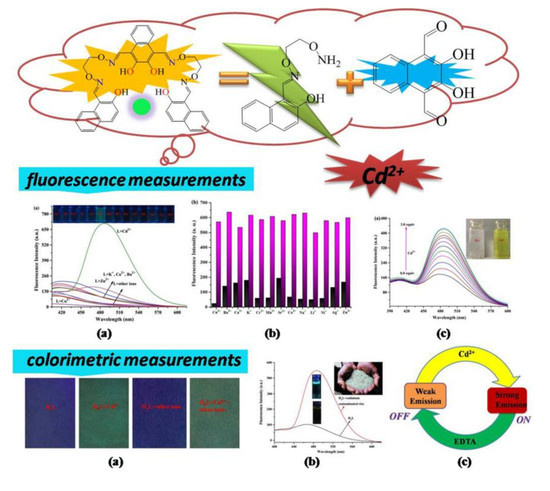
A Reversible Bis(Salamo)-Based Fluorescence Sensor for Selective Detection of Cd2+ in Water-Containing Systems and Food Samples
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
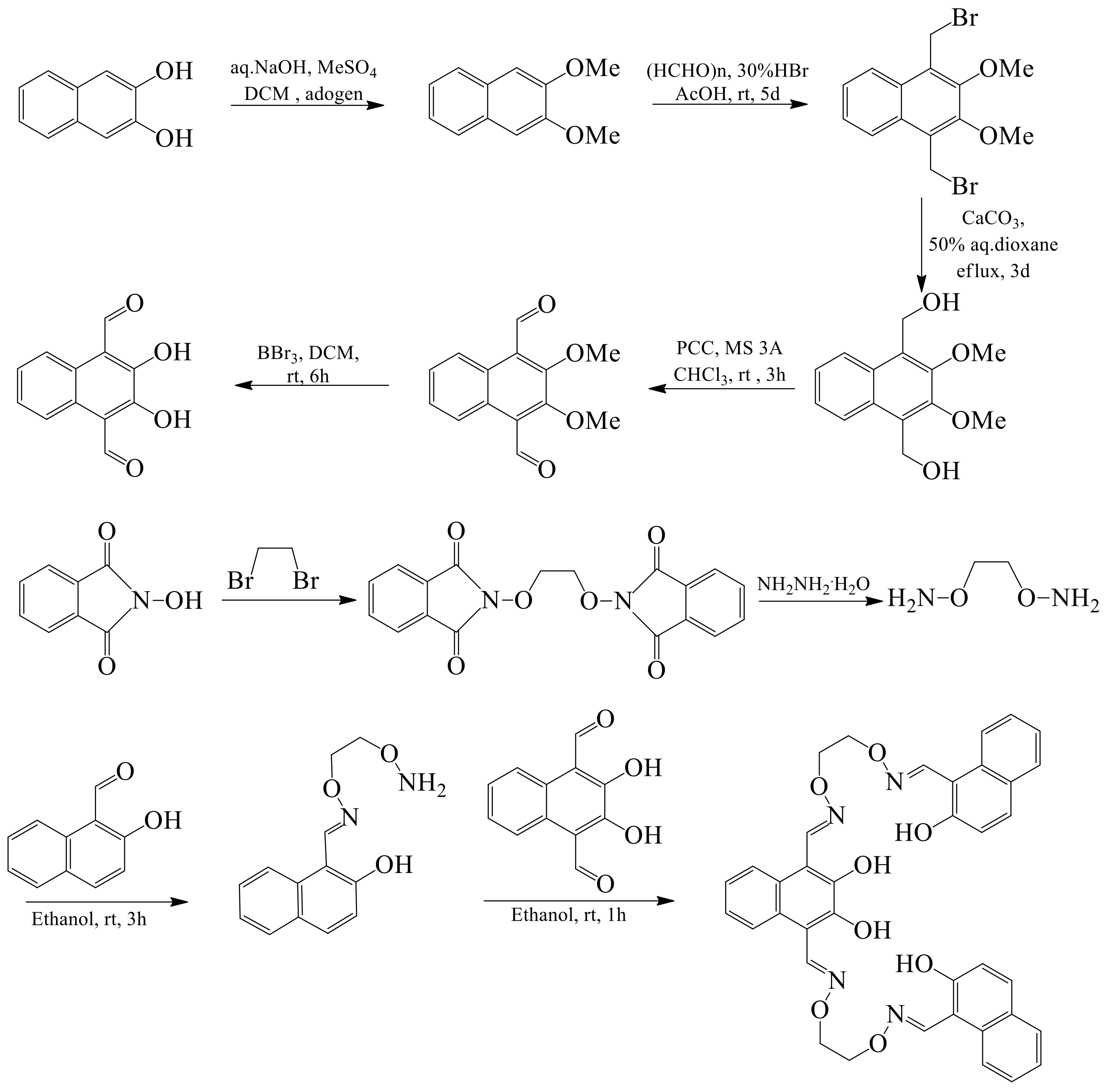
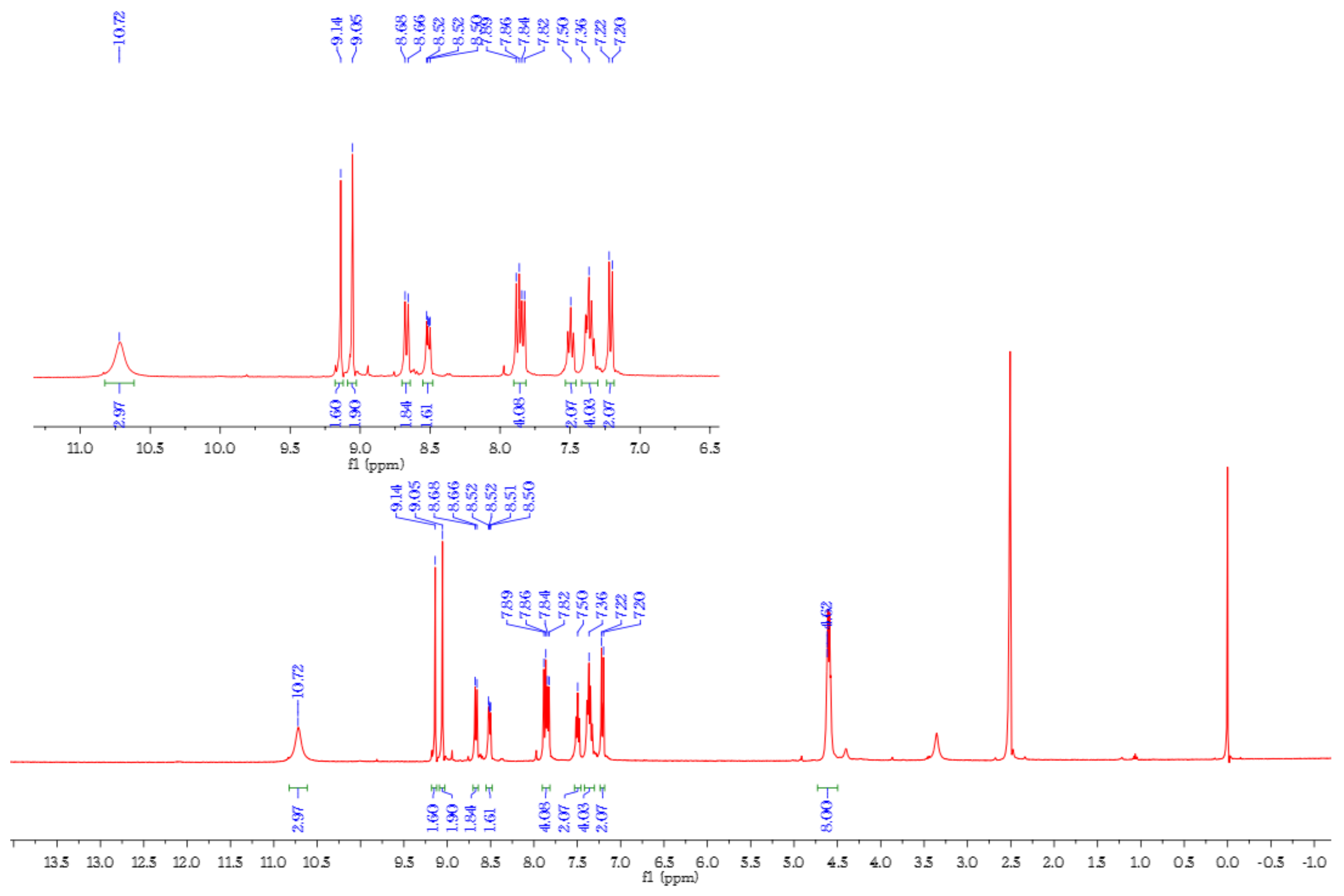
2.2. Preperation of Sensor H4L
3. Results and Discussion
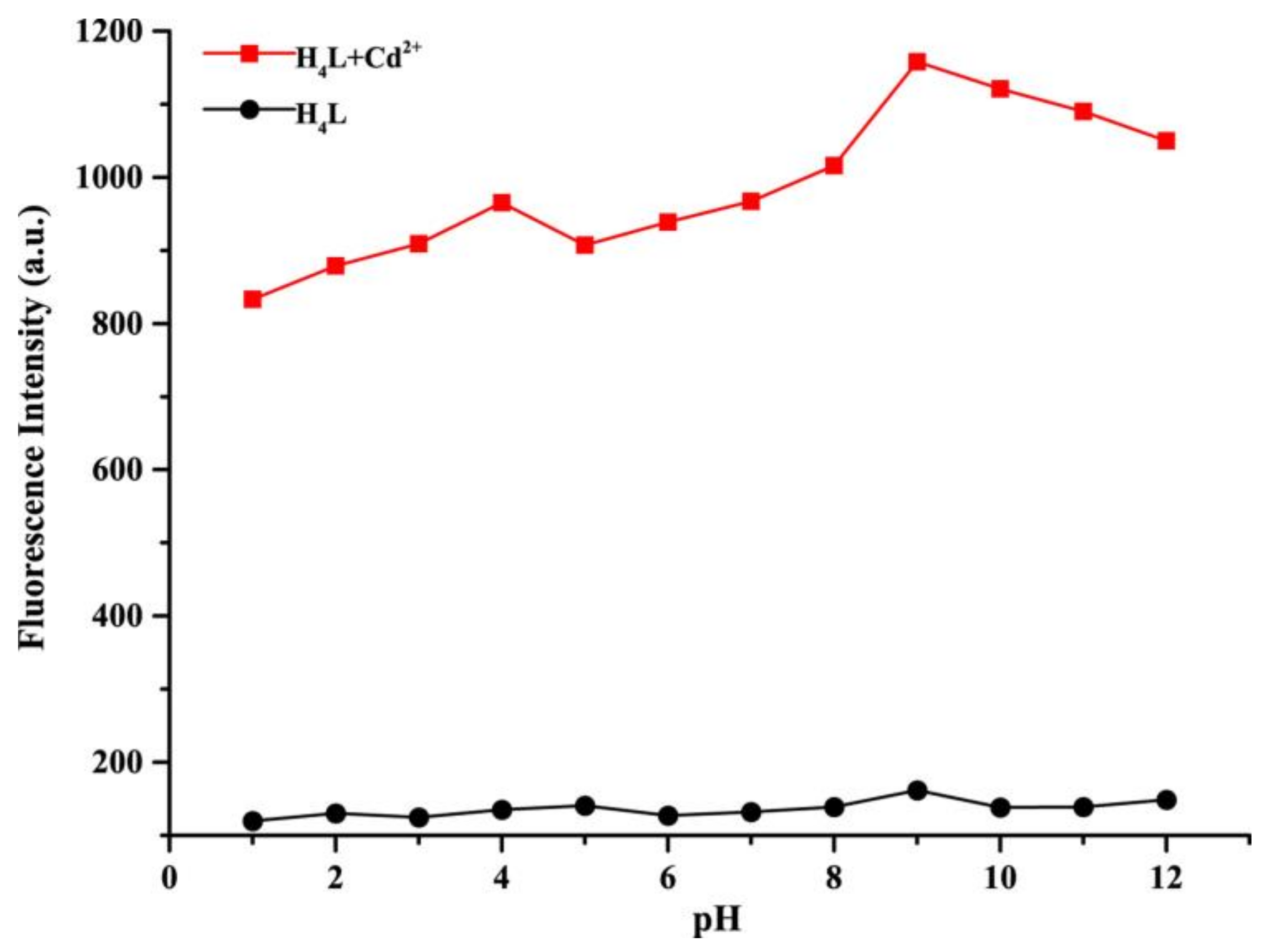
3.1. pH Effect of Sensor H4L
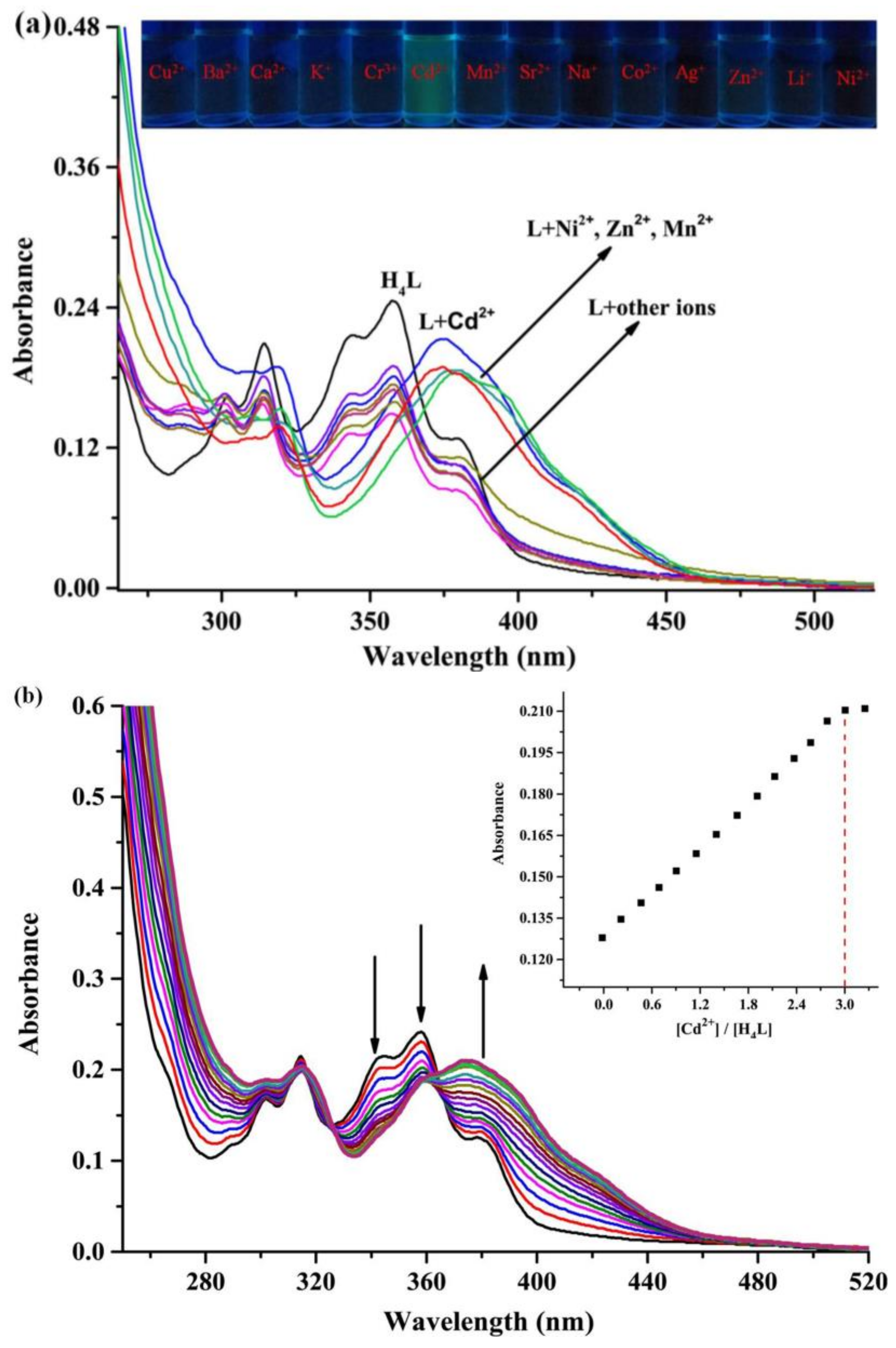
3.2. General UV–vis Measurements
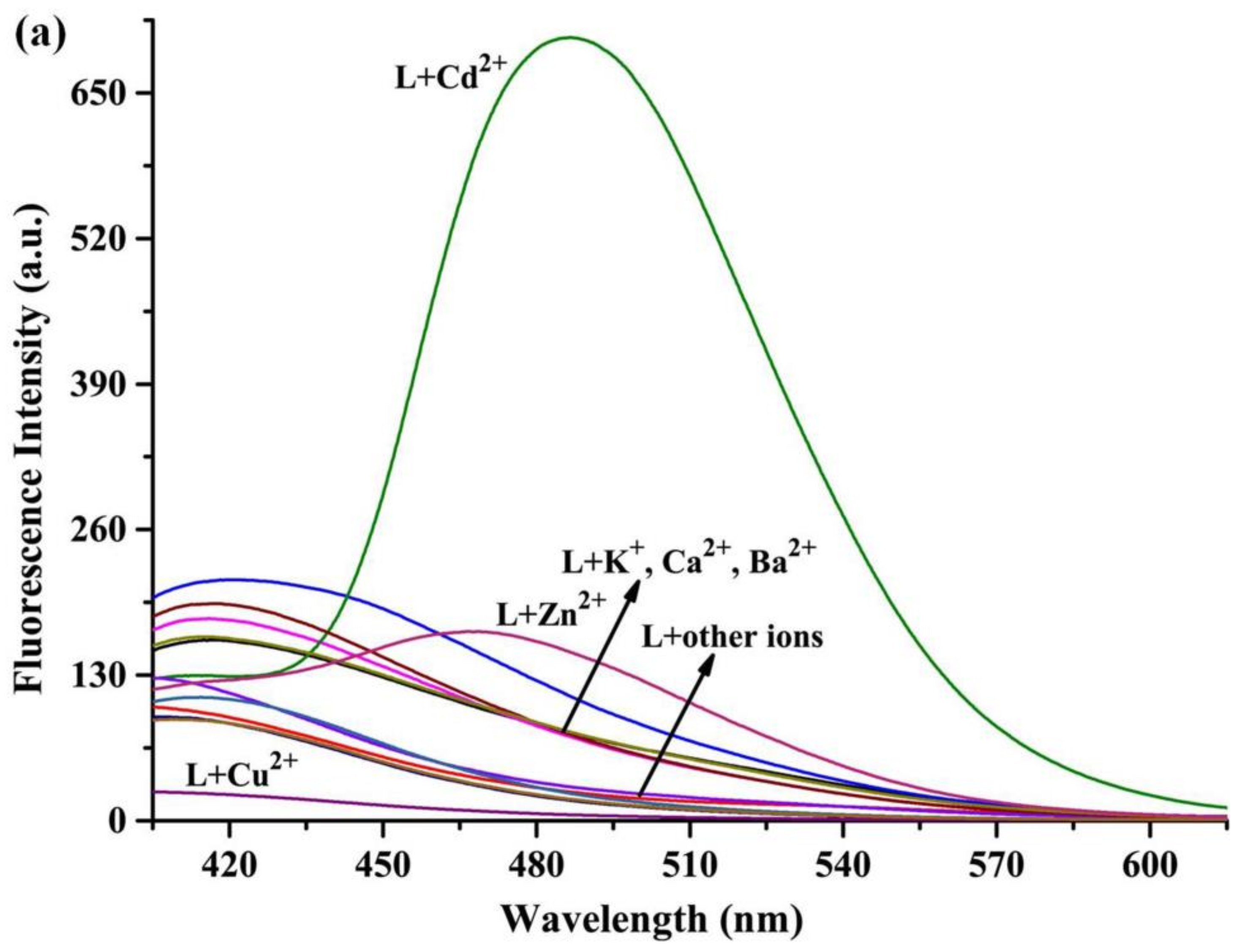
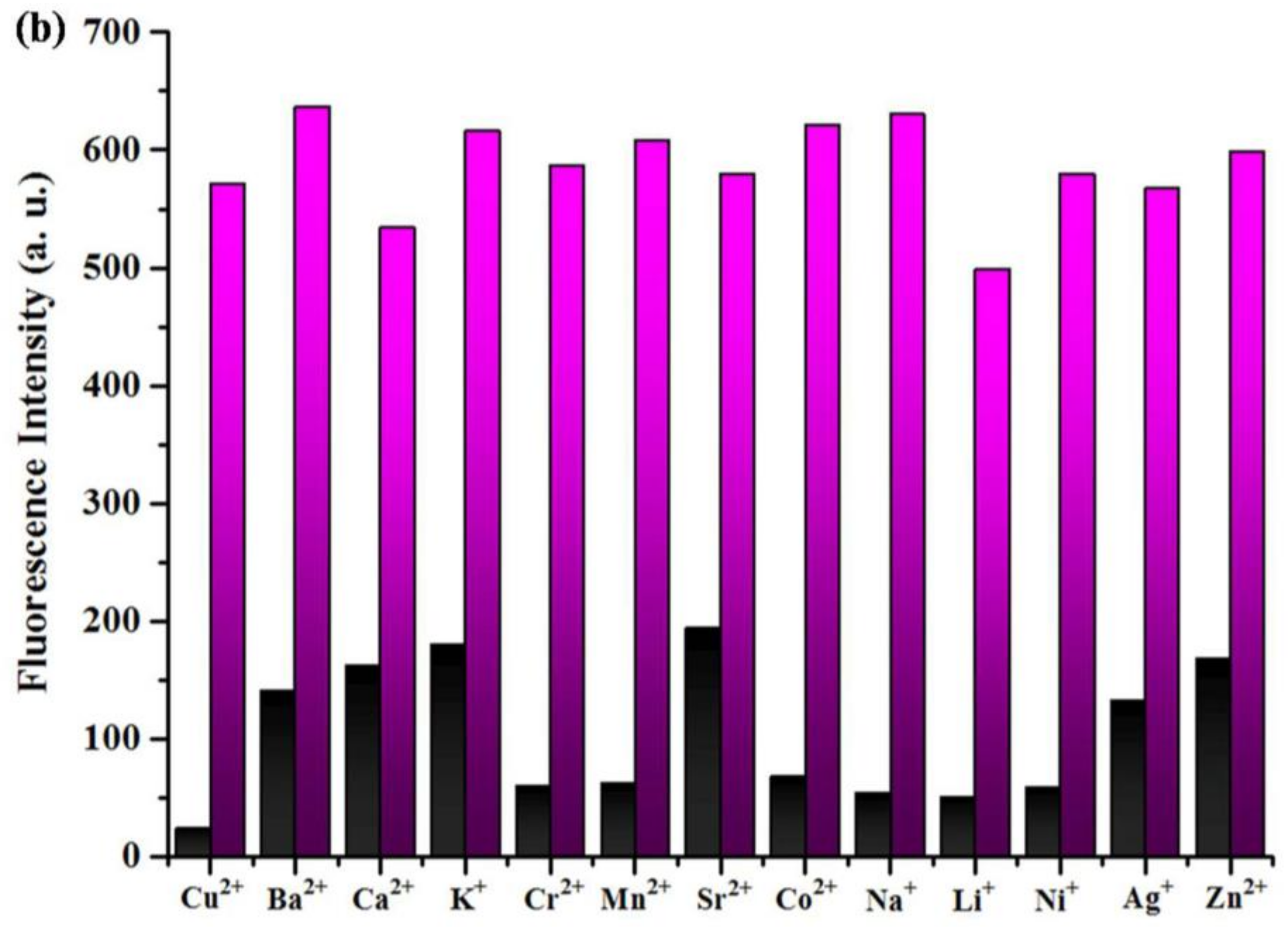
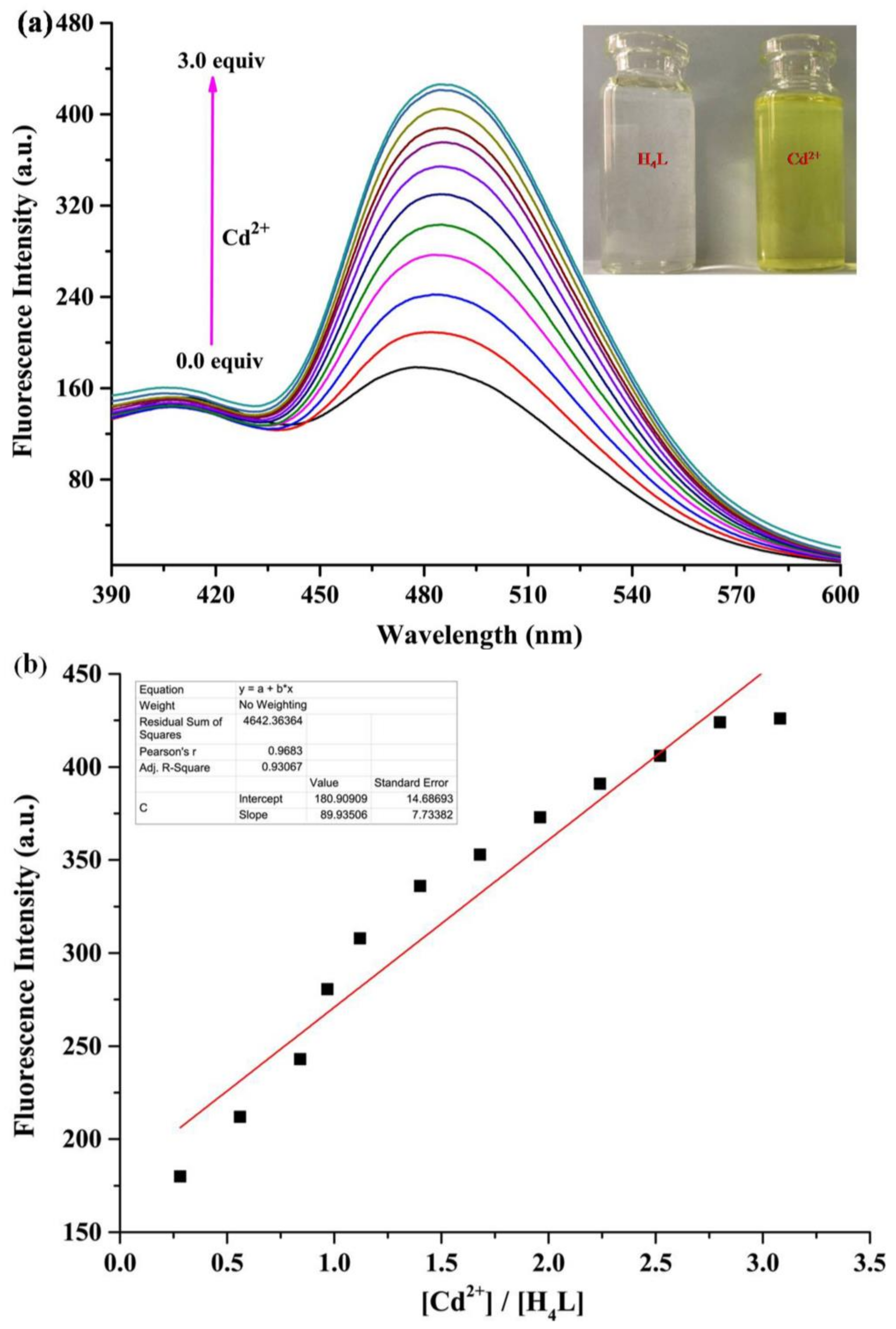
3.3. General Fluorescence Measurements
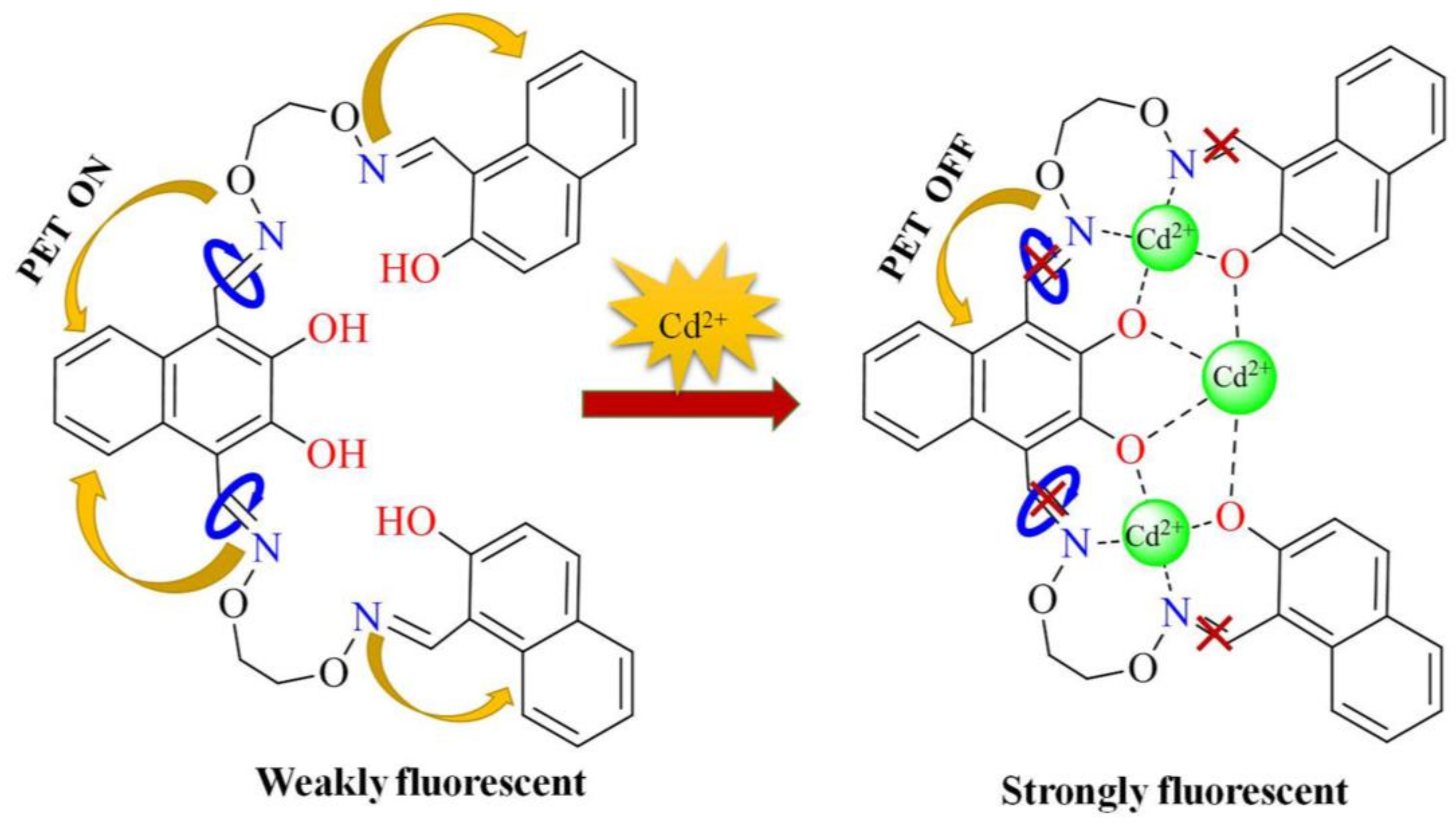
3.4. The Detecting Mechanism of H4L for Cd2+
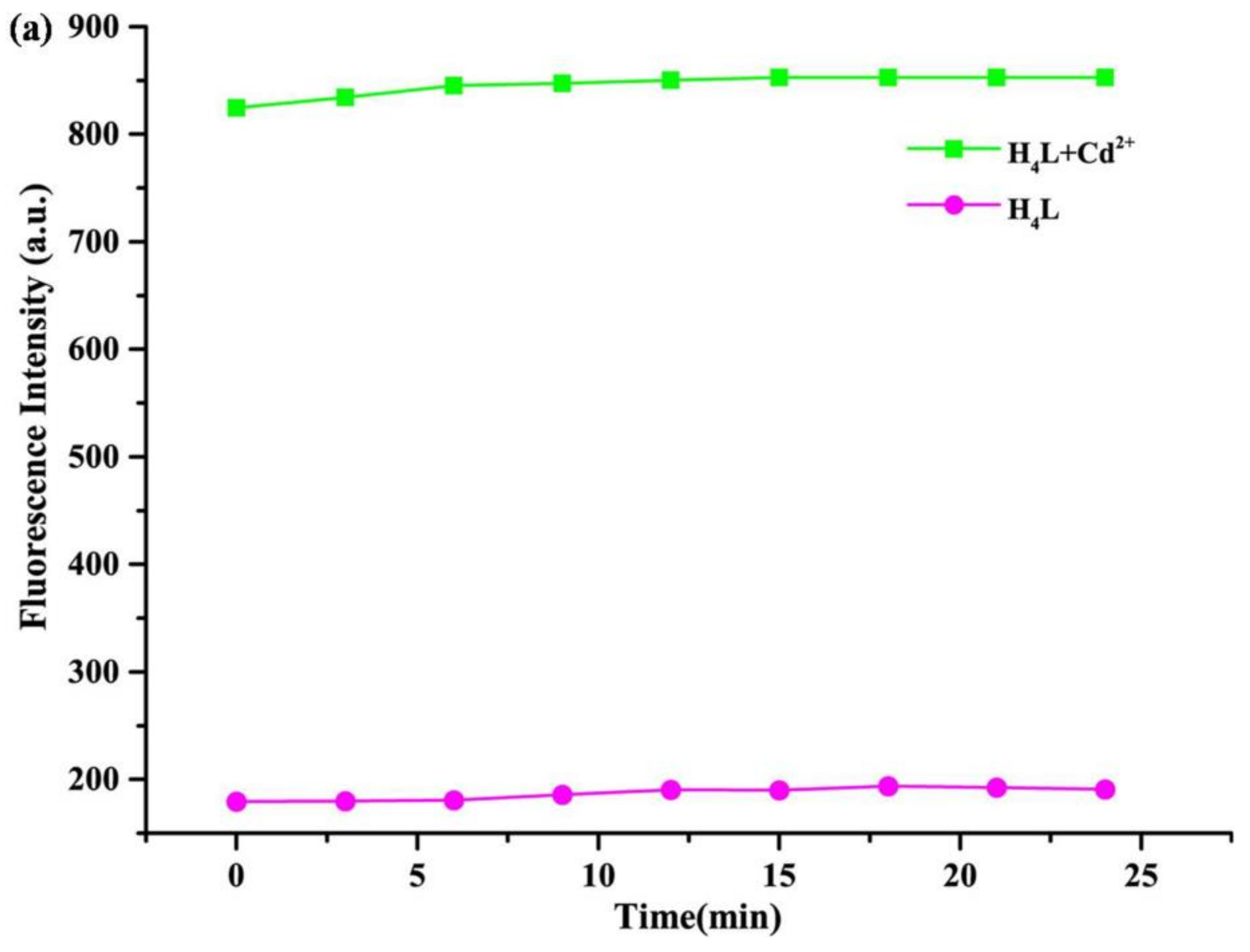
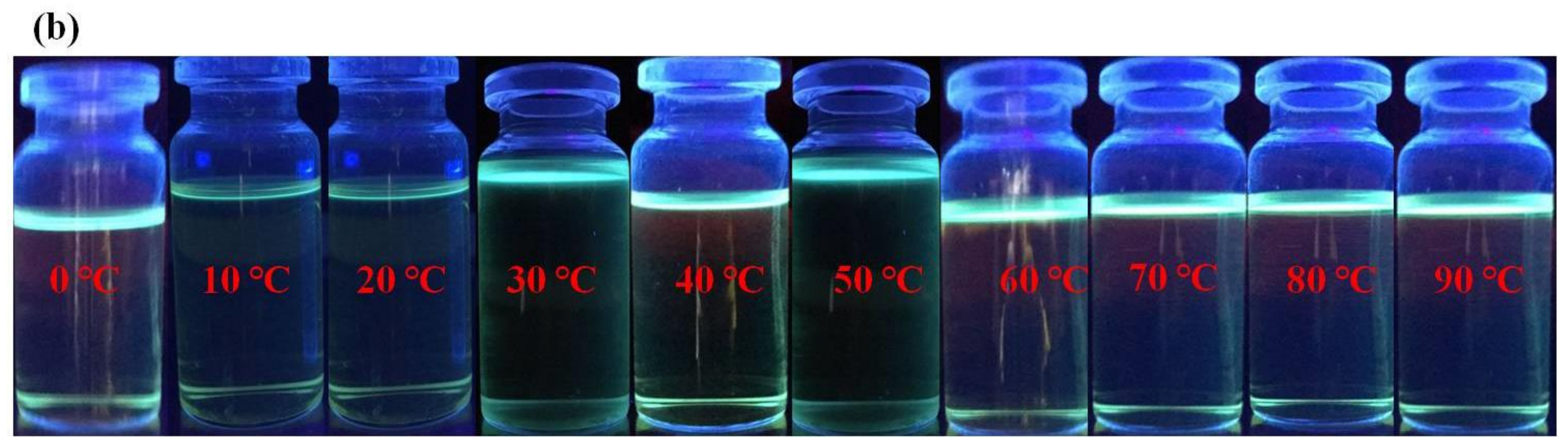
3.5. Time and Temperature Effect of Probe H4L
3.6. Test Strips Measurements
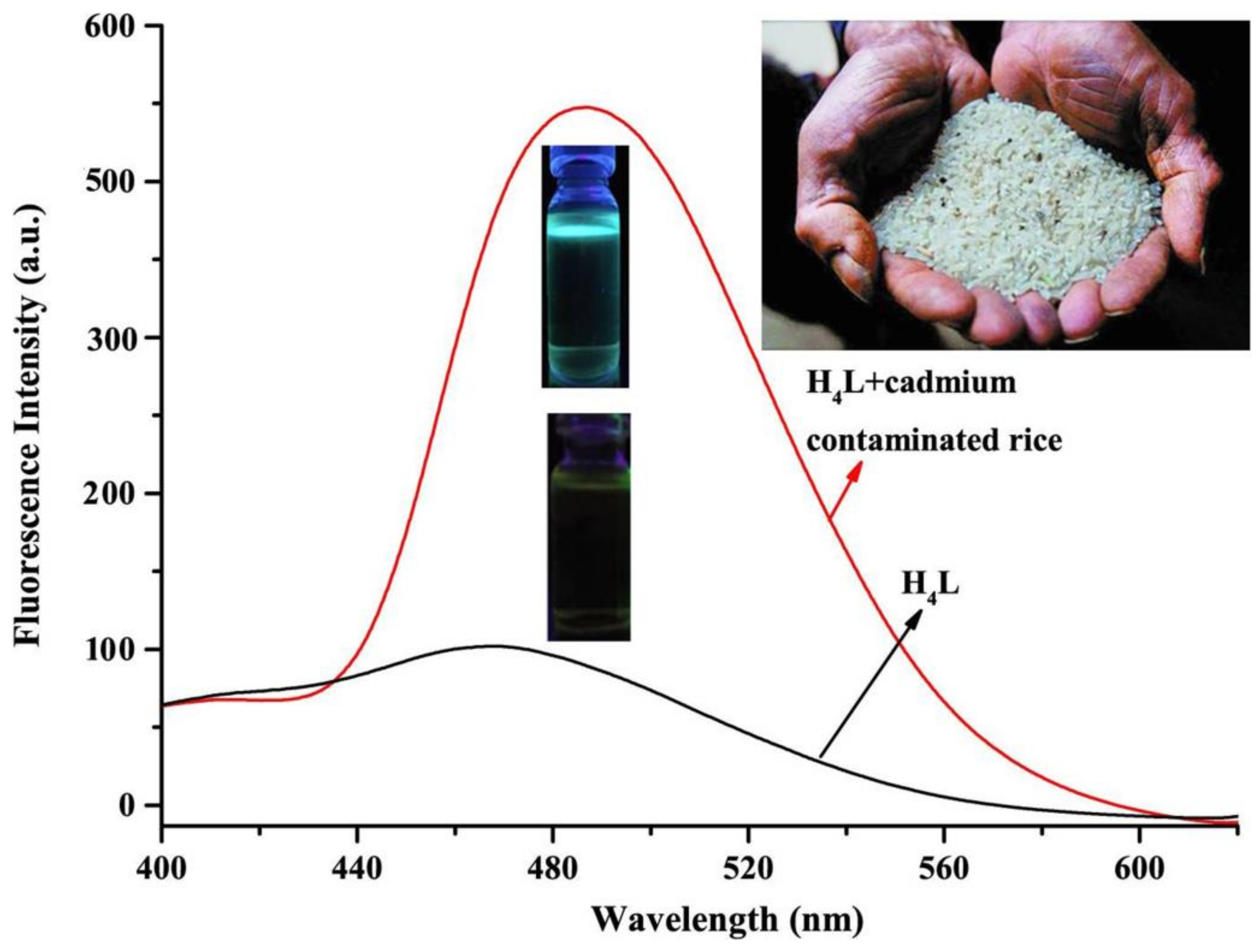
3.7. Application in Food Samples
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chai, L.Q.; Tang, L.J.; Chen, L.C.; Huang, J.J. Structural, spectral, electrochemical and DFT studies of two mononuclear manganese(II) and zinc(II) complexes. Polyhedron 2017, 122, 228–240. [Google Scholar] [CrossRef]
- Chai, L.Q.; Zhang, K.Y.; Tang, L.J.; Zhang, J.Y.; Zhang, H.S. Two mono- and dinuclear Ni(II) complexes constructed from quinazoline-type ligands: Synthesis, X-ray structures, spectroscopic, electrochemical, thermal, and antimicrobial studies. Polyhedron 2017, 130, 100–107. [Google Scholar] [CrossRef]
- Chai, L.Q.; Zhang, J.Y.; Chen, L.C.; Li, Y.X.; Tang, L.J. Synthesis, crystal structure, spectroscopic properties and DFT calculations of a new schiff base-type zinc(II) complex. Res. Chem. Intermed. 2016, 42, 3473–3488. [Google Scholar] [CrossRef]
- Chai, L.Q.; Li, Y.X.; Chen, L.C.; Zhang, J.Y.; Huang, J.J. Synthesis, X-ray structure, spectroscopic, electrochemical properties and DFT calculation of a bridged dinuclear copper(II) complex. Inorg. Chim. Acta 2016, 444, 193–201. [Google Scholar] [CrossRef]
- Liu, X.; Wang, P.; Fu, J.; Yao, K.; Xue, K.; Xu, K. Turn-on fluorescent sensor for zinc and cadmium ions based on quinolone and its sequential response to phosphate. J. Lumin. 2017, 186, 16–22. [Google Scholar] [CrossRef]
