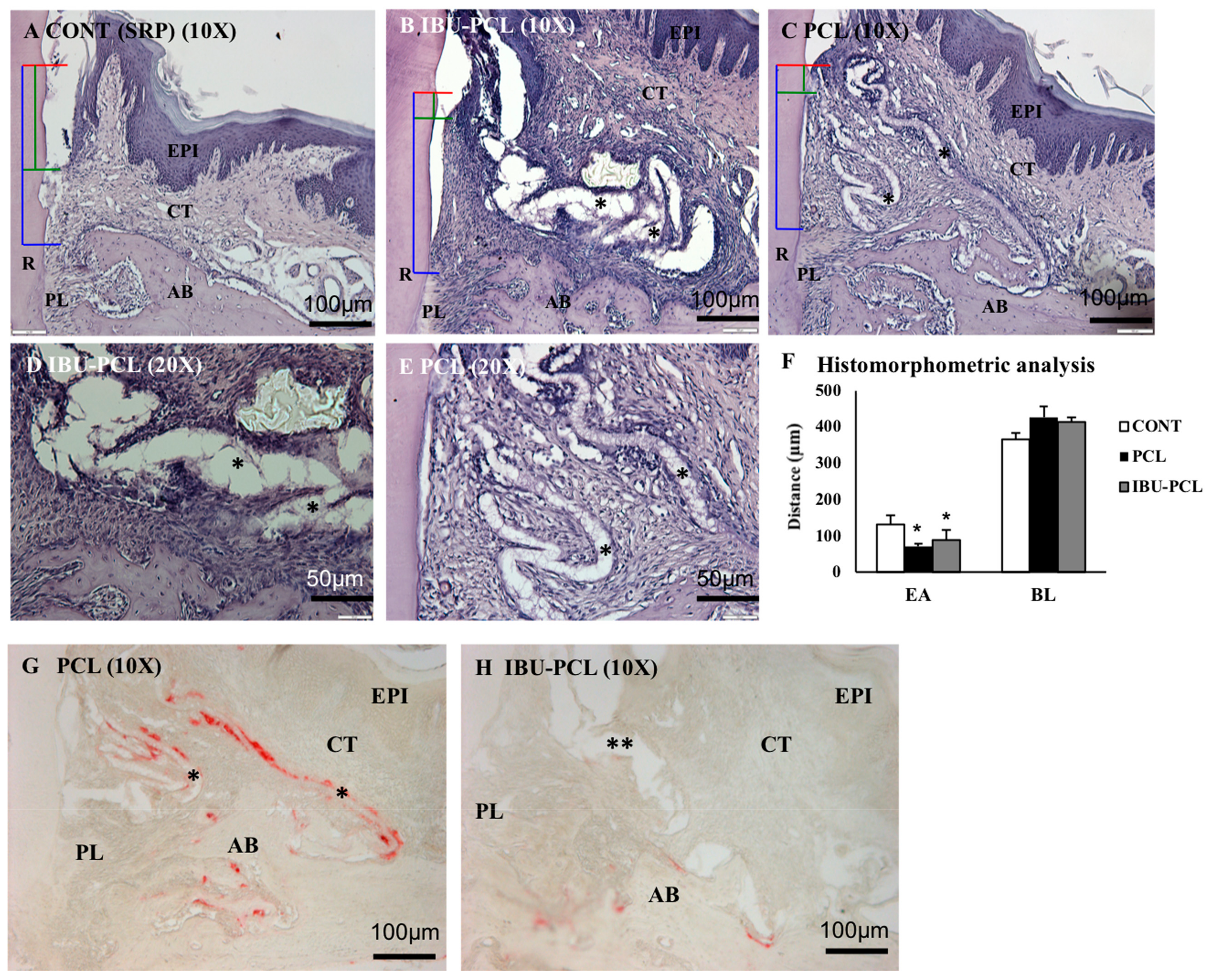
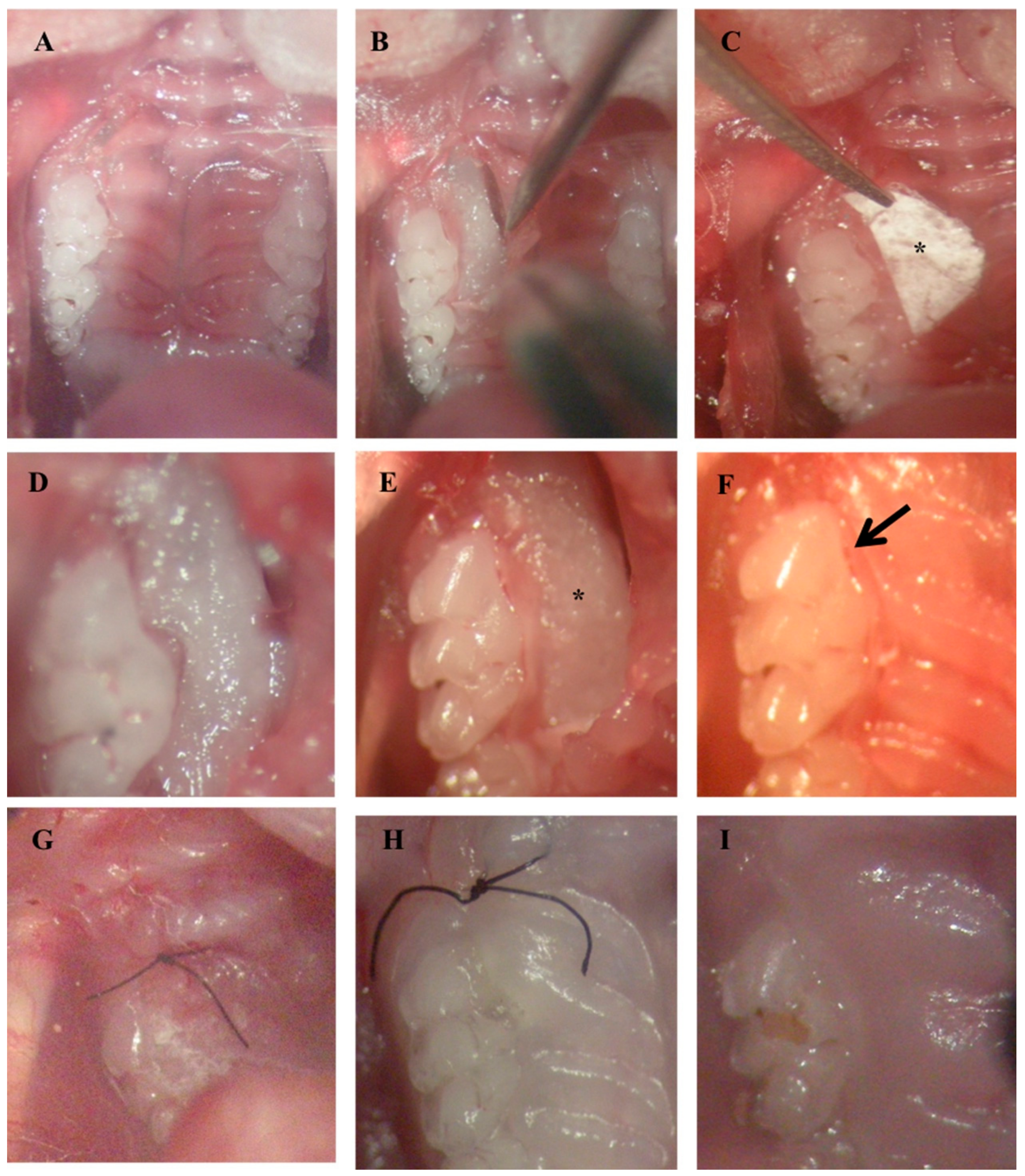
2.4. IBU-PCL Membrane Improves Wound Healing in an Induced Periodontitis Mouse Model
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Bacterial Culture
4.3. Stimulation of Cells with Porphyromonas Gingivalis-Lipopolysaccharide
4.4. Electrospinning and Functionalization
4.5. Scanning and Transmission Electron Microscopy
4.6. Cell Viability Assay
4.7. Wound Closure Assay
4.8. Real-Time qPCR
4.9. Experimental Periodontitis Induction in Mouse Model
4.10. Treatment of Periodontal Defect
4.11. Tissue Preparation
4.12. Histomorphometric Analysis
4.13. Tartrate-Resistant Acid Phosphatase Activity Assay (TRAP)
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jin, L.J.; Lamster, I.B.; Greenspan, J.S.; Pitts, N.B.; Scully, C.; Warnakulasuriya, S. Global burden of oral diseases: Emerging concepts, management and interplay with systemic health. Oral Dis. 2016, 22, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Jepsen, S.; Jin, L.; Otomo-Corgel, J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J. Clin. Periodontol. 2017, 44, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Bosshardt, D.D. The periodontal pocket: Pathogenesis, histopathology and consequences. Periodontology. 2018, 76, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Nunn, M.E.; Fan, J.; Su, X.; Levine, R.A.; Lee, H.-J.; McGuire, M.K. Development of prognostic indicators using classification and regression trees for survival. Periodontology 2012, 58, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primer 2017, 3, 17038. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I.; Lambris, J.D.; Hajishengallis, G. Porphyromonas gingivalis disturbs host-commensal homeostasis by changing complement function. J. Oral Microbiol. 2017, 9, 1340085. [Google Scholar] [CrossRef] [PubMed]
- Feres, M.; Figueiredo, L.C.; Soares, G.M.S.; Faveri, M. Systemic antibiotics in the treatment of periodontitis. Periodontology 2015, 67, 131–186. [Google Scholar] [CrossRef] [PubMed]
- Martin-Cabezas, R.; Davideau, J.-L.; Tenenbaum, H.; Huck, O. Clinical efficacy of probiotics as an adjunctive therapy to non-surgical periodontal treatment of chronic periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2016, 43, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.J.A.; Lang, N.P. Surgical and nonsurgical periodontal therapy. Learned and unlearned concepts. Periodontology 2013, 62, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Ivanovski, S.; Vaquette, C.; Gronthos, S.; Hutmacher, D.W.; Bartold, P.M. Multiphasic scaffolds for periodontal tissue engineering. J. Dent. Res. 2014, 93, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Bosshardt, D.D.; Sculean, A. Does periodontal tissue regeneration really work? Periodontology 2009, 51, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, P.; Buti, J.; Pini Prato, G.; Tonetti, M.S. Periodontal regeneration compared with access flap surgery in human intra-bony defects 20-year follow-up of a randomized clinical trial: Tooth retention, periodontitis recurrence and costs. J. Clin. Periodontol. 