Cross-Linked Poly(vinylidene fluoride-co-hexafluoropropene) (PVDF-co-HFP) Gel Polymer Electrolyte for Flexible Li-Ion Battery Integrated with Organic Light Emitting Diode (OLED)
Abstract
:1. Introduction
2. Materials and Methods
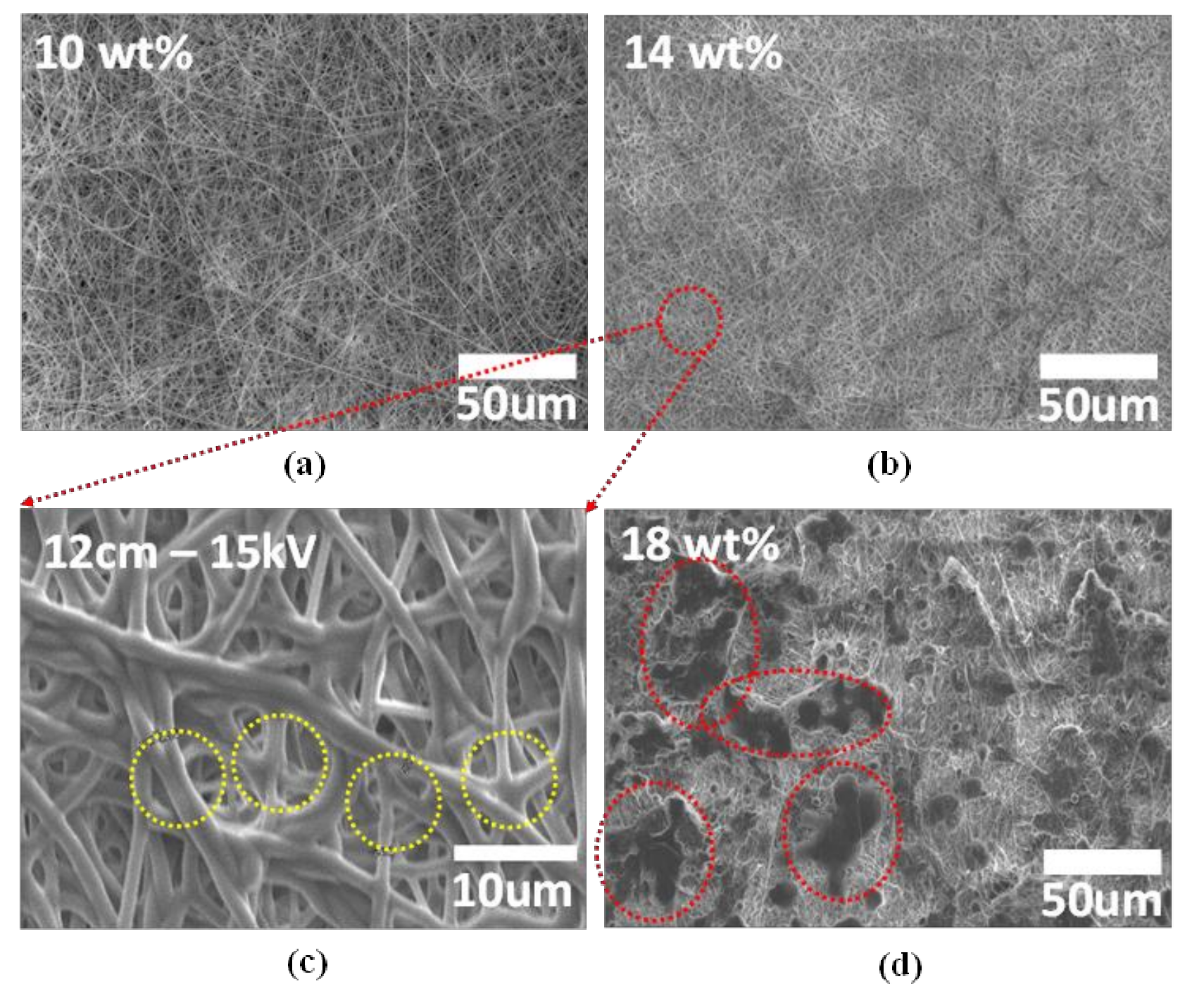
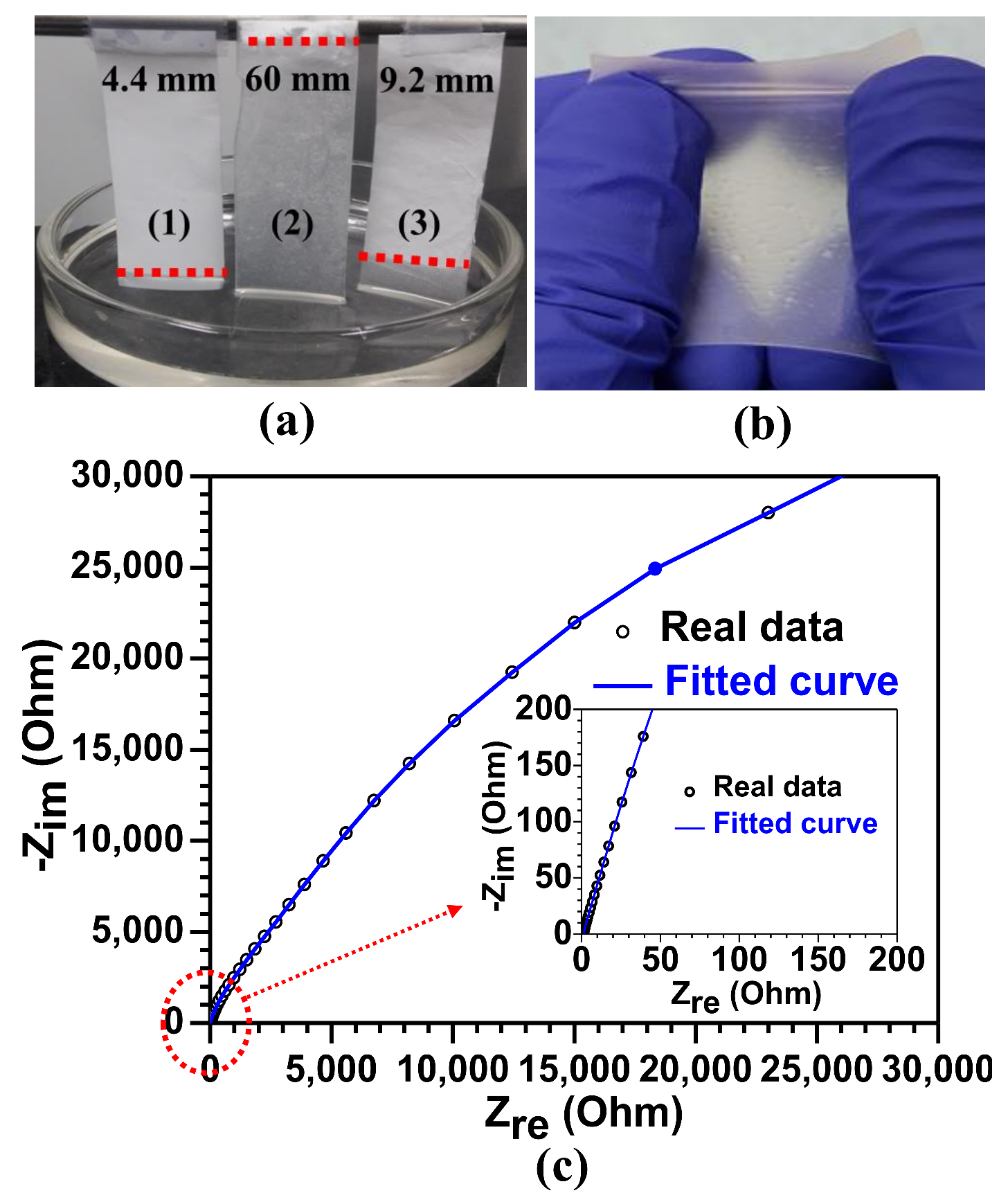
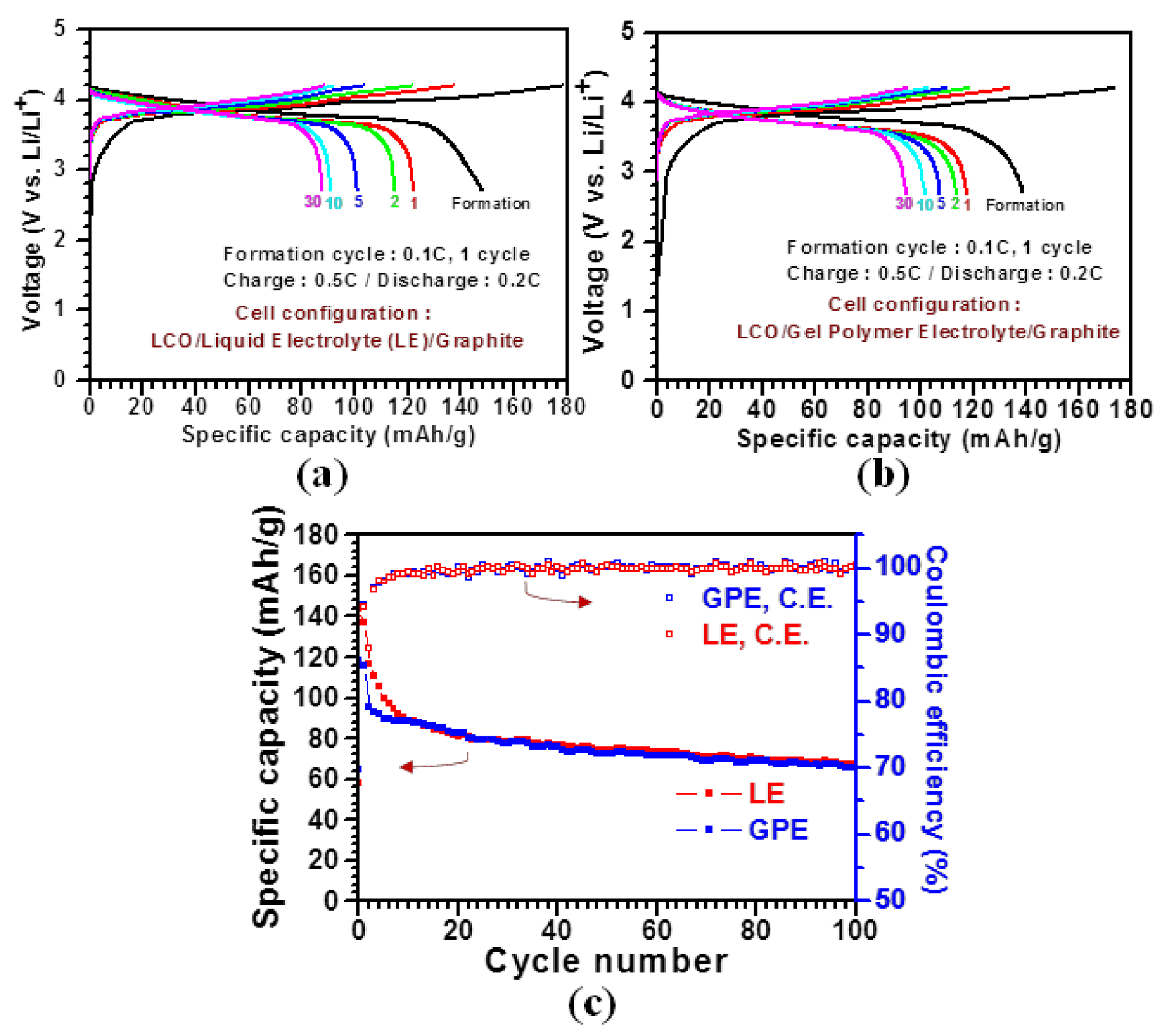
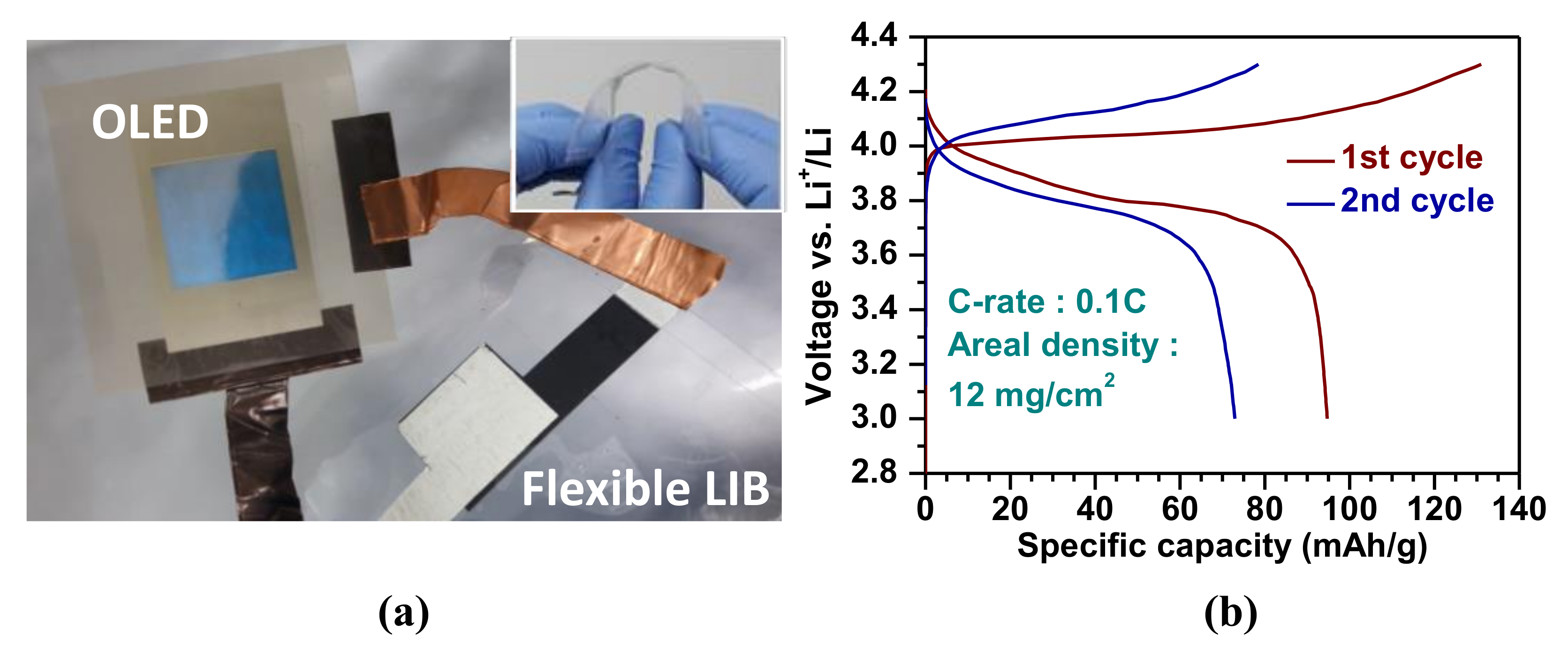
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhou, G.; Li, F.; Cheng, H.-M. Progress in flexible lithium batteries and future prospects. Energy Environ. Sci. 2014, 7, 1307–1338. [Google Scholar] [CrossRef]
- Kim, J.R.; Choi, S.W.; Jo, S.M.; Lee, W.S.; Kim, B.C. Electrospun PVdF-based fibrous polymer electrolytes for lithium ion polymer batteries. Electrochim. Acta 2004, 50, 69–75. [Google Scholar] [CrossRef]
- Gao, K.; Hu, X.; Dai, C.; Yi, T. Crystal structures of electrospun PVDF membranes and its separator application for rechargeable lithium metal cells. Mater. Sci. Eng. B 2006, 131, 100–105. [Google Scholar] [CrossRef]
- Cho, T.-H.; Tanaka, M.; Onishi, H.; Kondo, Y.; Nakamura, T.; Yamazaki, H.; Tanase, S.; Sakai, T. Battery performances and thermal stability of polyacrylonitrile nano-fiber-based nonwoven separators for Li-ion battery. J. Power Sources 2008, 181, 155–160. [Google Scholar] [CrossRef]
- Raghavan, P.; Manuel, J.; Zhao, X.; Kim, D.-S.; Ahn, J.-H.; Nah, C. Preparation and electrochemical characterization of gel polymer electrolyte based on electrospun polyacrylonitrile nonwoven membranes for lithium batteries. J. Power Sources 2011, 196, 6742–6749. [Google Scholar] [CrossRef]