- Marettová, E.; Maretta, M.; Legáth, J. Toxic effects of cadmium on testis of birds and mammals: A review. Anim. Reprod. Sci. 2015, 155, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Al-Khaldi, F.A.; Abusharkh, B.; Khaled, M.; Atieh, M.A.; Nasser, M.S. Adsorptive removal of cadmium(II) ions from liquid phase using acid modified carbon-based adsorbents. J. Mol. Liq. 2015, 204, 255–263. [Google Scholar]
- Akesson, A.; Julin, B.; Wolk, A. Long-term dietary cadmium intake and postmenopausal endometrial cancer incidence: A population-based prospective cohort study. Cancer Res. 2008, 68, 6435–6441. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.L.; Liu, X.M.; Liu, K.; Liu, H.; Zhao, F.Y.; Ruan, W.J. A polypyridyl-pyrene based off-on Cd2+ fluorescent sensor for aqueous phase analysis and living cell imaging. Talanta 2014, 128, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Vinod, K.; Gupta, M.R.; Ganjali, P.; Norouzi, H.; Khani, A.N.; Shilpi, A. Electrochemical analysis of some toxic metals by ion–selective electrodes. Crit. Rev. Anal. Chem. 2011, 41, 282–313. [Google Scholar]
- Gupta, V.K.; Jain, A.K.; Agarwal, S.; Maheshwari, G. An iron(III) ion-selective sensor based on a mu-bis(tridentate) ligand. Talanta 2007, 71, 1964–1968. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Chandra, S.; Lang, H. A highly selective mercury electrode based on a diamine donor ligand. Talanta 2005, 66, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Sethi, B.; Sharma, R.A.; Agarwal, S.; Bharti, A. Mercury selective potentiometric sensor based on low rim functionalized thiacalix-arene as a cationic receptor. J. Mol. Liq. 2013, 177, 114–118. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, L.; Guo, R.; Xiang, T.; Wu, C.; Zheng, Z. A highly sensitive and selective colorimetric and off–on fluorescent chemosensor for Cu2+, based on rhodamine derivative. Sens. Actuators B 2011, 156, 546–552. [Google Scholar] [CrossRef]
- Jun, M.E.; Roy, B.; Ahn, K.H. “Turn-On” fluorescent sensing with “Reactive” probes. Chem. Commun. 2011, 47, 7583–7601. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Hu, M.; Fan, J.; Peng, X. Cheminform abstract: Fuorescent chemodosimeters using “mild” chemical events for the detection of small anions and cations in biological and environmental media. Chem. Soc. Rev. 2012, 41, 4511–4535. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Pradhan, T.; Wang, F.; Kim, J.S.; Yoon, J. Fluorescent chemosensors based on spiroring-opening of xanthenes and related derivatives. Chem. Rev. 2012, 112, 1910–1956. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Chen, L.; Gao, L.; Zhang, Y.; Akogun, S.F.; Dong, W.K. Syntheses, crystal structures and catalytic activities of two solvent-induced homotrinuclear Co(II) complexes with a naphthalenediol-based bis(Salamo)-type tetraoxime ligand. RSC Adv. 2017, 7, 35905–35916. [Google Scholar] [CrossRef]
- Wang, L.; Ma, J.C.; Dong, W.K.; Zhu, L.C.; Zhang, Y. A novel Self–assembled nickel(II)–cerium(III) heterotetranuclear dimer constructed from N2O2-type bisoxime and terephthalic acid: Synthesis, structure and photophysical properties. Z. Anorg. Allg. Chem. 2016, 642, 834–839. [Google Scholar] [CrossRef]
- Ma, J.C.; Dong, X.Y.; Dong, W.K.; Zhang, Y.; Zhu, L.C.; Zhang, J.T. An unexpected dinuclear Cu(II) complex with a bis(Salamo) chelating ligand: Synthesis, crystal structure, and photophysical properties. J. Coord. Chem. 2016, 69, 149–159. [Google Scholar] [CrossRef]
- Tao, C.H.; Ma, J.C.; Zhu, L.C.; Zhang, Y.; Dong, W.K. Heterobimetallic 3d–4f Zn(II)–Ln(III) (Ln = Sm, Eu, Tb and Dy) complexes with a N2O4 bisoxime chelate ligand and a simple auxiliary ligand Py: Syntheses, structures and luminescence properties. Polyhedron 2017, 128, 38–45. [Google Scholar] [CrossRef]
- Dong, Y.J.; Dong, X.Y.; Dong, W.K.; Zhang, Y.; Zhang, L.S. Three asymmetric Salamo-type copper(II) and cobalt(II) complexes: Syntheses, structures, fluorescent properties. Polyhedron 2017, 123, 305–315. [Google Scholar] [CrossRef]
- Dong, Y.J.; Ma, J.C.; Zhu, L.C.; Dong, W.K.; Zhang, Y. Four 3d–4f heteromultinuclear zinc(II)–lanthanide(III) complexes constructed from a distinct hexadentate N2O2-type ligand: Syntheses, structures and photophysical properties. J. Coord. Chem. 2017, 70, 103–115. [Google Scholar] [CrossRef]
- Song, X.Q.; Liu, P.P.; Liu, Y.A.; Zhou, J.J.; Wang, X.L. Two dodecanuclear heterometallic [Zn6Ln6] clusters constructed by a multidentate salicylamide salen-like ligand: Synthesis, structure, luminescence and magnetic properties. Dalton Trans. 2016, 45, 8154–8163. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.P.; Sheng, L.; Song, X.Q.; Xu, W.Y.; Liu, Y.A. Synthesis, structure and magnetic properties of a new one dimensional manganese coordination polymer constructed by a new asymmetrical ligand. Inorg. Chim. Acta 2015, 434, 252–257. [Google Scholar] [CrossRef]