2017, 44, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Ravi, S.; Malaiappan, S.; Varghese, S.; Jayakumar, N.D.; Prakasam, G. Additive Effect of Plasma Rich in Growth Factors with Guided Tissue Regeneration in Treatment of Intrabony Defects in Patients with Chronic Periodontitis: A Split-Mouth Randomized Controlled Clinical Trial. J. Periodontol. 2017, 88, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Jenabian, N.; Haghanifar, S.; Ehsani, H.; Zahedi, E.; Haghpanah, M. Guided tissue regeneration and platelet rich growth factor for the treatment of Grade II furcation defects: A randomized double-blinded clinical trial—A pilot study. Dent. Res. J. 2017, 14, 363–369. [Google Scholar]
- Bottino, M.C.; Thomas, V. Membranes for Periodontal Regeneration—A Materials Perspective. Front. Oral Biol. 2015, 17, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Parrish, L.C.; Miyamoto, T.; Fong, N.; Mattson, J.S.; Cerutis, D.R. Non-bioabsorbable vs. bioabsorbable membrane: Assessment of their clinical efficacy in guided tissue regeneration technique. A systematic review. J. Oral Sci. 2009, 51, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, N.; Tohya, T.; Mori, A.; Koide, M.; Kawase, H.; Takada, T.; Inagaki, K.; Noguchi, T. Inflammatory cell population and bacterial contamination of membranes used for guided tissue regenerative procedures. J. Periodontol. 1998, 69, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar-Mohammadi, M.; Zamani, M.; Prabhakaran, M.P.; Bahrami, S.H.; Ramakrishna, S. Electrospinning of PLGA/gum tragacanth nanofibers containing tetracycline hydrochloride for periodontal regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Yamano, S.; Haku, K.; Yamanaka, T.; Dai, J.; Takayama, T.; Shohara, R.; Tachi, K.; Ishioka, M.; Hanatani, S.; Karunagaran, S.; et al. The effect of a bioactive collagen membrane releasing PDGF or GDF-5 on bone regeneration. Biomaterials 2014, 35, 2446–2453. [Google Scholar] [CrossRef] [PubMed]
- Yar, M.; Farooq, A.; Shahzadi, L.; Khan, A.S.; Mahmood, N.; Rauf, A.; Chaudhry, A.A.; Rehman, I.U. Novel meloxicam releasing electrospun polymer/ceramic reinforced biodegradable membranes for periodontal regeneration applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 64, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Morand, D.N.; Davideau, J.-L.; Clauss, F.; Jessel, N.; Tenenbaum, H.; Huck, O. Cytokines during periodontal wound healing: Potential application for new therapeutic approach. Oral Dis. 2017, 23, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Cekici, A.; Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontology 2014, 64, 57–80. [Google Scholar] [CrossRef] [PubMed]