- Ali, A.M.M.; Subban, R.H.Y.; Bahron, H.; Yahya, M.Z.A.; Kamisan, A.S. Investigation on modified natural rubber gel polymer electrolytes for lithium polymer battery. J. Power Sources 2013, 244, 636–640. [Google Scholar] [CrossRef]
- Balo, L.; Gupta, H.; Singh, V.K.; Singh, R.K. Flexible gel polymer electrolyte based on ionic liquid EMIMTFSI for rechargeable battery application. Electrochim. Acta 2017, 230, 123–131. [Google Scholar] [CrossRef]
- Baskakova, Y.V.; Ol’ga, V.Y.; Efimov, O.N. Polymer gel electrolytes for lithium batteries. Russ. Chem. Rev. 2012, 81, 367–380. [Google Scholar] [CrossRef]
- Park, J.K. 3.3 Electrolytes. In Principles and Applications of Lithium Secondary Batteries, 1st ed.; Park, J.K., Ed.; Wiley: Hoboken, NJ, USA, 2012; p. 143. ISBN 978-3-527-33151-2. [Google Scholar]
- Ye, H.; Huang, J.; Xu, J.J.; Khalfan, A.; Greenbaum, S.G. Li ion conducting polymer gel electrolytes based on ionic liquid/PVDF-HFP blends. J. Electrochem. Soc. 2007, 154, A1048–A1057. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X. A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy Environ. Sci. 2014, 7, 3857–3886. [Google Scholar] [CrossRef]
- Ferrari, S.; Quartarone, E.; Mustarelli, P.; Magistris, A.; Fagnoni, M.; Protti, S.; Gerbaldi, C.; Spinella, A. Lithium ion conducting PVdF-HFP composite gel electrolytes based on N-methoxyethyl-N-methylpyrrolidinium bis(trifluoromethanesulfonyl)-imide ionic liquid. J. Power Sources 2010, 195, 559–566. [Google Scholar] [CrossRef]