- Song, X.Q.; Liu, P.P.; Xiao, Z.R.; Li, X.; Liu, Y.A. Four polynuclear complexes based on a versatile salicylamide salen-like ligand: Synthesis, structural variations and magnetic properties. Inorg. Chim. Acta 2015, 438, 232–244. [Google Scholar] [CrossRef]
- Song, X.Q.; Peng, Y.J.; Chen, G.Q.; Wang, X.R.; Liu, P.P.; Xu, W.Y. Substituted group-directed assembly of Zn(II) coordination complexes based on two new structural related pyrazolone based Salen ligands: Syntheses, structures and fluorescence properties. Inorg. Chim. Acta 2015, 427, 13–21. [Google Scholar] [CrossRef]
- Liu, P.P.; Wang, C.Y.; Zhang, M.; Song, X.Q. Pentanuclear sandwich-type ZnII-LnIII clusters based on a new Salen-like salicylamide ligand: Structure, near-infrared emission and magnetic properties. Polyhedron 2017, 129, 133–140. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, L. Synthesis, structure and spectroscopic properties of the trinuclear cobalt(II) and nickel(II) complexes based on 2-hydroxynaphthaldehyde and bis(aminooxy)alkane. Spectrochim. Acta Part A 2015, 135, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhao, L. An infinite 2D supramolecular cobalt(II) complex based on an asymmetric Salamo-type ligand: Synthesis, crystal structure, and spectral properties. Synth. React. Inorg. Met-Org. Nano-MetChem. 2016, 46, 1095–1101. [Google Scholar] [CrossRef]
- Hu, J.H.; Li, J.B.; Qi, J.; Sun, Y. Selective colorimetric and “turn-on” fluorimetric detection of cyanide using an acylhydrazone sensor in aqueous media. New J. Chem. 2015, 39, 4041–4046. [Google Scholar] [CrossRef]
- Hu, J.H.; Li, J.B.; Qi, J.; Chen, J.J. Highly selective and effective mercury(II) fluorescent sensor. New J. Chem. 2015, 39, 843–848. [Google Scholar] [CrossRef]
- Li, J.B.; Hu, J.H.; Chen, J.J.; Qi, J. Cyanide detection using a benzimidazole derivative in aqueous media. Spectrochim. Acta A 2014, 133, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.H.; Li, J.B.; Qi, J.; Sun, Y. Acylhydrazone based fluorescent chemosensor for zinc in aqueous solution with high selectivity and sensitivity. Sens. Actuators B Chem. 2015, 208, 581–587. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, J.H.; Qi, J.; Li, J.B. A highly selective colorimetric and “turn-on” fluorimetric chemosensor for detecting CN—Based on unsymmetrical azine derivatives in aqueous media. Spectrochim. Acta A 2016, 167, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.H.; Chen, J.J.; Li, J.B.; Qi, J. A cyanide ion probe based on azosalicylic aldehyde of benzoyl hydrazone. Chin. J. Inorg. Chem. 2014, 30, 2544–2548. [Google Scholar]
- Qi, J.; Hu, J.H.; Chen, J.J.; Sun, Y.; Li, J.B. Cyanide Detection using Azo-acylhydrazone in Aqueous Media with High Sensitivity and Selectivity. Curr. Anal. Chem. 2016, 12, 119–123. [Google Scholar] [CrossRef]
- Hu, J.H.; Li, J.B.; Qi, J.; Sun, Y. Studies on the crystal structure and characterization of n-(4-acetylphenyl)-na-(2-nitrobenzoyl)-thiourea. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 984–987. [Google Scholar] [CrossRef]
- Hu, J.H.; Sun, Y.; Qi, J.; Pei, P.X.; Lin, Q.; Zhang, Y.M. A colorimetric and “turn-on” fluorimetric chemosensor for the selective detection of cyanide and its application in food samples. RSC Adv. 2016, 6, 100401–100406. [Google Scholar] [CrossRef]
- Hu, J.H.; Sun, Y.; Qi, J.; Li, Q.; Wei, T.B. A new unsymmetrical azine derivative based on coumarin group as dual-modal sensor for CN−, and fluorescent “off–on” for Zn2+. Spectrochim. Acta A 2017, 175, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.L.; Pan, G.L.; Wang, H.; Wang, X.L.; Bai, Y.C.; Zhang, Y.H. Study on synthesis, crystal structure, antioxidant and DNA-binding of mono-, di- and poly-nuclear lanthanides complexes with bis(N-salicylidene)-3-oxapentane-1,5-diamine. J. Photochem. Photobiol. B Biol. 2014, 135, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.L.; Bai, Y.C.; Zhang, Y.H.; Li, Z.; Wu, M.C.; Chen, C.Y.; Zhang, J.W. Synthesis, crystal structure, antioxidation and DNA-binding properties of a dinuclear copper(II) complex with bis(N-salicylidene)-3-oxapentane-1,5-diamine. J. Coord. Chem. 2014, 67, 3054–3066. [Google Scholar] [CrossRef]
- Wu, H.L.; Pan, G.L.; Bai, Y.C.; Wang, H.; Kong, J. Synthesis, structure, antioxidation, and DNA-bindingstudies of a binuclear ytterbium(III) complex with bis(N-salicylidene)-3-oxapentane-1,5-diamine. Res. Chem. Intermed. 2015, 41, 3375–3388. [Google Scholar] [CrossRef]
