Luminescent Lanthanide MOFs: A Unique Platform for Chemical Sensing
Abstract
1. Introduction
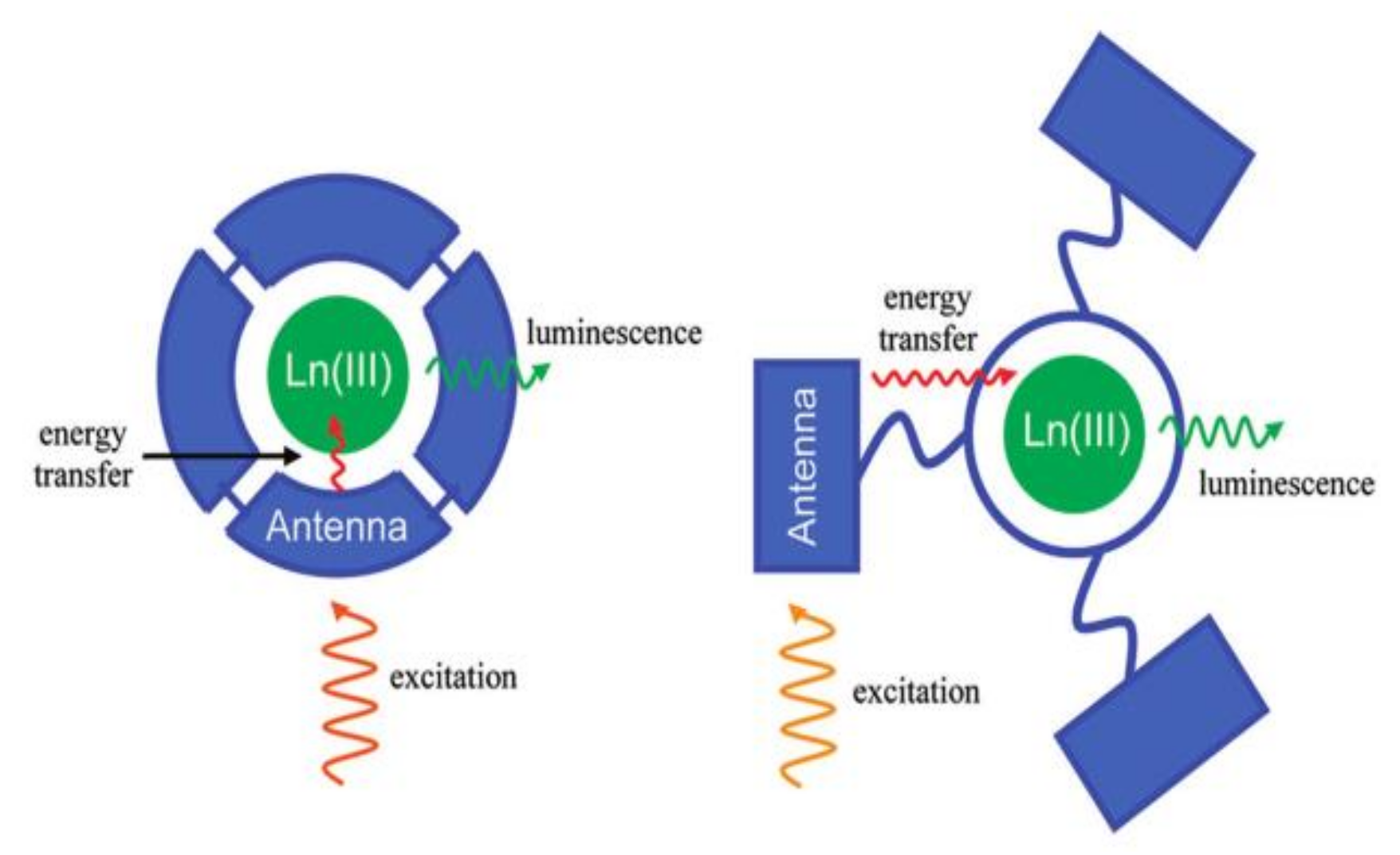
2. Luminescent Properties of LnMOFs
3. LnMOFs for Chemical Sensing
3.1. LnMOFs for Cation Sensing
3.2. LnMOFs for Anion Sensing
3.3. LnMOFs for Small Molecule Sensing
3.4. LnMOFs for Nitroaromatic Explosive Sensing
3.5. LnMOFs for Gas and Vapor Sensing
3.6. LnMOFs for pH Sensing
3.7. LnMOFs for Temperature Sensing
3.8. LnMOFs for Biosensing
4. Conclusions and Outlook
Acknowledgments
Conflicts of Interest
Abbreviations
| Ad | adenine |
| BCA | 2,2′-biquinoline-4,4′-dicarboxylate |
| H2BDC | 1,4-benzenedicarboxylic acid |
| H2BDC-F4 | 2,3,5,6-tetrafluoro-1,4-benzenedicarboxylate |
| bipy | 4,4′-bipyridine |
| H2BPDC | 2,2′-bipyridine-3,3′-dicarboxylic acid |
| BPT | biphenyl-3,4′,5-tricarboxylate |
| bpydc | 2,2′-bipyridine-5,5′-dicarboxylic acid |
| H3BTB | 1,3,5-benzenetribenzoate |
| BTC | benzene-1,3,5- tricarboxylate |
| H4btec | pyromellitic acid |
| H4BTMIPA | 5,5′-methylenebis(2,4,6-trimethylisophthalic acid) |
| CB | conduction band |
| CDs | carbon dots |
| HCHO | formaldehyde |
| CPNPs | coordination polymer nanoparticles |
| H2CPOC | 5-(4′-carboxylphenoxy) nicotinic acid |
| DMA | dimethylacetamide |
| DMBDC | 2,5-dimethoxy-1,4-benzenedicarboxylate |
| DMF | N′N-dimethylformamide |
| DPA | dipicolinic acid |
| H2FDC | 9,9-dimethyl-2,7-fluorenedicarboxylic acid |
| FBPT | 2′-fluoro-biphenyl-3,4′,5-tricarboxylate |
| FTIR | Fourier-transform infrared spectroscopy |
| fum | fumarate |
| ICT | intramolecular-charge-transfer |
| H2ipbpBr | 1-(3,5-dicarboxyphenyl)-4,4′-bipyridinium bromide |
| ITO | indium–tin–oxide |
| H2L1 | 2,5-di(pyridin-4-yl)terephthalic acid |
| H2L2− | 3, 5-dicarboxy-phenol anion ligand |
| L3 | 4,4′-dicarboxylate-2,2′-dipyridine anion |
| H3L4 | p-terphenyl-3,4″,5-tricarboxylic acid |
| H3L5 | 4-(2-carboxyphenoxy)benzene-1,3-dioic acid |
| H2L6 | 5-(4H-1,2,4-triazol-4-yl)benzene-1,3-dicarboxylic acid |
| L7 | 2′,5′-bis(methoxymethyl)-[1,1′:4′,1″-terphenyl]-4,4″-dicarboxylate |
| LMCT | ligand-to-metal charge transfer |
| LnMOFs | lanthanide metal–organic frameworks |
| LUMOs | lowest unoccupied molecular orbitals |
| Mg-MOF | {[Mg3(ndc)2.5(HCO2)2(H2O)][NH2Me2]⋅2H2O⋅DMF} |
| MIL-61 | Ga(OH)(btec)·0.5H2O |
| MLCT | metal-to-ligand charge transfer |
| MOFs | Metal–organic frameworks |
| mpca | 2-pyrazine-5-methyl-carboxylate |
| H4mtb | 4-[tris(4-carboxyphenyl)methyl]benzoic acid |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide |
| H2NDC | 2,6-naphthalenedicarboxylate |
| NB | nitrobenzene |
| 1,4-ndc | 1,4-naphthalenedicarboxylate |
| NFAs | nitrofuran antibiotics |
| NFT | nitrofurantoin |
| NFZ | nitrofurazone |
| 2,4-NP | 2,4-dinitrophenol |
| 3-NP | 3-nitrophenol |
| 4-NP | 4-nitrophenol |
| N-GQDs | N atom-doped graphene quantum dots |
| m-NT | m-nitrotoluene |
| o-NT | o-nitrotoluene |
| NTA | nitrilotriacetate |
| OBA | 4,4′-oxybis(benzoate) |
| ODA | oxydiacetic acid |
| ox | oxalate |
| HPAN | hydrolyzed polyacrylonitrile |
| PDC | pyridine-3,5-dicarboxylate |
| PET | photoinduced electron transfer |
| Phen | 1,10-phenanthroline |
| QPTCA | 1,1′:4′,1″:4″,1′″-quaterphenyl-3,3′″,5,5′″-tetracarboxylic acid |
| H2S | hydrogen sulfide |
| tctpH3 | tris(p-carboxylato)triphenylphosphine |
| TDGA | thiodiglycolic acid |
| TNP | 2,4,6-trinitrophenol |
| TNT | 2,4,6-trinitrotoluene |
| VCM | vinyl chloride monomer |
| ZIF-8 | zeolitic imidazolate framework-8 |
References
- Colon, Y.J.; Snurr, R.Q. High-throughput computational screening of metal-organic frameworks. Chem. Soc. Rev. 2014, 43, 5735–5749. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Bu, X.; Nguyen, E.T.; Zhai, Q.G.; Mao, C.; Feng, P. Multivariable Modular Design of Pore Space Partition. J. Am. Chem. Soc. 2016, 138, 15102–15105. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhou, W.; Qian, G.; Chen, B. Methane storage in metal-organic frameworks. Chem. Soc. Rev. 2014, 43, 5657–5678. [Google Scholar] [CrossRef] [PubMed]
- Flaig, R.W.; Osborn Popp, T.M.; Fracaroli, A.M.; Kapustin, E.A.; Kalmutzki, M.J.; Altamimi, R.M.; Fathieh, F.; Reimer, J.A.; Yaghi, O.M. The Chemistry of CO2 Capture in an Amine-Functionalized Metal-Organic Framework under Dry and Humid Conditions. J. Am. Chem. Soc. 2017, 139, 12125–12128. [Google Scholar] [CrossRef] [PubMed]