- Yang, P.; Cui, W.; Li, L.; Liu, L.; An, M. Characterization and properties of ternary P(VdF-HFP)-LiTFSI-EMITFSI ionic liquid polymer electrolytes. Solid State Sci. 2012, 14, 598–606. [Google Scholar] [CrossRef]
- Kim, J.; Choi, S.; Jo, S.; Lee, W.; Kim, B. Characterization and properties of P (VdF-HFP)-based fibrous polymer electrolyte membrane prepared by electrospinning. J. Electrochem. Soc. 2005, 152, A295–A300. [Google Scholar] [CrossRef]
- Raghavan, P.; Zhao, X.; Kim, J.-K.; Manuel, J.; Chauhan, G.S.; Ahn, J.-H.; Nah, C. Ionic conductivity and electrochemical properties of nanocomposite polymer electrolytes based on electrospun poly (vinylidene fluoride-co-hexafluoropropylene) with nano-sized ceramic fillers. Electrochim. Acta 2008, 54, 228–234. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.; Zhang, P.; Wu, Y.; Zhou, X. Macroporous nanocomposite polymer electrolyte for lithium-ion batteries. J. Power Sources 2008, 184, 562–565. [Google Scholar] [CrossRef]
- Cheng, C.; Wan, C.; Wang, Y. Preparation of porous, chemically cross-linked, PVdF-based gel polymer electrolytes for rechargeable lithium batteries. J. Power Sources 2004, 134, 202–210. [Google Scholar] [CrossRef]
- Croce, F.; Focarete, M.L.; Hassoun, J.; Meschini, I.; Scrosati, B. A safe, high-rate and high-energy polymer lithium-ion battery based on gelled membranes prepared by electrospinning. Energy Environ. Sci. 2011, 4, 921–927. [Google Scholar] [CrossRef]