- Wu, H.L.; Wang, C.P.; Wang, F.; Peng, H.P.; Zhang, H.; Bai, Y.C. A new manganese(III) complex from bis(5-methylsalicylaldehyde)-3-oxapentane-1,5-diamine: Synthesis, characterization, antioxidant activity and luminescence. J. Chin. Chem. Soc. 2015, 62, 1028–1034. [Google Scholar] [CrossRef]
- Chen, C.Y.; Zhang, J.W.; Zhang, Y.H.; Yang, Z.H.; Wu, H.L. Gadolinium(III) and dysprosium(III) complexes with a Schiff base bis(N-salicylidene)-3-oxapentane-1,5-diamine: Synthesis, characterization, antioxidation, and DNA-binding studies. J. Coord. Chem. 2015, 68, 1054–1071. [Google Scholar] [CrossRef]
- Wu, H.L.; Bai, Y.C.; Zhang, Y.H.; Pan, G.L.; Kong, J.; Shi, F.; Wang, X.L. Two lanthanide(III) complexes based on the schiff base N,N-Bis(salicylidene)-1,5-diamino-3-oxapentane: Synthesis, characterization, DNA-binding properties, and antioxidation. Z. Anorg. Allg. Chem. 2014, 640, 2062–2071. [Google Scholar] [CrossRef]
- Wu, H.L.; Pan, G.L.; Bai, Y.C.; Wang, H.; Kong, J.; Shi, F.; Zhang, Y.H.; Wang, X.L. Preparation, structure, DNA-binding properties, and antioxidant activities of a homodinuclear erbium(III) complex with a pentadentate Schiff base ligand. J. Chem. Res. 2014, 38, 211–217. [Google Scholar] [CrossRef]
- Wang, B.J.; Dong, W.K.; Zhang, Y.; Akogun, S.F. A novel relay-sensor for highly sensitive and selective detection of Zn2+/Pic- and fluorescence on/off switch response of H+/OH−. Sens. Actuators B 2017, 247, 254–264. [Google Scholar] [CrossRef]
- Dong, Y.J.; Li, X.L.; Zhang, Y.; Dong, W.K. A highly selective visual and fluorescent sensor for Pb2+ and Zn2+ and crystal structure of Cu2+ complex based-on a novel single-armed Salamo-type bisoxime. Supramol. Chem. 2017, 29, 518–527. [Google Scholar] [CrossRef]
- Dong, W.K.; Ma, J.C.; Zhu, L.C.; Zhang, Y.; Li, X.L. Four new nickel(II) complexes based on an asymmetric Salamo-type ligand: Synthesis, structure, solvent effect and electrochemical property. Inorg. Chim. Acta 2016, 445, 140–148. [Google Scholar] [CrossRef]
- Dong, W.K.; Lan, P.F.; Zhou, W.M.; Zhang, Y. Salamo-type trinuclear and tetranuclear cobalt(II) complexes based on a new asymmetry salamo-type ligand: Syntheses, crystal structures and fluorescence properties. J. Coord. Chem. 2016, 65, 1272–1283. [Google Scholar] [CrossRef]