- Brägger, U.; Mühle, T.; Fourmousis, I.; Lang, N.P.; Mombelli, A. Effect of the NSAID flurbiprofen on remodelling after periodontal surgery. J. Periodontal Res. 1997, 32, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Agossa, K.; Morand, D.-N.; Tenenbaum, H.; Davideau, J.-L.; Huck, O. Systemic Application of Anti-inflammatory Agents in Periodontal Treatment. Clin. Anti-Inflamm. Anti-Allergy Drugs 2015, 2, 3–13. Available online: http://www.eurekaselect.com/142258/article (accessed on 30 November 2017). [CrossRef]
- Potrč, T.; Baumgartner, S.; Roškar, R.; Planinšek, O.; Lavrič, Z.; Kristl, J.; Kocbek, P. Electrospun polycaprolactone nanofibers as a potential oromucosal delivery system for poorly water-soluble drugs. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2015, 75, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Sundararaj, S.C.; Thomas, M.V.; Peyyala, R.; Dziubla, T.D.; Puleo, D.A. Design of a Multiple Drug Delivery System Directed at Periodontitis. Biomaterials 2013, 34, 8835–8842. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Jiang, H.; Wang, R.; Xie, Y.; Zhao, C. Fabrication of metronidazole loaded poly (ε-caprolactone)/zein core/shell nanofiber membranes via coaxial electrospinning for guided tissue regeneration. J. Colloid Interface Sci. 2017, 490, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Morand, D.-N.; Huck, O.; Keller, L.; Jessel, N.; Tenenbaum, H.; Davideau, J.-L. Active Nanofibrous Membrane Effects on Gingival Cell Inflammatory Response. Materials 2015, 8, 7217–7229. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Xue, J.; He, M.; Chen, D.; Zhang, L.; Tian, W. Structure, physical properties, biocompatibility and in vitro/vivo degradation behavior of anti-infective polycaprolactone-based electrospun membranes for guided tissue/bone regeneration. Polym. Degrad. STable 2014, 109, 293–306. [Google Scholar] [CrossRef]
- Basar, A.O.; Castro, S.; Torres-Giner, S.; Lagaron, J.M.; Turkoglu Sasmazel, H. Novel poly(ε-caprolactone)/gelatin wound dressings prepared by emulsion electrospinning with controlled release capacity of Ketoprofen anti-inflammatory drug. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 81, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Farooq, A.; Yar, M.; Khan, A.S.; Shahzadi, L.; Siddiqi, S.A.; Mahmood, N.; Rauf, A.; Qureshi, Z.-A.; Manzoor, F.; Chaudhry, A.A.; et al. Synthesis of piroxicam loaded novel electrospun biodegradable nanocomposite scaffolds for periodontal regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 56, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Hortensius, R.A.; Harley, B.A. Naturally derived biomaterials for addressing inflammation in tissue regeneration. Exp. Biol. Med. 2016, 241, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, K.; Ishikawa, I. The roles of cyclooxygenase-2 and prostaglandin E2 in periodontal disease. Periodontology 2007, 43, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Van Dyke, T.E.; Hasturk, H.; Kantarci, A.; Freire, M.O.; Nguyen, D.; Dalli, J.; Serhan, C.N. Proresolving nanomedicines activate bone regeneration in periodontitis. J. Dent. Res. 2015, 94, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Gupte, R.; Zelkha, S.; Amar, S. Receptor activator of nuclear factor kappa B ligand antagonists inhibit tissue inflammation and bone loss in experimental periodontitis. J. Clin. Periodontol. 