- Wu, N.; Cao, Q.; Wang, X.; Li, X.; Deng, H. A novel high-performance gel polymer electrolyte membrane basing on electrospinning technique for lithium rechargeable batteries. J. Power Sources 2011, 196, 8638–8643. [Google Scholar] [CrossRef]
- Gopalan, A.I.; Santhosh, P.; Manesh, K.M.; Nho, J.H.; Kim, S.H.; Hwang, C.-G.; Lee, K.-P. Development of electrospun PVdF–PAN membrane-based polymer electrolytes for lithium batteries. J. Membr. Sci. 2008, 325, 683–690. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, Z. Characterization of the polymer electrolyte based on the blend of poly (vinylidene fluoride-co-hexafluoropropylene) and poly (vinyl pyrrolidone) for lithium ion battery. Mater. Chem. Phys. 2003, 82, 16–20. [Google Scholar] [CrossRef]
- Capiglia, C.; Saito, Y.; Yamamoto, H.; Kageyama, H.; Mustarelli, P. Transport properties and microstructure of gel polymer electrolytes. Electrochim. Acta 2000, 45, 1341–1345. [Google Scholar] [CrossRef]
- Cui, Z.-Y.; Xu, Y.-Y.; Zhu, L.-P.; Wang, J.-Y.; Xi, Z.-Y.; Zhu, B.-K. Preparation of PVDF/PEO-PPO-PEO blend microporous membranes for lithium ion batteries via thermally induced phase separation process. J. Membr. Sci. 2008, 325, 957–963. [Google Scholar] [CrossRef]
- Cheng, C.L.; Wan, C.C.; Wang, Y.Y. Microporous PVdF-HFP based gel polymer electrolytes reinforced by PEGDMA network. Electrochem. Commun. 2004, 6, 531–535. [Google Scholar] [CrossRef]