- Dong, W.K.; Ma, J.C.; Zhu, L.C.; Zhang, Y. Self-assembled zinc(II)-lanthanide(III) heteromultinuclear complexes constructed from 3-MeOsalamo ligand: Syntheses, structures and luminescent properties. Cryst. Growth Des. 2016, 16, 6903–6914. [Google Scholar] [CrossRef]
- Dong, X.Y.; Kang, Q.P.; Jin, B.X.; Dong, W.K. A dinuclear nickel(II) complex derived from an asymmetric Salamo-type N2O2 chelate ligand: Synthesis, structure and optical properties. Z. Naturforsch. 2017, 72, 415–420. [Google Scholar] [CrossRef]
- Dong, W.K.; Zhang, J.T.; Dong, Y.J.; Zhang, Y.; Wang, Z.K. Construction of mononuclear copper(II) and trinuclear cobalt(II) complexes based on asymmetric Salamo-type ligands. Z. Anorg. Allg. Chem. 2016, 642, 189–196. [Google Scholar] [CrossRef]
- Dong, W.K.; Li, X.L.; Wang, L.; Zhang, Y.; Ding, Y.J. A new application of Salamo-type bisoximes: As a relay-sensor for Zn2+/Cu2+ and its novel complexes for successive sensing of H+/OH−. Sens. Actuators B 2016, 229, 370–378. [Google Scholar] [CrossRef]
- Dong, W.K.; Zhang, J.; Zhang, Y.; Li, N. Novel multinuclear transition metal(II) complexes based on an asymmetric Salamo-type ligand: Syntheses, structure characterizations and fluorescent properties. Inorg. Chim. Acta 2016, 444, 95–102. [Google Scholar] [CrossRef]
- Dong, W.K.; Ma, J.C.; Dong, Y.J.; Zhu, L.C.; Zhang, Y. Di-and tetranuclear heterometallic 3d-4f cobalt(II)-lanthanide(III) complexes derived from a hexadentate bisoxime: Syntheses, structures and magnetic properties. Polyhedron 2016, 115, 228–235. [Google Scholar] [CrossRef]
- Dong, W.K.; Ma, J.C.; Zhu, L.C.; Zhang, Y. Nine self-assembled nickel(II)-lanthanide(III) heterometallic complexes constructed from a Salamo-type bisoxime and bearing N- or O-donor auxiliary ligand: Syntheses, structures and magnetic properties. New J. Chem. 2016, 40, 6998–7010. [Google Scholar] [CrossRef]
- Gao, L.; Wang, F.; Zhao, Q.; Zhang, Y.; Dong, W.K. Mononuclear Zn(II) and trinuclear Ni(II) complexes derived from a coumarin-containing N2O2 ligand: Syntheses, crystal structures and fluorescence properties. Polyhedron 2018, 139, 7–16. [Google Scholar] [CrossRef]
- Chen, L.; Dong, W.K.; Zhang, H.; Zhang, Y.; Sun, Y.X. Structural variation and luminescence properties of tri- and dinuclear CuII and ZnII complexes constructed from a naphthalenediol-based bis(Salamo)-type ligand. Cryst. Growth Des. 2017, 17, 3636–3648. [Google Scholar] [CrossRef]