2011, 38, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Zupancic, S.; Kocbek, P.; Baumgartner, S.; Kristl, J. Contribution of Nanotechnology to Improved Treatment of Periodontal Disease. Curr. Pharm. Des. 2015, 21, 3257–3271. [Google Scholar] [CrossRef] [PubMed]
- Eap, S.; Ferrand, A.; Palomares, C.M.; Hébraud, A.; Stoltz, J.-F.; Mainard, D.; Schlatter, G.; Benkirane-Jessel, N. Electrospun nanofibrous 3D scaffold for bone tissue engineering. Biomed. Mater. Eng. 2012, 22, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Vaquette, C.; Fan, W.; Xiao, Y.; Hamlet, S.; Hutmacher, D.W.; Ivanovski, S. A biphasic scaffold design combined with cell sheet technology for simultaneous regeneration of alveolar bone/periodontal ligament complex. Biomaterials 2012, 33, 5560–5573. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.; Vaquette, C.; Theodoropoulos, C.; Hamlet, S.M.; Hutmacher, D.W.; Ivanovski, S. Decellularized periodontal ligament cell sheets with recellularization potential. J. Dent. Res. 2014, 93, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Eap, S.; Keller, L.; Schiavi, J.; Huck, O.; Jacomine, L.; Fioretti, F.; Gauthier, C.; Sebastian, V.; Schwinté, P.; Benkirane-Jessel, N. A living thick nanofibrous implant bifunctionalized with active growth factor and stem cells for bone regeneration. Int. J. Nanomed. 2015, 10, 1061–1075. [Google Scholar] [CrossRef]
- Mathew, A.; Vaquette, C.; Hashimi, S.; Rathnayake, I.; Huygens, F.; Hutmacher, D.W.; Ivanovski, S. Antimicrobial and Immunomodulatory Surface-Functionalized Electrospun Membranes for Bone Regeneration. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Ferrand, A.; Eap, S.; Richert, L.; Lemoine, S.; Kalaskar, D.; Demoustier-Champagne, S.; Atmani, H.; Mély, Y.; Fioretti, F.; Schlatter, G.; et al. Osteogenetic properties of electrospun nanofibrous PCL scaffolds equipped with chitosan-based nanoreservoirs of growth factors. Macromol. Biosci. 2014, 14, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Wang, Y.; Sun, T.; Yue, W.; Zhang, H. Electrospun PCL/gelatin composite nanofiber structures for effective guided bone regeneration membranes. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.X.F.; Savalani, M.M.; Teoh, S.-H.; Hutmacher, D.W. Dynamics of in vitro polymer degradation of polycaprolactone-based scaffolds: Accelerated versus simulated physiological conditions. Biomed. Mater. 2008, 3, 034108. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Hsiao, B.S.; Chu, B. Functional electrospun nanofibrous scaffolds for biomedical applications. Adv. Drug Deliv. Rev. 2007, 59, 1392–1412. [Google Scholar] [CrossRef] [PubMed]