- Cavaliere, S.; Subianto, S.; Savych, I.; Jones, D.J.; Rozière, J. Electrospinning: designed architectures for energy conversion and storage devices. Energy Environ. Sci. 2011, 4, 4761–4785. [Google Scholar] [CrossRef]
- Padmaraj, O.; Rao, B.N.; Venkateswarlu, M.; Satyanarayana, N. Electrochemical characterization of electrospun nanocomposite polymer blend electrolyte fibrous membrane for lithium battery. J. Phys. Chem. B 2015, 119, 5299–5308. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Choi, S.W.; Jo, S.M.; Chin, B.D.; Kim, D.Y.; Lee, K.Y. Electrochemical properties and cycle performance of electrospun poly (vinylidene fluoride)-based fibrous membrane electrolytes for Li-ion polymer battery. J. Power Sources 2006, 163, 41–46. [Google Scholar] [CrossRef]
- Raghavan, P.; Zhao, X.; Shin, C.; Baek, D.-H.; Choi, J.-W.; Manuel, J.; Heo, M.-Y.; Ahn, J.-H.; Nah, C. Preparation and electrochemical characterization of polymer electrolytes based on electrospun poly (vinylidene fluoride-co-hexafluoropropylene)/polyacrylonitrile blend/composite membranes for lithium batteries. J. Power Sources 2010, 195, 6088–6094. [Google Scholar] [CrossRef]
- Padmaraj, O.; Venkateswarlu, M.; Satyanarayana, N. Characterization and Electrochemical Properties of P (VdF-co-HFP) Based Electrospun Nanocomposite Fibrous Polymer Electrolyte Membrane for Lithium Battery Applications. Electroanalysis 2014, 26, 2373–2379. [Google Scholar] [CrossRef]