- Medishetty, R.; Nalla, V.; Nemec, L.; Henke, S.; Mayer, D.; Sun, H.; Reuter, K.; Fischer, R.A. A New Class of Lasing Materials: Intrinsic Stimulated Emission from Nonlinear Optically Active Metal-Organic Frameworks. Adv. Mater. 2017, 29, 1605637. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Hao, Z.M.; Song, X.Z.; Meng, X.; Zhao, S.N.; Song, S.Y.; Zhang, H.J. A new type of double-chain based 3D lanthanide(III) metal-organic framework demonstrating proton conduction and tunable emission. Chem. Commun. 2014, 50, 1912–1914. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.V.; Liao, Y.-T.; Kang, T.-C.; Chen, J.E.; Yoshikawa, T.; Nakasaka, Y.; Masuda, T.; Wu, K.C.W. A metal-free, high nitrogen-doped nanoporous graphitic carbon catalyst for an effective aerobic HMF-to-FDCA conversion. Green Chem. 2016, 18, 5957–5961. [Google Scholar] [CrossRef]
- Fan, S.; Dong, W.; Huang, X.; Gao, H.; Wang, J.; Jin, Z.; Tang, J.; Wang, G. In Situ-Induced Synthesis of Magnetic Cu-CuFe2O4@HKUST-1 Heterostructures with Enhanced Catalytic Performance for Selective Aerobic Benzylic C–H Oxidation. ACS Catal. 2016, 7, 243–249. [Google Scholar] [CrossRef]
- Du, P.-Y.; Gu, W.; Liu, X. A three-dimensional Nd(III)-based metal–organic framework as a smart drug carrier. New J. Chem. 2016, 40, 9017–9020. [Google Scholar] [CrossRef]
- Vallejo, J.; Fortea-Pérez, F.R.; Pardo, E.; Benmansour, S.; Castro, I.; Krzystek, J.; Armentano, D.; Cano, J. Guest-dependent single-ion magnet behaviour in a cobalt(II) metal–organic framework. Chem. Sci. 2016, 7, 2286–2293. [Google Scholar] [CrossRef]
- Zhao, S.N.; Song, X.Z.; Zhu, M.; Meng, X.; Wu, L.L.; Feng, J.; Song, S.Y.; Zhang, H.J. Encapsulation of Ln(III) Ions/Dyes within a Microporous Anionic MOF by Post-synthetic Ionic Exchange Serving as a Ln(III) Ion Probe and Two-Color Luminescent Sensors. Chem. Eur. J. 2015, 21, 9748–9752. [Google Scholar] [CrossRef] [PubMed]
- Wales, D.J.; Grand, J.; Ting, V.P.; Burke, R.D.; Edler, K.J.; Bowen, C.R.; Mintova, S.; Burrows, A.D. Gas sensing using porous materials for automotive applications. Chem. Soc. Rev. 2015, 44, 4290–4321. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Yang, G.; Song, X.; Zhu, M.; Meng, X.; Zhao, S.; Song, S.; Zhang, H. A europium(III) based metal–organic framework: Bifunctional properties related to sensing and electronic conductivity. J. Mater. Chem. A 2014, 2, 237–244. [Google Scholar] [CrossRef]
- Niu, S.; Wang, Z.; Zhou, T.; Yu, M.; Yu, M.; Qiu, J. A Polymetallic Metal-Organic Framework-Derived Strategy toward Synergistically Multidoped Metal Oxide Electrodes with Ultralong Cycle Life and High Volumetric Capacity. Adv. Funct. Mater. 2017, 27, 1605332. [Google Scholar] [CrossRef]
- Zhao, S.-N.; Song, X.-Z.; Song, S.-Y.; Zhang, H.-J. Highly efficient heterogeneous catalytic materials derived from metal-organic framework supports/precursors. Coord. Chem. Rev. 2017, 337, 80–96. [Google Scholar] [CrossRef]
- Salunkhe, R.R.; Kaneti, Y.V.; Kim, J.; Kim, J.H.; Yamauchi, Y. Nanoarchitectures for Metal-Organic Framework-Derived Nanoporous Carbons toward Supercapacitor Applications. Acc. Chem. Res. 2016, 49, 2796–2806. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Hohman, J.N.; Li, F.H.; Jia, C.; Solis-Ibarra, D.; Wu, B.; Dahl, J.E.; Carlson, R.M.; Tkachenko, B.A.; Fokin, A.A.; et al. Hybrid metal-organic chalcogenide nanowires with electrically conductive inorganic core through diamondoid-directed assembly. Nat. Mater. 2017, 16, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yue, Z.; Liu, Y. Incorporation of imidazole within the metal-organic framework UiO-67 for enhanced anhydrous proton conductivity. Dalton Trans. 2015, 44, 12976–12980. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tang, J.; He, L.; Liu, Y.; Liu, Y.; Chen, C.; Tang, Z. Core-Shell Upconversion Nanoparticle@Metal-Organic Framework Nanoprobes for Luminescent/Magnetic Dual-Mode Targeted Imaging. Adv. Mater. 2015, 27, 4075–4080. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent functional metal-organic frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, T.; Lin, W. Rational synthesis of noncentrosymmetric metal-organic frameworks for second-order nonlinear optics. Chem. Rev. 2012, 112, 1084–1104. [Google Scholar] [CrossRef] [PubMed]
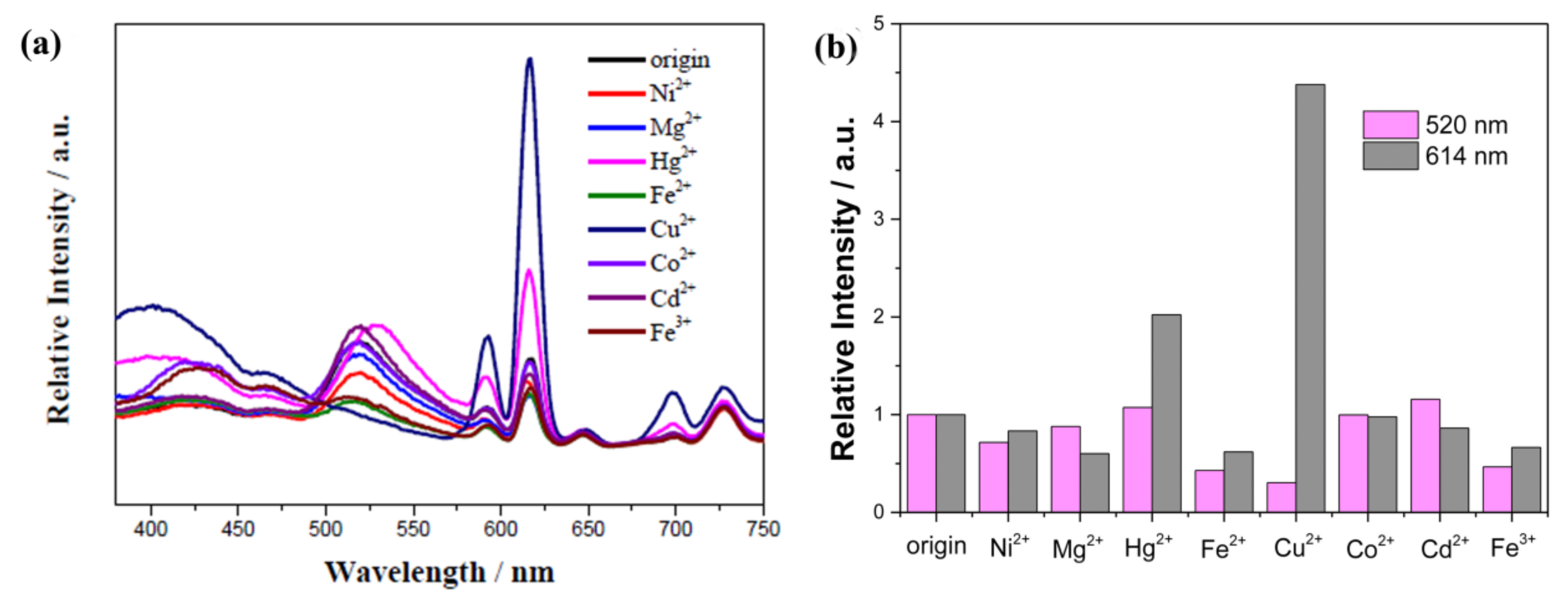