- Salvi, G.E.; Lang, N.P. The effects of non-steroidal anti-inflammatory drugs (selective and non-selective) on the treatment of periodontal diseases. Curr. Pharm. Des. 2005, 11, 1757–1769. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, S.B.; Hakki, S.S.; Hakki, E.E.; Durak, Y.; Kantarci, A. Porphyromonas gingivalis Lipopolysaccharide Induces a Pro-inflammatory Human Gingival Fibroblast Phenotype. Inflammation 2017, 40, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Ara, T.; Kurata, K.; Hirai, K.; Uchihashi, T.; Uematsu, T.; Imamura, Y.; Furusawa, K.; Kurihara, S.; Wang, P.-L. Human gingival fibroblasts are critical in sustaining inflammation in periodontal disease. J. Periodontal Res. 2009, 44, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Huang, P.; Chen, M.W. Curcumin attenuates cyclooxygenase-2 expression via inhibition of the NF-κB pathway in lipopolysaccharide-stimulated human gingival fibroblasts. Cell Biol. Int. 2013, 37, 443–448. [Google Scholar] [CrossRef] [PubMed]
- McClure, R.; Massari, P. TLR-Dependent Human Mucosal Epithelial Cell Responses to Microbial Pathogens. Front. Immunol. 2014, 5, 386. [Google Scholar] [CrossRef] [PubMed]
- Spiekstra, S.W.; Breetveld, M.; Rustemeyer, T.; Scheper, R.J.; Gibbs, S. Wound-healing factors secreted by epidermal keratinocytes and dermal fibroblasts in skin substitutes. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2007, 15, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Kocgozlu, L.; Elkaim, R.; Tenenbaum, H.; Werner, S. Variable cell responses to P. gingivalis lipopolysaccharide. J. Dent. Res. 2009, 88, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-L.; Ohura, K. Porphyromonas gingivalis lipopolysaccharide signaling in gingival fibroblasts-CD14 and Toll-like receptors. Crit. Rev. Oral Biol. Med. Off. Publ. Am. Assoc. Oral Biol. 2002, 13, 132–142. [Google Scholar]
- Cantón, I.; Mckean, R.; Charnley, M.; Blackwood, K.A.; Fiorica, C.; Ryan, A.J.; MacNeil, S. Development of an Ibuprofen-releasing biodegradable PLA/PGA electrospun scaffold for tissue regeneration. Biotechnol. Bioeng. 2010, 105, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Kasaj, A.; Reichert, C.; Götz, H.; Röhrig, B.; Smeets, R.; Willershausen, B. In vitro evaluation of various bioabsorbable and nonresorbable barrier membranes for guided tissue regeneration. Head Face Med. 2008, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Larjava, H.; Koivisto, L.; Häkkinen, L.; Heino, J. Epithelial integrins with special reference to oral epithelia. J. Dent. Res. 2011, 90, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Gräber, H.G.; Conrads, G.; Wilharm, J.; Lampert, F. Role of interactions between integrins and extracellular matrix components in healthy epithelial tissue and establishment of a long junctional epithelium during periodontal wound healing: A review. J. Periodontol. 1999, 70, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Preeja, C.; Janam, P.; Nayar, B.R. Fibrin clot adhesion to root surface treated with tetracycline hydrochloride and ethylenediaminetetraacetic acid: A scanning electron microscopic study. Dent. Res. J. 2013, 10, 382–388. [Google Scholar]