- Koo, M.; Park, K.-I.; Lee, S.H.; Suh, M.; Jeon, D.Y.; Choi, J.W.; Kang, K.; Lee, K.J. Bendable inorganic thin-film battery for fully flexible electronic systems. Nano Lett. 2012, 12, 4810–4816. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S.; Kobayashi, T.; Asaoka, T. High lithium ionic conductivity in the garnet-type oxide Li7− XLa3 (Zr2− X, NbX)O12 (X= 0–2). J. Power Sources 2011, 196, 3342–3345. [Google Scholar] [CrossRef]
- Ye, Y.-S.; Rick, J.; Hwang, B.-J. Ionic liquid polymer electrolytes. J. Mater. Chem. A 2013, 1, 2719–2743. [Google Scholar] [CrossRef]
- Yang, C.; Jia, Z.; Guan, Z.; Wang, L. Polyvinylidene fluoride membrane by novel electrospinning system for separator of Li-ion batteries. J. Power Sources 2009, 189, 716–720. [Google Scholar] [CrossRef]
- Rao, M.; Geng, X.; Liao, Y.; Hu, S.; Li, W. Preparation and performance of gel polymer electrolyte based on electrospun polymer membrane and ionic liquid for lithium ion battery. J. Memb. Sci. 2012, 399, 37–42. [Google Scholar] [CrossRef]
- Cowie, J.M.G.; Arrighi, V. Structure-Property Relations. In Polymers: Chemistry and Physics of Modern Materials, 3rd ed.; CRC press: Boca Raton, FL, USA, 2007; p. 422. ISBN 9780849398131. [Google Scholar]

| Parameters | Values |
|---|---|
| Copolymer concentration in solution | 14–16 wt % |
| Spinning solution volume | 9 mL |
| Spinning rate | 3 mL/h |
| Spinning time | 3 h |
| Working distance between the capillary and the collector | 15 cm |
| Electrical potential | 17 kV |
| The rotation frequency of the collector | 100 rpm |