- Hao, Z.; Song, X.; Zhu, M.; Meng, X.; Zhao, S.; Su, S.; Yang, W.; Song, S.; Zhang, H. One-dimensional channel-structured Eu-MOF for sensing small organic molecules and Cu2+ ion. J. Mater. Chem. A 2013, 1, 11043–11050. [Google Scholar] [CrossRef]
- Liu, W.; Huang, X.; Xu, C.; Chen, C.; Yang, L.; Dou, W.; Chen, W.; Yang, H.; Liu, W. A Multi-responsive Regenerable Europium-Organic Framework Luminescent Sensor for Fe3+, CrVI Anions, and Picric Acid. Chem. Eur. J. 2016, 22, 18769–18776. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.X.; Yan, B. Eu(III)-functionalized In-MOF (In(OH)bpydc) as fluorescent probe for highly selectively sensing organic small molecules and anions especially for CHCl3 and MnO4. J. Colloid Interface Sci. 2017, 504, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.-N.; Yan, B. Rapid and facile ratiometric detection of CO32− based on heterobimetallic metal-organic frameworks (Eu/Pt-MOFs). Dyes Pigment. 2017, 142, 1–7. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal-organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.M.; Cheng, R.R.; Xu, H.; Wang, W.M.; Zhao, B. A Sensitive Luminescent Acetylacetone Probe Based on Zn-MOF with Six-Fold Interpenetration. Chem. Eur. J. 2017, 23, 13289–13293. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Chen, B.; Qian, G. Lanthanide metal-organic frameworks for luminescent sensing and light-emitting applications. Coord. Chem. Rev. 2014, 273–274, 76–86. [Google Scholar] [CrossRef]
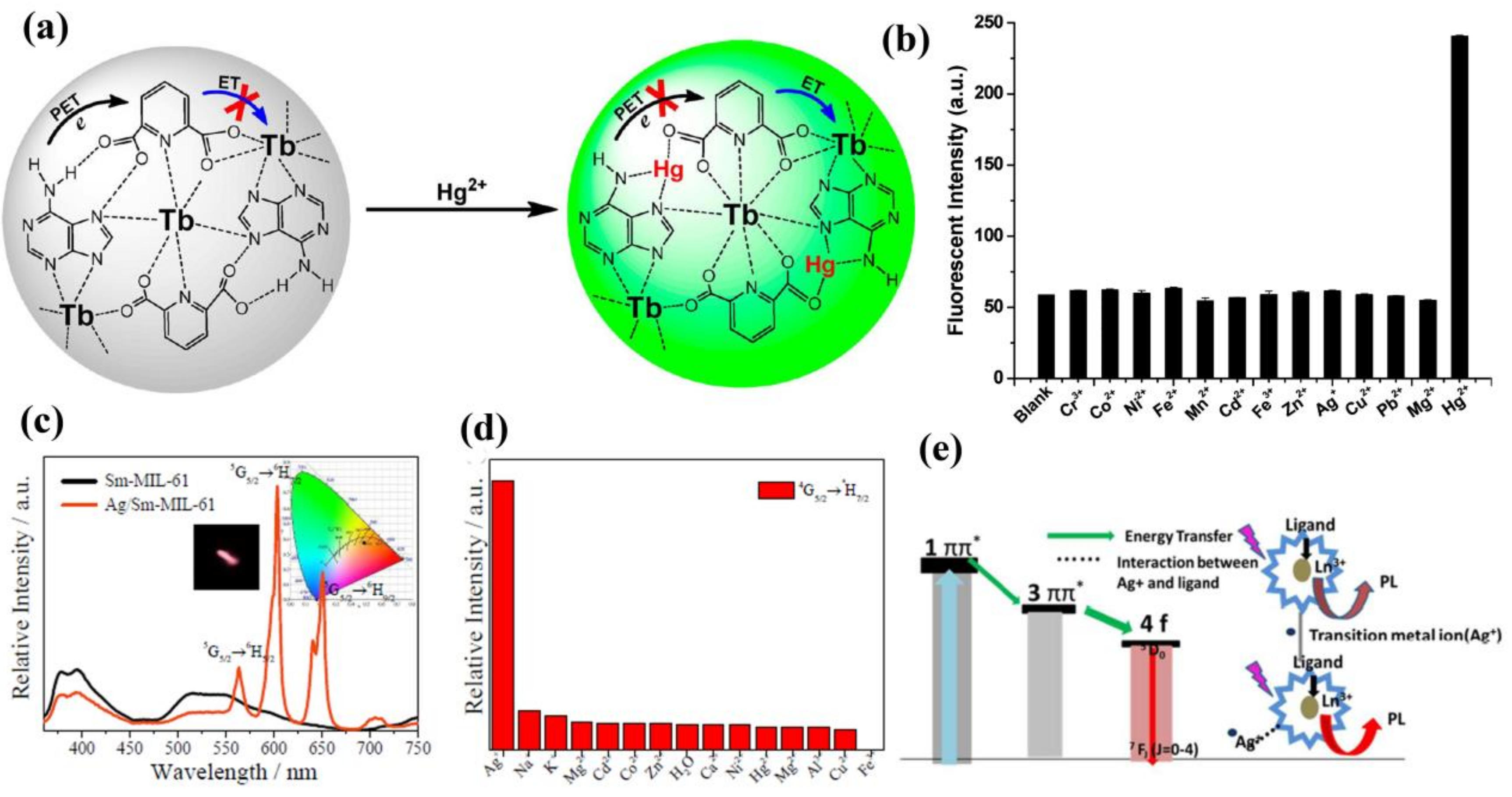
- Zhao, S.-N.; Wu, L.-L.; Feng, J.; Song, S.-Y.; Zhang, H.-J. An ideal detector composed of a 3D Gd-based coordination polymer for DNA and Hg2+ ion. Inorg. Chem. Front. 2016, 3, 376–380. [Google Scholar] [CrossRef]
- Doonan, C.; Ricco, R.; Liang, K.; Bradshaw, D.; Falcaro, P. Metal-Organic Frameworks at the Biointerface: Synthetic Strategies and Applications. Acc. Chem. Res. 2017, 50, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Roales, J.; Moscoso, F.G.; Gamez, F.; Lopes-Costa, T.; Sousaraei, A.; Casado, S.; Castro-Smirnov, J.R.; Cabanillas-Gonzalez, J.; Almeida, J.; Queiros, C.; et al. Preparation of Luminescent Metal-Organic Framework Films by Soft-Imprinting for 2,4-Dinitrotoluene Sensing. Materials 2017, 10, 992. [Google Scholar] [CrossRef] [PubMed]
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal-organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-L.; Chen, X.; Hou, G.-H.; Guan, R.-F.; Shao, R.; Xie, M.-H. A Multiresponsive Metal-Organic Framework: Direct Chemiluminescence, Photoluminescence, and Dual Tunable Sensing Applications. Adv. Funct. Mater. 2016, 26, 393–398. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, B.; Wang, X.; Xie, L.H.; Li, J.; Xie, Y.; Li, J.R. A Copper(II)-Paddlewheel Metal-Organic Framework with Exceptional Hydrolytic Stability and Selective Adsorption and Detection Ability of Aniline in Water. ACS Appl. Mater. Interfaces 2017, 9, 27027–27035. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, J.; Liu, Z. A chemically stable europium metal-organic framework for bifunctional chemical sensor and recyclable on–off–on vapor response. J. Solid State Chem. 2017, 251, 243–247. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Jiang, M.; Liu, Y.; Zhang, L.; Wu, P. A Highly Chemically Stable Metal-Organic Framework as a Luminescent Probe for the Regenerable Ratiometric Sensing of pH. Chem. Eur. J. 2016, 22, 13023–13027. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Xu, H.; Yue, Y.; Guo, Z.; Yu, J.; Chen, Z.; Gao, J.; Yang, Y.; Qian, G.; Chen, B. A luminescent mixed-lanthanide metal-organic framework thermometer. J. Am. Chem. Soc. 2012, 134, 3979–3982. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, D.; Cui, Y.; Yang, Y.; Qian, G. A Eu/Tb-mixed MOF for luminescent high-temperature sensing. J. Solid State Chem. 2017, 246, 341–345. [Google Scholar] [CrossRef]