- Fairweather, M.; Heit, Y.I.; Buie, J.; Rosenberg, L.M.; Briggs, A.; Orgill, D.P.; Bertagnolli, M.M. Celecoxib inhibits early cutaneous wound healing. J. Surg. Res. 2015, 194, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Khil, M.S.; Kim, H.Y.; Lee, H.U.; Jahng, K.Y. An improved hydrophilicity via electrospinning for enhanced cell attachment and proliferation. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 78, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, P.; Bondioli, F.; Messori, M.; Bartoli, C.; Dinucci, D.; Chiellini, F. Porous scaffolds of polycaprolactone reinforced with in situ generated hydroxyapatite for bone tissue engineering. J. Mater. Sci. Mater. Med. 2010, 21, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-P.; Chang, Y.-S. Preparation and characterization of composite nanofibers of polycaprolactone and nanohydroxyapatite for osteogenic differentiation of mesenchymal stem cells. Colloids Surf. B Biointerfaces 2011, 86, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.I.; Sultana, N. Characterization, drug loading and antibacterial activity of nanohydroxyapatite/polycaprolactone (nHA/PCL) electrospun membrane. Biotech 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Groppo, M.F.; Caria, P.H.; Freire, A.R.; Figueroba, S.R.; Ribeiro-Neto, W.A.; Bretas, R.E.S.; Prado, F.B.; Haiter-Neto, F.; Aguiar, F.H.; Rossi, A.C. The effect of a hydroxyapatite impregnated PCL membrane in rat subcritical calvarial bone defects. Arch. Oral Biol. 2017, 82, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Kim, T.-I.; Ku, Y.; Chung, C.-P.; Han, S.-B.; Yu, J.-H.; Lee, S.-P.; Kim, H.-W.; Lee, H.-H. Effect of hydroxyapatite-coated nanofibrous membrane on the responses of human periodontal ligament fibroblast. J. Ceram. Soc. Jpn. 2008, 116, 31–35. [Google Scholar] [CrossRef]
- Plazas Bonilla, C.E.; Trujillo, S.; Demirdögen, B.; Perilla, J.E.; Murat Elcin, Y.; Gómez Ribelles, J.L. New porous polycaprolactone–silica composites for bone regeneration. Mater. Sci. Eng. C 2014, 40, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Petrauskaite, O.; Gomes, P.D.S.; Fernandes, M.H.; Juodzbalys, G.; Stumbras, A.; Maminskas, J.; Liesiene, J.; Cicciù, M. Biomimetic mineralization on a macroporous cellulose-based matrix for bone regeneration. BioMed Res. Int. 2013, 2013, 452750. [Google Scholar] [CrossRef] [PubMed]
- Saadi-Thiers, K.; Huck, O.; Simonis, P.; Tilly, P.; Fabre, J.-E.; Tenenbaum, H.; Davideau, J.-L. Periodontal and systemic responses in various mice models of experimental periodontitis: Respective roles of inflammation duration and Porphyromonas gingivalis infection. J. Periodontol. 2013, 84, 396–406. [Google Scholar] [CrossRef] [PubMed]
- De Molon, R.S.; Mascarenhas, V.I.; de Avila, E.D.; Finoti, L.S.; Toffoli, G.B.; Spolidorio, D.M.P.; Scarel-Caminaga, R.M.; Tetradis, S.; Cirelli, J.A. Long-term evaluation of oral gavage with periodontopathogens or ligature induction of experimental periodontal disease in mice. Clin. Oral Investig. 2016, 20, 1203–1216. [Google Scholar] [CrossRef] [PubMed]