| Width of as-electrospun fiber sheet | 110–150 cm |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, I.; Kim, B.S.; Nam, S.; Lee, H.-J.; Chung, H.K.; Cho, S.M.; Luu, T.H.T.; Hyun, S.; Kang, C. Cross-Linked Poly(vinylidene fluoride-co-hexafluoropropene) (PVDF-co-HFP) Gel Polymer Electrolyte for Flexible Li-Ion Battery Integrated with Organic Light Emitting Diode (OLED). Materials 2018, 11, 543. https://doi.org/10.3390/ma11040543
Kim I, Kim BS, Nam S, Lee H-J, Chung HK, Cho SM, Luu THT, Hyun S, Kang C. Cross-Linked Poly(vinylidene fluoride-co-hexafluoropropene) (PVDF-co-HFP) Gel Polymer Electrolyte for Flexible Li-Ion Battery Integrated with Organic Light Emitting Diode (OLED). Materials. 2018; 11(4):543. https://doi.org/10.3390/ma11040543
Chicago/Turabian StyleKim, Ilhwan, Bong Sung Kim, Seunghoon Nam, Hoo-Jeong Lee, Ho Kyoon Chung, Sung Min Cho, Thi Hoai Thuong Luu, Seungmin Hyun, and Chiwon Kang. 2018. "Cross-Linked Poly(vinylidene fluoride-co-hexafluoropropene) (PVDF-co-HFP) Gel Polymer Electrolyte for Flexible Li-Ion Battery Integrated with Organic Light Emitting Diode (OLED)" Materials 11, no. 4: 543. https://doi.org/10.3390/ma11040543
APA StyleKim, I., Kim, B. S., Nam, S., Lee, H.-J., Chung, H. K., Cho, S. M., Luu, T. H. T., Hyun, S., & Kang, C. (2018). Cross-Linked Poly(vinylidene fluoride-co-hexafluoropropene) (PVDF-co-HFP) Gel Polymer Electrolyte for Flexible Li-Ion Battery Integrated with Organic Light Emitting Diode (OLED). Materials, 11(4), 543. https://doi.org/10.3390/ma11040543