- Rao, X.; Song, T.; Gao, J.; Cui, Y.; Yang, Y.; Wu, C.; Chen, B.; Qian, G. A highly sensitive mixed lanthanide metal-organic framework self-calibrated luminescent thermometer. J. Am. Chem. Soc. 2013, 135, 15559–15564. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, G.; Geng, D.; Shang, M.; Peng, C.; Lin, J. Color-tunable emission and energy transfer in Ca3Gd7(PO4)(SiO4)5O2: Ce3+/Tb3+/Mn2+ phosphors. Inorg. Chem. 2012, 51, 11655–11664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Geng, D.; Kang, X.; Shang, M.; Wu, Y.; Li, X.; Lian, H.; Cheng, Z.; Lin, J. Rapid, large-scale, morphology-controllable synthesis of YOF:Ln3+ (Ln = Tb, Eu, Tm, Dy, Ho, Sm) nano-/microstructures with multicolor-tunable emission properties. Inorg. Chem. 2013, 52, 12986–12994. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Li, K.; Lian, H.; Shang, M.; Lin, J. Crystal-site engineering control for the reduction of Eu3+ to Eu2+ in CaYAlO4: Structure refinement and tunable emission properties. ACS Appl. Mater. Interfaces 2015, 7, 2715–2725. [Google Scholar] [CrossRef] [PubMed]
- Mao, J. Structures and luminescent properties of lanthanide phosphonates. Coord. Chem. Rev. 2007, 251, 1493–1520. [Google Scholar] [CrossRef]
- Song, J.L.; Mao, J.G. New types of blue, red or near IR luminescent phosphonate-decorated lanthanide oxalates. Chem. Eur. J. 2005, 11, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Parmentier, A.B.; Smet, P.F.; Poelman, D. Broadband Luminescence in Rare Earth Doped Sr2SiS4: Relating Energy Levels of Ce3+ and Eu2+. Materials 2013, 6, 3663–3675. [Google Scholar] [CrossRef] [PubMed]
- Binnemans, K. Lanthanide-based luminescent hybrid materials. Chem. Rev. 2009, 109, 4283–4374. [Google Scholar] [CrossRef] [PubMed]
- Choppin, G.R.; Peterman, D.R. Applications of lanthanide luminescence spectroscopy to solution studies of coordination chemistry. Coord. Chem. Rev. 1998, 174, 283–299. [Google Scholar] [CrossRef]
- Moore, E.G.; Samuel, A.P.S.; Raymond, K.N. From Antenna to Assay: Lessons Learned in Lanthanide Luminescence. Acc. Chem. Res. 2009, 42, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Bünzli, J.-C.G. Lanthanide Luminescence for Biomedical Analyses and Imaging. Chem. Rev. 2010, 110, 2729–2755. [Google Scholar] [CrossRef] [PubMed]
- Eliseeva, S.V.; Bunzli, J.C. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef] [PubMed]
- Bunzli, J.C.; Piguet, C. Taking advantage of luminescent lanthanide ions. Chem. Soc. Rev. 2005, 34, 1048–1077. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.-J.; Xu, G.-T.; Chen, Z.-N. Recent advances in lanthanide luminescence with metal-organic chromophores as sensitizers. Coord. Chem. Rev. 2014, 273–274, 47–62. [Google Scholar] [CrossRef]
- Weng, H.; Yan, B. N-GQDs and Eu3+ co-encapsulated anionic MOFs: Two-dimensional luminescent platform for decoding benzene homologues. Dalton Trans. 2016, 45, 8795–8801. [Google Scholar] [CrossRef] [PubMed]
- Parker, D. Luminescent lanthanide sensors for pH, pO2 and selected anions. Coord. Chem. Rev. 2000, 205, 109–130. [Google Scholar] [CrossRef]
- Sun, Y.-G.; Gu, X.-F.; Ding, F.; Smet, P.F.; Gao, E.-J.; Poelman, D.; Verpoort, F. Synthesis, Crystal Structures, and Properties of Novel Heterometallic La/Pr−Cu−K and Sm/Eu/Tb−Cu Coordination Polymers. Cryst. Growth Des. 2010, 10, 1059–1067. [Google Scholar] [CrossRef]
- Liu, D.; Lu, K.; Poon, C.; Lin, W. Metal-organic frameworks as sensory materials and imaging agents. Inorg. Chem. 2014, 53, 1916–1924. [Google Scholar] [CrossRef] [PubMed]
- Yi, F.-Y.; Chen, D.; Wu, M.-K.; Han, L.; Jiang, H.-L. Chemical Sensors Based on Metal-Organic Frameworks. ChemPlusChem 2016, 81, 675–690. [Google Scholar] [CrossRef]
- Gaetke, L.M.; Chow-Johnson, H.S.; Chow, C.K. Copper: Toxicological relevance and mechanisms. Arch. Toxicol. 2014, 88, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Qu, K.; Xu, C.; Ren, J.; Qu, X. Visual and quantitative detection of copper ions using magnetic silica nanoparticles clicked on multiwalled carbon nanotubes. Chem. Commun. 2010, 46, 6572–6574. [Google Scholar] [CrossRef] [PubMed]
- Wen, R.M.; Han, S.D.; Ren, G.J.; Chang, Z.; Li, Y.W.; Bu, X.H. A flexible zwitterion ligand based lanthanide metal-organic framework for luminescence sensing of metal ions and small molecules. Dalton Trans. 2015, 44, 10914–10917. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Yang, W.; Zhou, N.; Qian, R.; Zhang, Y.; Lou, K.; Wang, R.; Wang, W. Fluorescent theranostic agents for Hg2+ detection and detoxification treatment. Chem. Commun. 2015, 51, 4443–4446. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Che, J.X.; Hu, Y.Z.; Dong, X.W.; Liu, X.Y.; Che, C.M. Alkenyl/thiol-derived metal-organic frameworks (MOFs) by means of postsynthetic modification for effective mercury adsorption. Chem. Eur. J. 2014, 20, 14090–14095. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wang, L.; Xiao, Y.; Fronczek, F.R.; Xue, M.; Cui, Y.; Qian, G. A luminescent metal-organic framework with Lewis basic pyridyl sites for the sensing of metal ions. Angew Chem. Int. Ed. 2009, 48, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Hou, L.; Wu, W.P.; Dou, A.N.; Wang, Y.Y. Highly selective luminescence sensing for Cu2+ ions and selective CO2 capture in a doubly interpenetrated MOF with Lewis basic pyridyl sites. Dalton Trans. 2015, 44, 4423–4427. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Liu, S.; Liu, Y.; Miao, J.; Li, S.; Zhang, L.; Shi, Z.; Zheng, Z. Cation sensing by a luminescent metal-organic framework with multiple Lewis basic sites. Inorg. Chem. 2013, 52, 2799–2801. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Liu, Q.K.; Ma, J.P.; Dong, Y.B. Cd(II)-coordination framework: Synthesis, anion-induced structural transformation, anion-responsive luminescence, and anion separation. Inorg. Chem. 2013, 52, 3225–3235. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, G.P.; Zhao, Y.; Wu, W.P.; Liu, B.; Wang, Y.Y. Three new solvent-directed Cd(II)-based MOFs with unique luminescent properties and highly selective sensors for Cu2+ cations and nitrobenzene. Dalton Trans. 2015, 44, 3271–3277. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.X.; Wu, Y.P.; Dong, W.W.; Zhao, J.; Li, D.S.; Zhang, J. An Ultrastable Europium(III)-Organic Framework with the Capacity of Discriminating Fe2+/Fe3+ Ions in Various Solutions. Inorg. Chem. 2016, 55, 10114–10117. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.; Yan, B. A Eu(III) doped metal-organic framework conjugated with fluorescein-labeled single-stranded DNA for detection of Cu(II) and sulfide. Anal. Chim. Acta 2017, 988, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Zhang, C.; Chen, S.; Han, L.; Zheng, H. Two Lanthanide Metal-Organic Frameworks as Remarkably Selective and Sensitive Bifunctional Luminescence Sensor for Metal Ions and Small Organic Molecules. ACS Appl. Mater. Interfaces 2017, 9, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Sun, Y.; Zhang, L.; Sun, D.; Liu, F.; Meng, Q.; Wang, R.; Sun, D. A tubular europium-organic framework exhibiting selective sensing of Fe3+ and Al3+ over mixed metal ions. Chem. Commun. 2013, 49, 11557–11559. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Liu, B.; Chen, Y. Lanthanide Coordination Polymer Nanoparticles for Sensing of Mercury(II) by Photoinduced Electron Transfer. ACS Nano 2012, 6, 10505–10511. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, Q.; Niu, Z.; Zhou, X.; Yang, T.; Huang, W. Lanthanide metal–organic frameworks assembled from a fluorene-based ligand: Selective sensing of Pb2+ and Fe3+ ions. J. Mater. Chem. C 2016, 4, 1900–1905. [Google Scholar] [CrossRef]
- Ji, G.; Liu, J.; Gao, X.; Sun, W.; Wang, J.; Zhao, S.; Liu, Z. A luminescent lanthanide MOF for selectively and ultra-high sensitively detecting Pb2+ ions in aqueous solution. J. Mater. Chem. A 2017, 5, 10200–10205. [Google Scholar] [CrossRef]
- Sun, N.; Yan, B. Ag+-induced photoluminescence enhancement in lanthanide post-functionalized MOFs and Ag+ sensing. Phys. Chem. Chem. Phys. 2017, 19, 9174–9180. [Google Scholar] [CrossRef] [PubMed]
- Mahata, P.; Mondal, S.K.; Singha, D.K.; Majee, P. Luminescent rare-earth-based MOFs as optical sensors. Dalton Trans. 2017, 46, 301–328. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Fan, J.; Shang, M.; Li, K.; Zhang, Y.; Lian, H.; Lin, J. Pechini-type sol–gel synthesis and multicolor-tunable emission properties of GdY(MoO4)3:RE3+ (RE = Eu, Dy, Sm, Tb) phosphors. Opt. Mater. 2016, 51, 162–170. [Google Scholar] [CrossRef]
- Chen, B.; Wang, L.; Zapata, F.; Qian, G.; Lobkovsky, E.B. A Luminescent Microporous Metal-Organic Framework for the Recognition and Sensing of Anions. J. Am. Chem. Soc. 2008, 130, 6718–6719. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.M.; Shi, W.; Xu, N.; Cheng, P. Highly selective luminescent sensing of fluoride and organic small-molecule pollutants based on novel lanthanide metal-organic frameworks. Inorg. Chem. 2013, 52, 8082–8090. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.F.; Hu, H.C.; Zhang, Z.Y.; Xiong, G.; Zhao, B. Heterometal-organic frameworks as highly sensitive and highly selective luminescent probes to detect I(−) ions in aqueous solutions. Chem. Commun. 2015, 51, 3985–3988. [Google Scholar] [CrossRef] [PubMed]