- Karimbux, N.Y.; Ramamurthy, N.S.; Golub, L.M.; Nishimura, I. The expression of collagen I and XII mRNAs in Porphyromonas gingivalis-induced periodontitis in rats: The effect of doxycycline and chemically modified tetracycline. J. Periodontol. 1998, 69, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Hajishengallis, G. Optimization of the ligature-induced periodontitis model in mice. J. Immunol. Methods 2013, 394, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Nagai, A.; Onitsuka, T.; Koga, T.; Fujiwara, T.; Kaya, H.; Hamada, S. Induction of experimental periodontitis in mice with Porphyromonas gingivalis-adhered ligatures. J. Periodontol. 2000, 71, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Noordijk, M.; Davideau, J.-L.; Eap, S.; Huck, O.; Fioretti, F.; Stoltz, J.-F.; Bacon, W.; Benkirane-Jessel, N.; Clauss, F. Bone defects and future regenerative nanomedicine approach using stem cells in the mutant Tabby mouse model. Biomed. Mater. Eng. 2015, 25, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Cicciù, M. Real Opportunity for the Present and a Forward Step for the Future of Bone Tissue Engineering. J. Craniofac. Surg. 2017, 28, 592–593. [Google Scholar] [CrossRef] [PubMed]
- Bugueno, I.M.; Batool, F.; Korah, L.; Benkirane-Jessel, N.; Huck, O. Porphyromonas gingivalis differentially modulates apoptosome APAF-1 in epithelial cells and fibroblasts. Am. J. Pathol. 2017. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batool, F.; Morand, D.-N.; Thomas, L.; Bugueno, I.M.; Aragon, J.; Irusta, S.; Keller, L.; Benkirane-Jessel, N.; Tenenbaum, H.; Huck, O. Synthesis of a Novel Electrospun Polycaprolactone Scaffold Functionalized with Ibuprofen for Periodontal Regeneration: An In Vitro andIn Vivo Study. Materials 2018, 11, 580. https://doi.org/10.3390/ma11040580
Batool F, Morand D-N, Thomas L, Bugueno IM, Aragon J, Irusta S, Keller L, Benkirane-Jessel N, Tenenbaum H, Huck O. Synthesis of a Novel Electrospun Polycaprolactone Scaffold Functionalized with Ibuprofen for Periodontal Regeneration: An In Vitro andIn Vivo Study. Materials. 2018; 11(4):580. https://doi.org/10.3390/ma11040580
Chicago/Turabian StyleBatool, Fareeha, David-Nicolas Morand, Lionel Thomas, Isaac Maximiliano Bugueno, Javier Aragon, Silvia Irusta, Laetitia Keller, Nadia Benkirane-Jessel, Henri Tenenbaum, and Olivier Huck. 2018. "Synthesis of a Novel Electrospun Polycaprolactone Scaffold Functionalized with Ibuprofen for Periodontal Regeneration: An In Vitro andIn Vivo Study" Materials 11, no. 4: 580. https://doi.org/10.3390/ma11040580
APA StyleBatool, F., Morand, D.-N., Thomas, L., Bugueno, I. M., Aragon, J., Irusta, S., Keller, L., Benkirane-Jessel, N., Tenenbaum, H., & Huck, O. (2018). Synthesis of a Novel Electrospun Polycaprolactone Scaffold Functionalized with Ibuprofen for Periodontal Regeneration: An In Vitro andIn Vivo Study. Materials, 11(4), 580. https://doi.org/10.3390/ma11040580