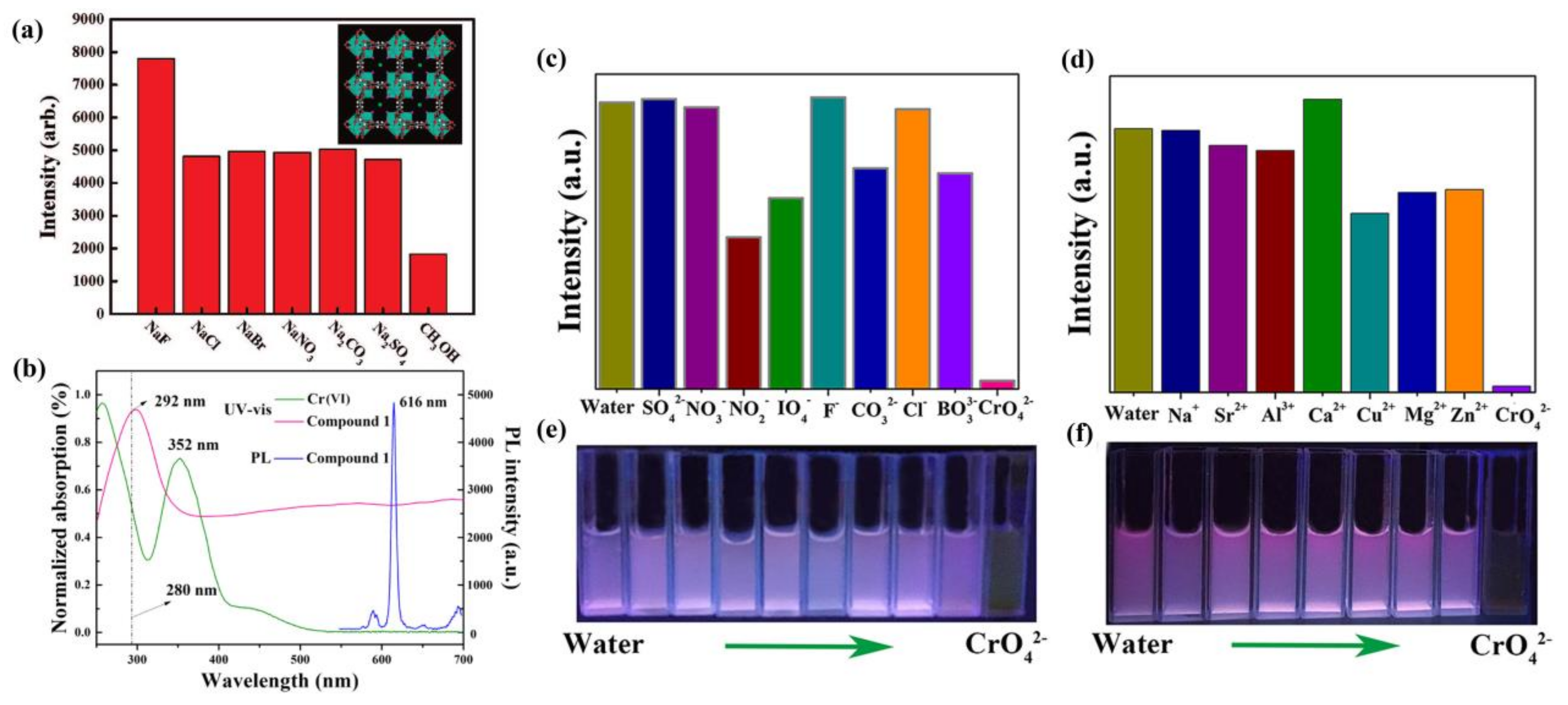
- Liu, W.; Wang, Y.; Bai, Z.; Li, Y.; Wang, Y.; Chen, L.; Xu, L.; Diwu, J.; Chai, Z.; Wang, S. Hydrolytically Stable Luminescent Cationic Metal Organic Framework for Highly Sensitive and Selective Sensing of Chromate Anions in Natural Water Systems. ACS Appl. Mater. Interfaces 2017, 9, 16448–16457. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.M.; Kirman, C.R.; Proctor, D.M.; Haws, L.C.; Suh, M.; Hays, S.M.; Hixon, J.G.; Harris, M.A. A chronic oral reference dose for hexavalent chromium-induced intestinal cancer. J. Appl. Toxicol. 2014, 34, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Sun, L.; Yan, Y.; Shi, H.; Wang, B.; Liang, Z.; Li, J. A novel photo- and hydrochromic europium metal–organic framework with good anion sensing properties. J. Mater. Chem. C 2017, 5, 8999–9004. [Google Scholar] [CrossRef]
- Ding, B.; Liu, S.X.; Cheng, Y.; Guo, C.; Wu, X.X.; Guo, J.H.; Liu, Y.Y.; Li, Y. Heterometallic Alkaline Earth-Lanthanide BaII-LaIII Microporous Metal-Organic Framework as Bifunctional Luminescent Probes of Al3+ and MnO4−. Inorg. Chem. 2016, 55, 4391–4402. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wan, X.; Song, H.; Hao, L.; Su, Y.; Lv, Y. Metal–organic frameworks (MOFs) combined with ZnO quantum dots as a fluorescent sensing platform for phosphate. Sens. Actuators B 2014, 197, 50–57. [Google Scholar] [CrossRef]
- Xu, H.; Xiao, Y.; Rao, X.; Dou, Z.; Li, W.; Cui, Y.; Wang, Z.; Qian, G. A metal–organic framework for selectively sensing of PO43− anion in aqueous solution. J. Alloys Compd. 2011, 509, 2552–2554. [Google Scholar] [CrossRef]
- Xu, H.; Cao, C.S.; Zhao, B. A water-stable lanthanide-organic framework as a recyclable luminescent probe for detecting pollutant phosphorus anions. Chem. Commun. 2015, 51, 10280–10283. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, S.; Pascual, L.; Licchelli, M.; Martinez-Manez, R.; Gil, S.; Costero, A.M.; Sancenon, F. Chromogenic Detection of Aqueous Formaldehyde Using Functionalized Silica Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 14318–14322. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Huang, J.; Zhu, H.; Liu, L.; Feng, Y.; Hu, G.; Yu, X. Dual-emitting fluorescence of Eu/Zr-MOF for ratiometric sensing formaldehyde. Sens. Actuators B 2017, 253, 275–282. [Google Scholar] [CrossRef]
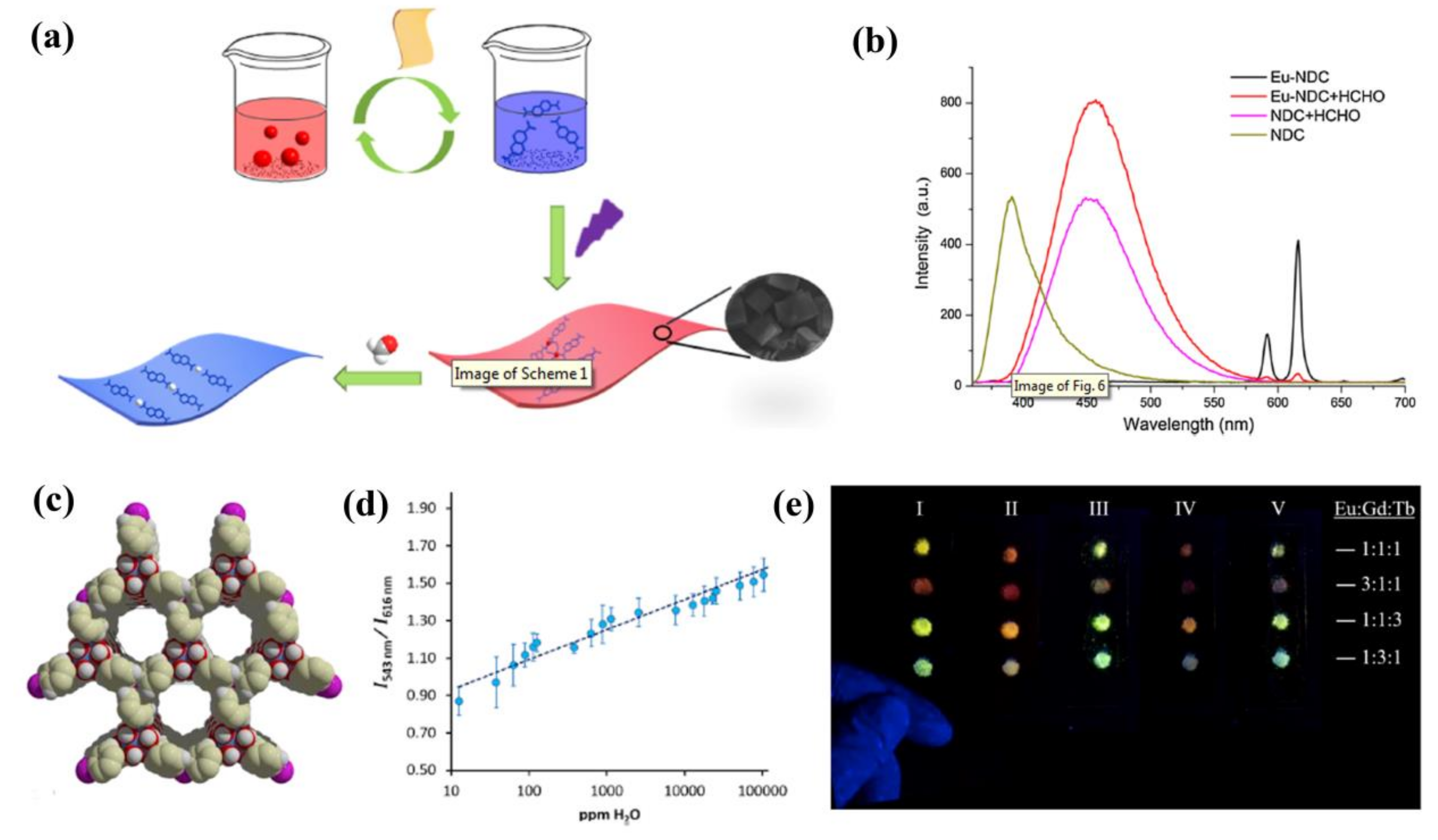
- Wang, Y.; Zhang, G.; Zhang, F.; Chu, T.; Yang, Y. A novel lanthanide MOF thin film: The highly performance self-calibrating luminescent sensor for detecting formaldehyde as an illegal preservative in aquatic product. Sens. Actuators B 2017, 251, 667–673. [Google Scholar] [CrossRef]
- Dunning, S.G.; Nuñez, A.J.; Moore, M.D.; Steiner, A.; Lynch, V.M.; Sessler, J.L.; Holliday, B.J.; Humphrey, S.M. A Sensor for Trace H2O Detection in D2O. Chem 2017, 2, 579–589. [Google Scholar] [CrossRef]
- Wiberg, K.B. The deuterium isotope effect. Chem. Rev. 1995, 55, 713–743. [Google Scholar]
- Wehner, T.; Seuffert, M.T.; Sorg, J.R.; Schneider, M.; Mandel, K.; Sextl, G.; Müller-Buschbaum, K. Composite materials combining multiple luminescent MOFs and superparamagnetic microparticles for ratiometric water detection. J. Mater. Chem. C 2017, 5, 10133–10142. [Google Scholar] [CrossRef]
- Guo, Z.; Xu, H.; Su, S.; Cai, J.; Dang, S.; Xiang, S.; Qian, G.; Zhang, H.; O’Keeffe, M.; Chen, B. A robust near infrared luminescent ytterbium metal-organic framework for sensing of small molecules. Chem. Commun. 2011, 47, 5551–5553. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.X.; Huang, X.F.; Song, X.Q.; Liu, W.S. Assembly of framework-isomeric 4 d–4 f heterometallic metal-organic frameworks with neutral/anionic micropores and guest-tuned luminescence properties. Chem. Eur. J. 2013, 19, 3590–3595. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.W.; Jian, B.R.; Huang, S.C.; Huang, C.H.; Hsu, K.F. Synthesis and characterization of three ytterbium coordination polymers featuring various cationic species and a luminescence study of a terbium analogue with open channels. Inorg. Chem. 2010, 49, 2316–2324. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shan, L.; Fan, Y.; Jia, J.; Xu, J.; Wang, L. Fabrication of Ln-MOFs with color-tunable photoluminescence and sensing for small molecules. J. Solid State Chem. 2017, 245, 132–137. [Google Scholar] [CrossRef]
- Wang, L.; Fan, G.; Xu, X.; Chen, D.; Wang, L.; Shi, W.; Cheng, P. Detection of polychlorinated benzenes (persistent organic pollutants) by a luminescent sensor based on a lanthanide metal–organic framework. J. Mater. Chem. A 2017, 5, 5541–5549. [Google Scholar] [CrossRef]
- Hu, Z.; Deibert, B.J.; Li, J. Luminescent metal-organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef] [PubMed]
- Salinas, Y.; Martinez-Manez, R.; Marcos, M.D.; Sancenon, F.; Costero, A.M.; Parra, M.; Gil, S. Optical chemosensors and reagents to detect explosives. Chem. Soc. Rev. 2012, 41, 1261–1296. [Google Scholar] [CrossRef] [PubMed]
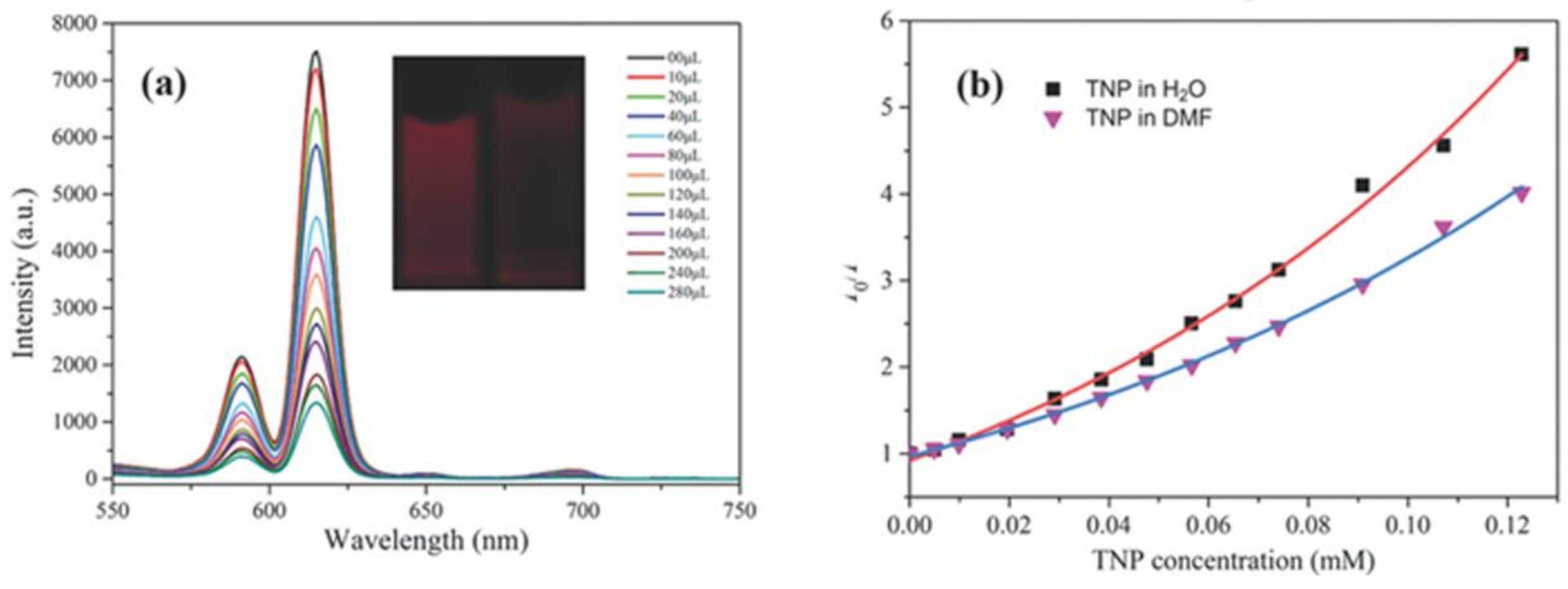
- Zhang, S.R.; Du, D.Y.; Qin, J.S.; Bao, S.J.; Li, S.L.; He, W.W.; Lan, Y.Q.; Shen, P.; Su, Z.M. A fluorescent sensor for highly selective detection of nitroaromatic explosives based on a 2D, extremely stable, metal-organic framework. Chem. Eur. J. 2014, 20, 3589–3594. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S.; Zheng, C.; Zhang, X.; Emge, T.J.; Li, J. New microporous metal-organic framework demonstrating unique selectivity for detection of high explosives and aromatic compounds. J. Am. Chem. Soc. 2011, 133, 4153–4155. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Li, Y.; Ma, Y.; Li, L. Dual-functional recyclable luminescent sensors based on 2D lanthanide-based metal-organic frameworks for highly sensitive detection of Fe3+ and 2,4-dinitrophenol. Dyes Pigment. 2017, 146, 263–271. [Google Scholar] [CrossRef]
- Zhao, S.-N.; Song, X.-Z.; Zhu, M.; Meng, X.; Wu, L.-L.; Song, S.-Y.; Wang, C.; Zhang, H.-J. Highly thermostable lanthanide metal–organic frameworks exhibiting unique selectivity for nitro explosives. RSC Adv. 2015, 5, 93–98. [Google Scholar] [CrossRef]
- Zhu, M.; Song, X.Z.; Song, S.Y.; Zhao, S.N.; Meng, X.; Wu, L.L.; Wang, C.; Zhang, H.J. A Temperature-Responsive Smart Europium Metal-Organic Framework Switch for Reversible Capture and Release of Intrinsic Eu3+ Ions. Adv. Sci. 2015, 2, 1500012. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Hao, X.-M.; Liu, J.-L.; Wu, L.-W.; Wang, H.; Wu, Y.-B.; Yang, D.; Guo, W.-L. Construction of Eu(III)- and Tb(III)-MOFs with photoluminescence for sensing small molecules based on furan-2,5-dicarboxylic acid. J. Solid State Chem. 2017, 255, 76–81. [Google Scholar] [CrossRef]
- Song, X.-Z.; Song, S.-Y.; Zhao, S.-N.; Hao, Z.-M.; Zhu, M.; Meng, X.; Wu, L.-L.; Zhang, H.-J. Single-Crystal-to-Single-Crystal Transformation of a Europium(III) Metal-Organic Framework Producing a Multi-responsive Luminescent Sensor. Adv. Funct. Mater. 2014, 24, 4034–4041. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, S.; Day, G.; Wang, X.; Yang, X.; Zhou, H.-C. Luminescent sensors based on metal-organic frameworks. Coord. Chem. Rev. 2018, 354, 28–45. [Google Scholar] [CrossRef]
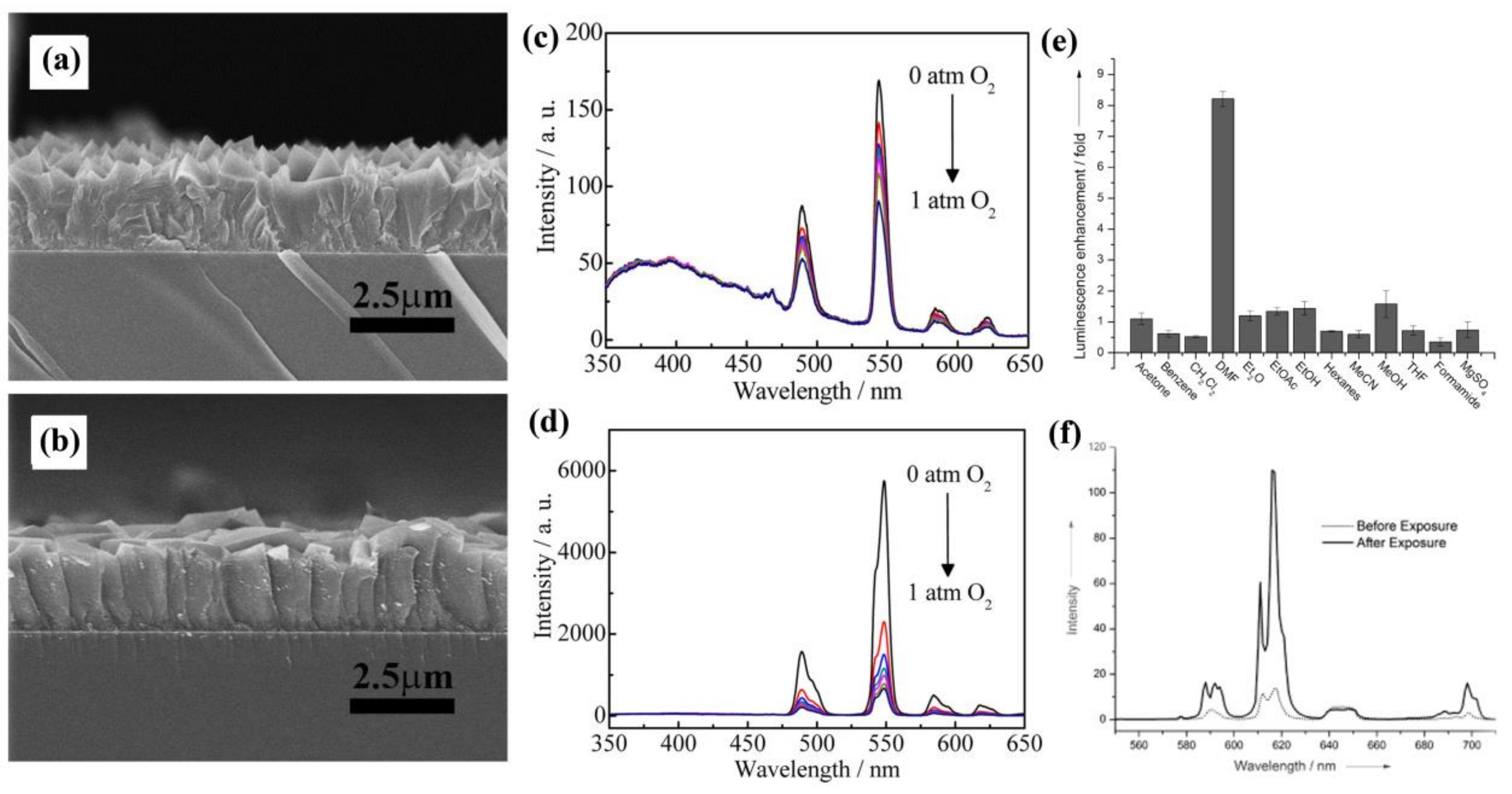
- Dou, Z.; Yu, J.; Cui, Y.; Yang, Y.; Wang, Z.; Yang, D.; Qian, G. Luminescent metal-organic framework films as highly sensitive and fast-response oxygen sensors. J. Am. Chem. Soc. 2014, 136, 5527–5530. [Google Scholar] [CrossRef] [PubMed]
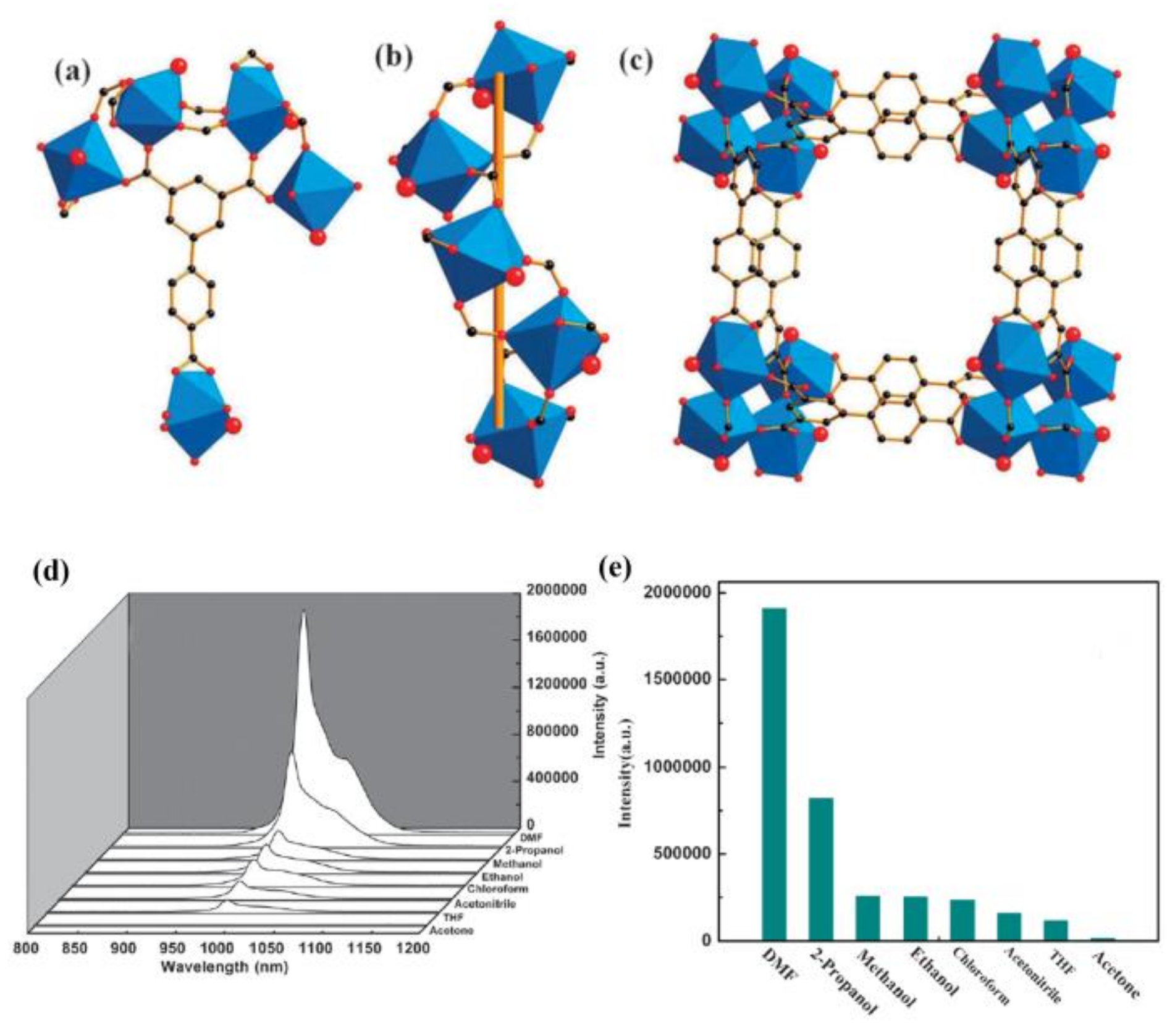
- Li, Y.; Zhang, S.; Song, D. A luminescent metal-organic framework as a turn-on sensor for DMF vapor. Angew. Chem. Int. Ed. 2013, 52, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhang, J.; Wang, S.; Zhu, A.; Hou, X. Sensing during in situ growth of Mn-doped ZnS QDs: A phosphorescent sensor for detection of H2S in biological samples. Chem. Eur. J. 2014, 20, 952–956. [Google Scholar] [CrossRef] [PubMed]
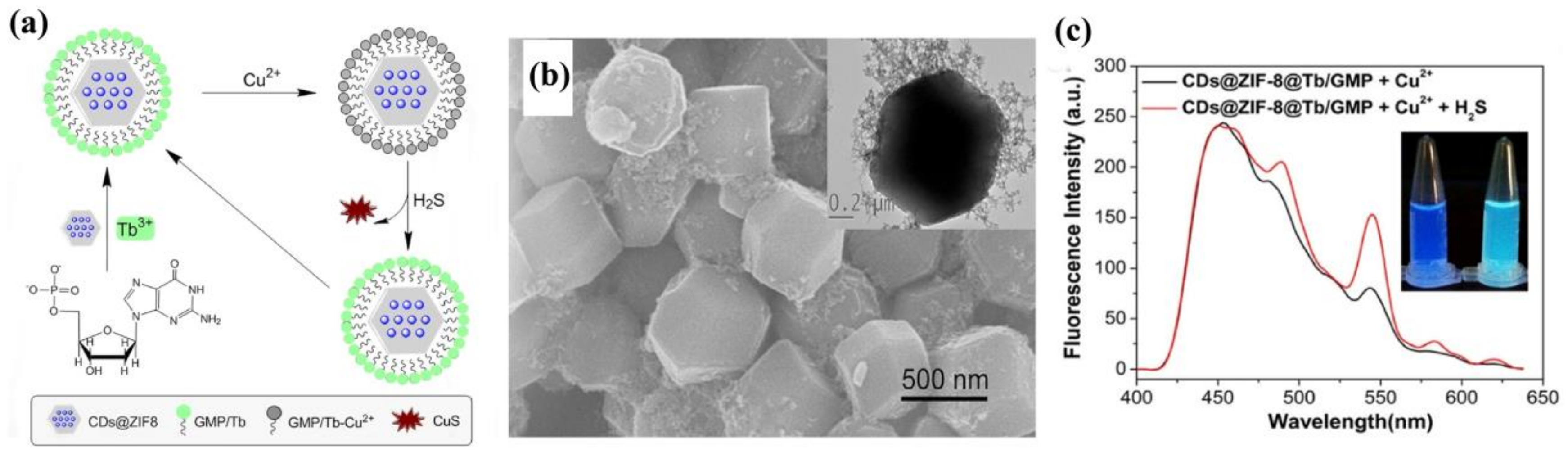
- Gao, J.; Li, Q.; Wang, C.; Tan, H. Copper (II)-mediated fluorescence of lanthanide coordination polymers doped with carbon dots for ratiometric detection of hydrogen sulfide. Sens. Actuators B 2017, 253, 27–33. [Google Scholar] [CrossRef]
- Zheng, X.; Fan, R.; Song, Y.; Wang, A.; Xing, K.; Du, X.; Wang, P.; Yang, Y. A highly sensitive turn-on ratiometric luminescent probe based on postsynthetic modification of Tb3+@Cu-MOF for H2S detection. J. Mater. Chem. C 2017, 5, 9943–9951. [Google Scholar] [CrossRef]
- Gao, Y.; Jing, P.; Yan, N.; Hilbers, M.; Zhang, H.; Rothenberg, G.; Tanase, S. Dual-mode humidity detection using a lanthanide-based metal-organic framework: Towards multifunctional humidity sensors. Chem. Commun. 2017, 53, 4465–4468. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Burgess, K. Fluorescent Indicators for Intracellular pH. Chem. Rev. 2010, 110, 2709–2728. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Pradhan, T.; Wang, F.; Kim, J.S.; Yoon, J. Fluorescent chemosensors based on spiroring-opening of xanthenes and related derivatives. Chem. Rev. 2012, 112, 1910–1956. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Chen, Y. Charge-transfer-based terbium MOF nanoparticles as fluorescent pH sensor for extreme acidity. Biosens. Bioelectron. 2017, 87, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Dimitriev, O.P.; Kislyuk, V.V. Processes of molecular association and excimeric emission in protonated N,N-dimethylformamide. Spectrochim. Acta Part A 2007, 68, 29–35. [Google Scholar] [CrossRef] [PubMed]
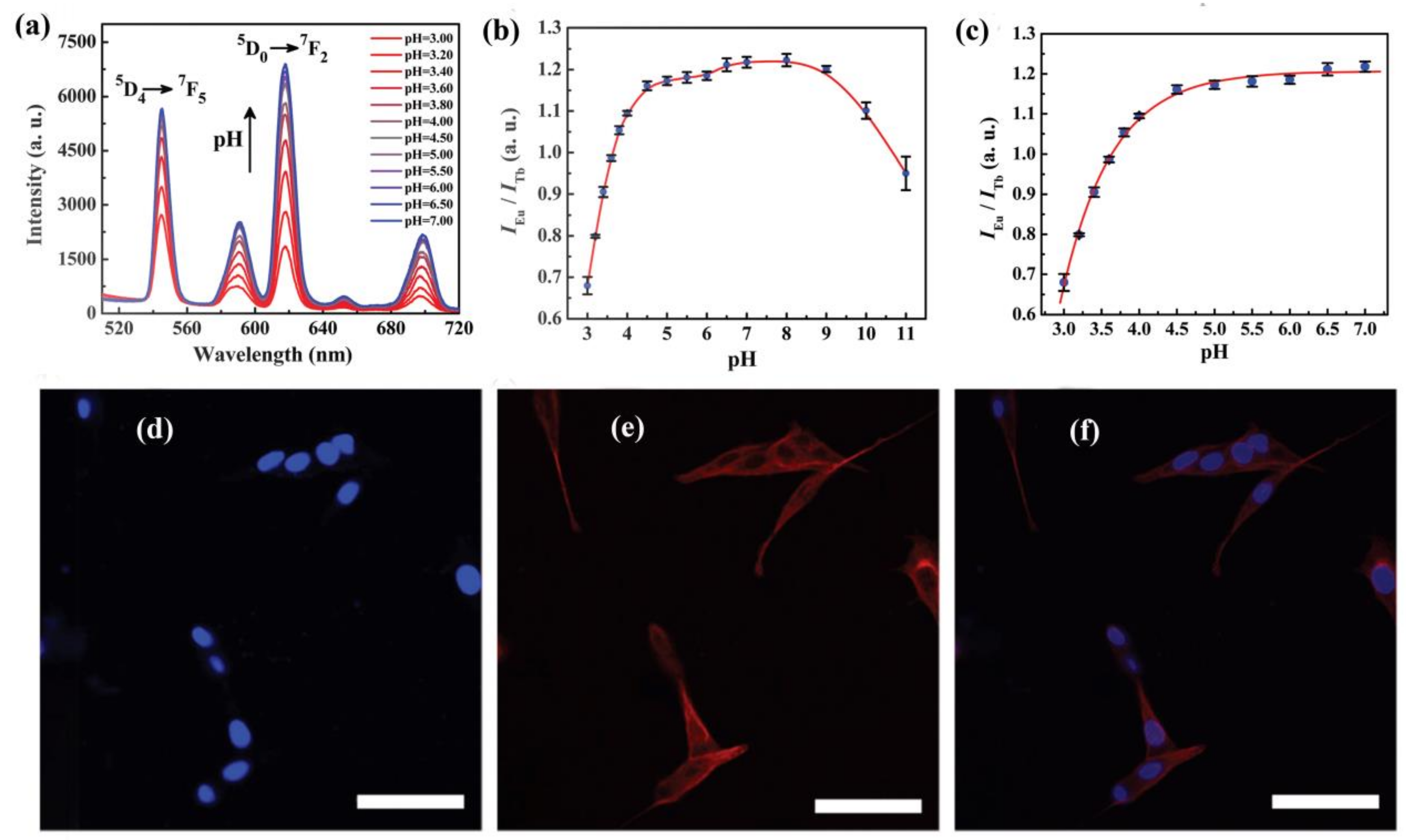
- Zhang, X.; Jiang, K.; He, H.; Yue, D.; Zhao, D.; Cui, Y.; Yang, Y.; Qian, G. A stable lanthanide-functionalized nanoscale metal-organic framework as a fluorescent probe for pH. Sens. Actuators B 2018, 254, 1069–1077. [Google Scholar] [CrossRef]
- Xia, T.; Zhu, F.; Jiang, K.; Cui, Y.; Yang, Y.; Qian, G. A luminescent ratiometric pH sensor based on a nanoscale and biocompatible Eu/Tb-mixed MOF. Dalton Trans. 2017, 46, 7549–7555. [Google Scholar] [CrossRef] [PubMed]
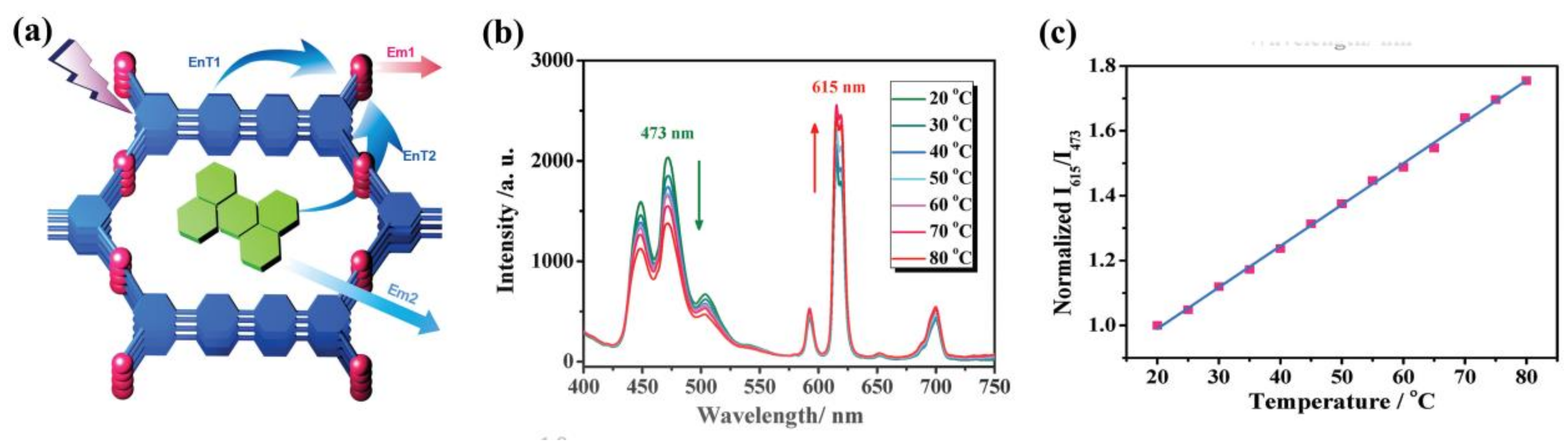
- Zhao, S.-N.; Li, L.-J.; Song, X.-Z.; Zhu, M.; Hao, Z.-M.; Meng, X.; Wu, L.-L.; Feng, J.; Song, S.-Y.; Wang, C.; et al. Lanthanide Ion Codoped Emitters for Tailoring Emission Trajectory and Temperature Sensing. Adv. Funct. Mater. 2015, 25, 1463–1469. [Google Scholar] [CrossRef]
- Cui, Y.; Zhu, F.; Chen, B.; Qian, G. Metal-organic frameworks for luminescence thermometry. Chem. Commun. 2015, 51, 7420–7431. [Google Scholar] [CrossRef] [PubMed]
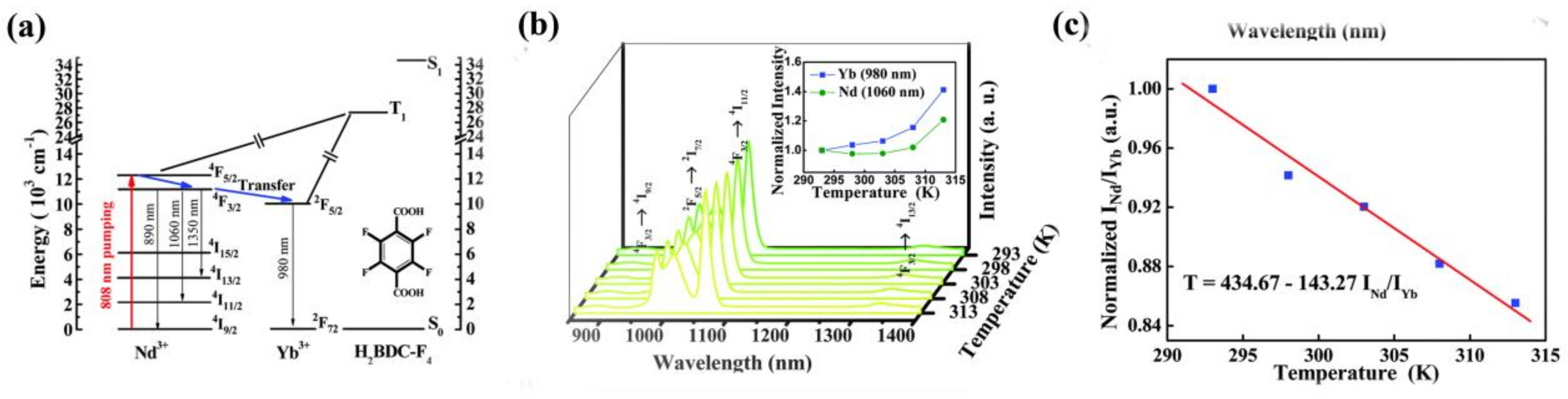
- Lian, X.; Zhao, D.; Cui, Y.; Yang, Y.; Qian, G. A near infrared luminescent metal-organic framework for temperature sensing in the physiological range. Chem. Commun. 2015, 51, 17676–17679. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Song, R.; Yu, J.; Liu, M.; Wang, Z.; Wu, C.; Yang, Y.; Wang, Z.; Chen, B.; Qian, G. Dual-emitting MOF supersetdye composite for ratiometric temperature sensing. Adv. Mater. 2015, 27, 1420–1425. [Google Scholar] [CrossRef] [PubMed]
- Taokaenchan, N.; Tangkuaram, T.; Pookmanee, P.; Phaisansuthichol, S.; Kuimalee, S.; Satienperakul, S. Enhanced electrogenerated chemiluminescence of tris(2,2′-bipyridyl)ruthenium(II) system by l-cysteine-capped CdTe quantum dots and its application for the determination of nitrofuran antibiotics. Biosens. Bioelectron. 2015, 66, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yao, H.; Chu, T.; Zhang, G.; Wang, Y.; Yang, Y. A Lanthanide MOF Thin-Film Fixed with Co3O4 Nano-Anchors as a Highly Efficient Luminescent Sensor for Nitrofuran Antibiotics. Chem. Eur. J. 2017, 23, 10293–10300. [Google Scholar] [CrossRef] [PubMed]
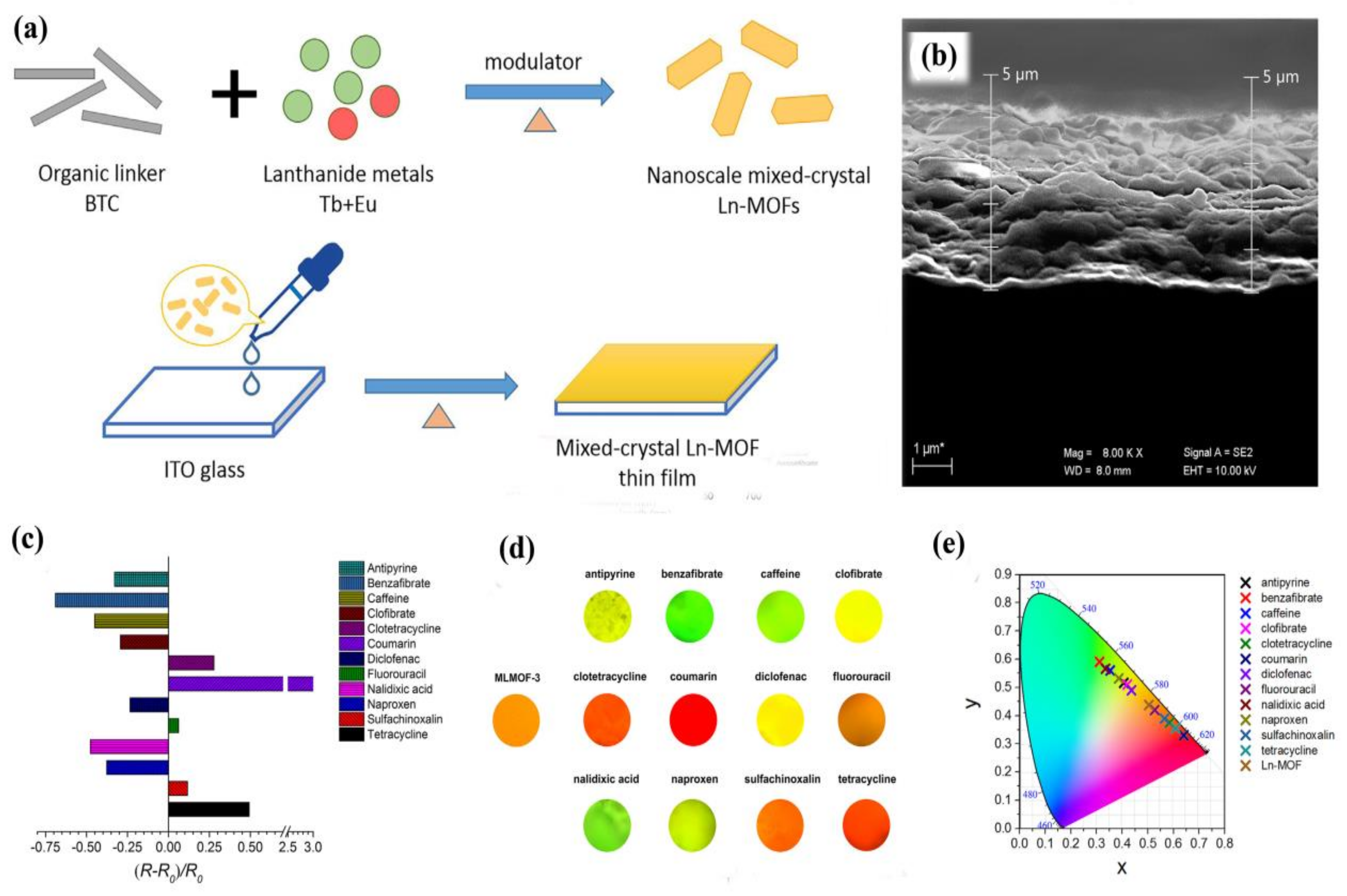
- Gao, Y.; Yu, G.; Liu, K.; Wang, B. Luminescent mixed-crystal Ln-MOF thin film for the recognition and detection of pharmaceuticals. Sens. Actuators B 2018, 257, 931–935. [Google Scholar] [CrossRef]
- Hao, J.N.; Xu, X.Y.; Lian, X.; Zhang, C.; Yan, B. A Luminescent 3d-4f-4d MOF Nanoprobe as a Diagnosis Platform for Human Occupational Exposure to Vinyl Chloride Carcinogen. Inorg. Chem. 2017, 56, 11176–11183. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.-N.; Wang, G.; Poelman, D.; Voort, P.V.D. Luminescent Lanthanide MOFs: A Unique Platform for Chemical Sensing. Materials 2018, 11, 572. https://doi.org/10.3390/ma11040572
Zhao S-N, Wang G, Poelman D, Voort PVD. Luminescent Lanthanide MOFs: A Unique Platform for Chemical Sensing. Materials. 2018; 11(4):572. https://doi.org/10.3390/ma11040572
Chicago/Turabian StyleZhao, Shu-Na, Guangbo Wang, Dirk Poelman, and Pascal Van Der Voort. 2018. "Luminescent Lanthanide MOFs: A Unique Platform for Chemical Sensing" Materials 11, no. 4: 572. https://doi.org/10.3390/ma11040572
APA StyleZhao, S.-N., Wang, G., Poelman, D., & Voort, P. V. D. (2018). Luminescent Lanthanide MOFs: A Unique Platform for Chemical Sensing. Materials, 11(4), 572. https://doi.org/10.3390/ma11040572