- Dong, W.K.; Akogun, S.F.; Zhang, Y.; Sun, Y.X.; Dong, X.Y. A reversible “turn-on” fluorescent sensor for selective detection of Zn2+. Sens. Actuators B 2017, 238, 723–734. [Google Scholar] [CrossRef]
- Hao, J.; Liu, L.Z.; Dong, W.K.; Zhang, J.; Zhang, Y. Three multinuclear Co.(II), Zn(II) and Cd(II) complexes based on a single-armed salamo-type bisoxime: Syntheses, structural characterizations and fluorescent properties. J. Coord. Chem. 2017, 11, 1936–1952. [Google Scholar] [CrossRef]
- Zheng, S.S.; Dong, W.K.; Zhang, Y.; Chen, L.; Ding, Y.J. Four Salamo-type 3d–4f hetero-bimetallic [ZnIILnIII] complexes: Syntheses, crystal structures, and luminescent and magnetic properties. New J. Chem. 2017, 41, 4966–4973. [Google Scholar] [CrossRef]
- Dong, W.K.; Zheng, S.S.; Zhang, J.T.; Zhang, Y.; Sun, Y.X. Luminescent properties of heterotrinuclear 3d–4f complexes constructed from a naphthalenediol-based acyclic bis(salamo)-type ligand. Spectrochim. Acta Part A 2017, 184, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Hao, J.; Liu, L.Z.; Zhou, W.M.; Dong, W.K. Syntheses, crystal structures and thermal behaviors of two supramolecular salamo-type cobalt(II) and zinc(II) complexes. Crystals 2017, 7, 217. [Google Scholar]
- Peng, Y.D.; Li, X.Y.; Kang, Q.P.; An, G.X.; Zhang, Y.; Dong, W.K. Synthesis and fluorescence properties of asymmetrical salamo-type tetranuclear zinc(II) complex. Crystals 2018, 8, 107. [Google Scholar] [CrossRef]
- Hao, J.; Li, L.L.; Zhang, J.T.; Akogun, S.F.; Wang, L.; Dong, W.K. Four homo- and hetero-bismetallic 3d/3d-2s complexes constructed from a naphthalenediol-based acyclic bis(salamo)-type tetraoxime ligand. Polyhedron 2017, 134, 1–10. [Google Scholar] [CrossRef]
- Wang, L.; Hao, J.; Zhai, L.X.; Zhang, Y.; Dong, W.K. Synthesis, crystal structure, luminescence, electrochemical and antimicrobial properties of bis(salamo)-based Co.(II) complex. Crystals 2017, 7, 277. [Google Scholar] [CrossRef]
- Wang, F.; Gao, L.; Zhao, Q.; Zhang, Y.; Dong, W.K.; Ding, Y.J. A highly selective fluorescent chemosensor for CN− based on a novel bis(salamo)-type tetraoxime ligand. Spectrochim. Acta Part A 2018, 190, 111–115. [Google Scholar] [CrossRef] [PubMed]
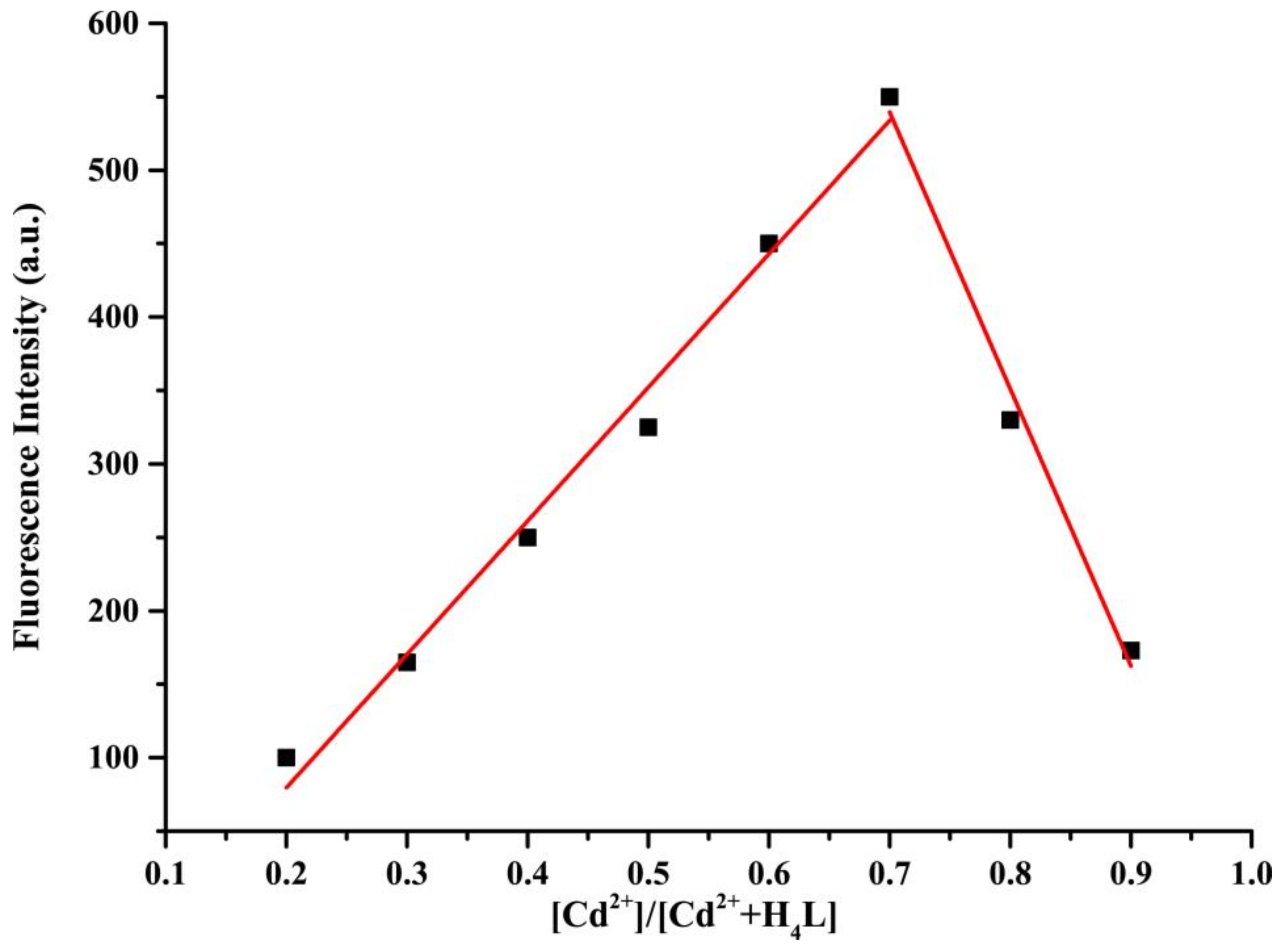
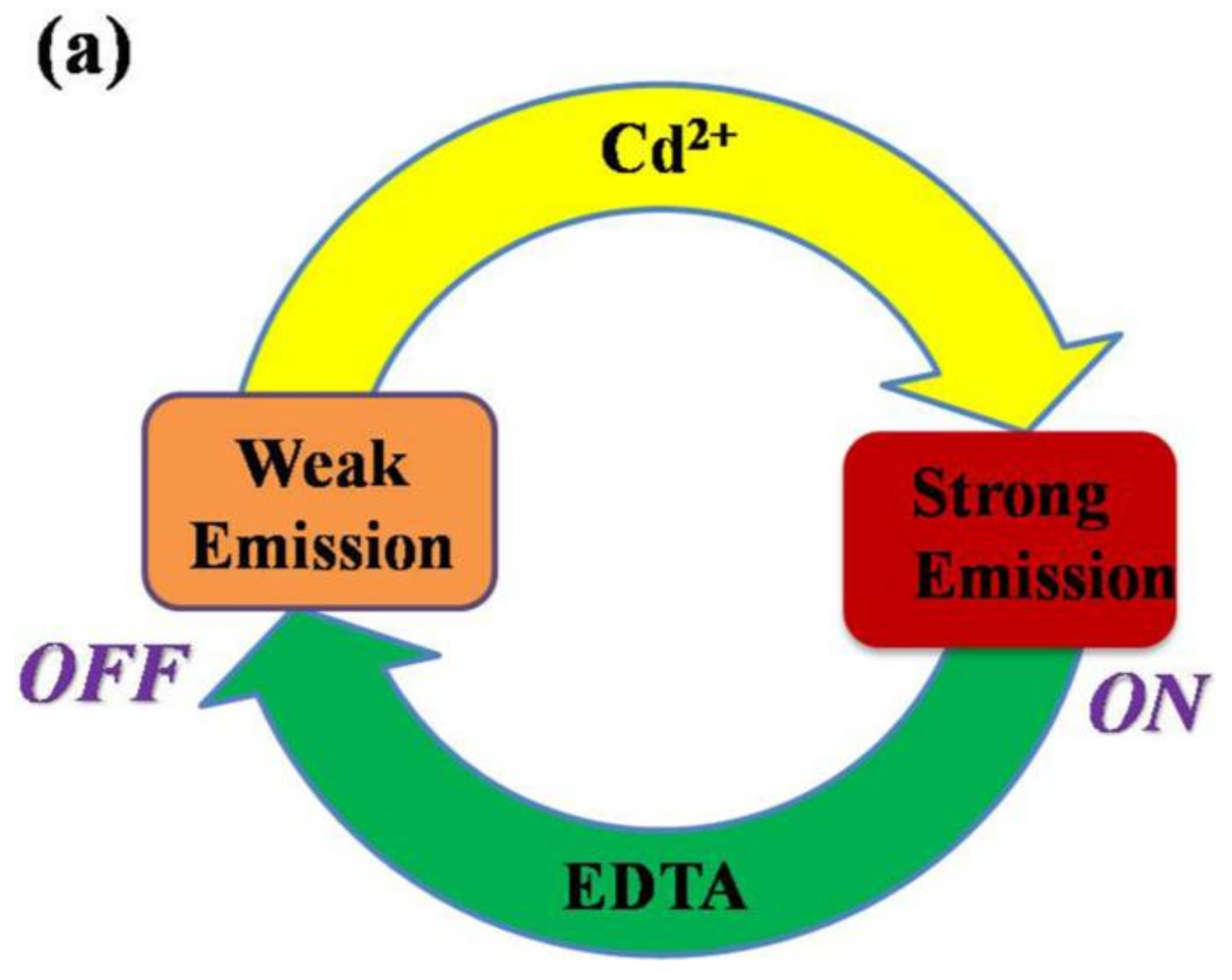
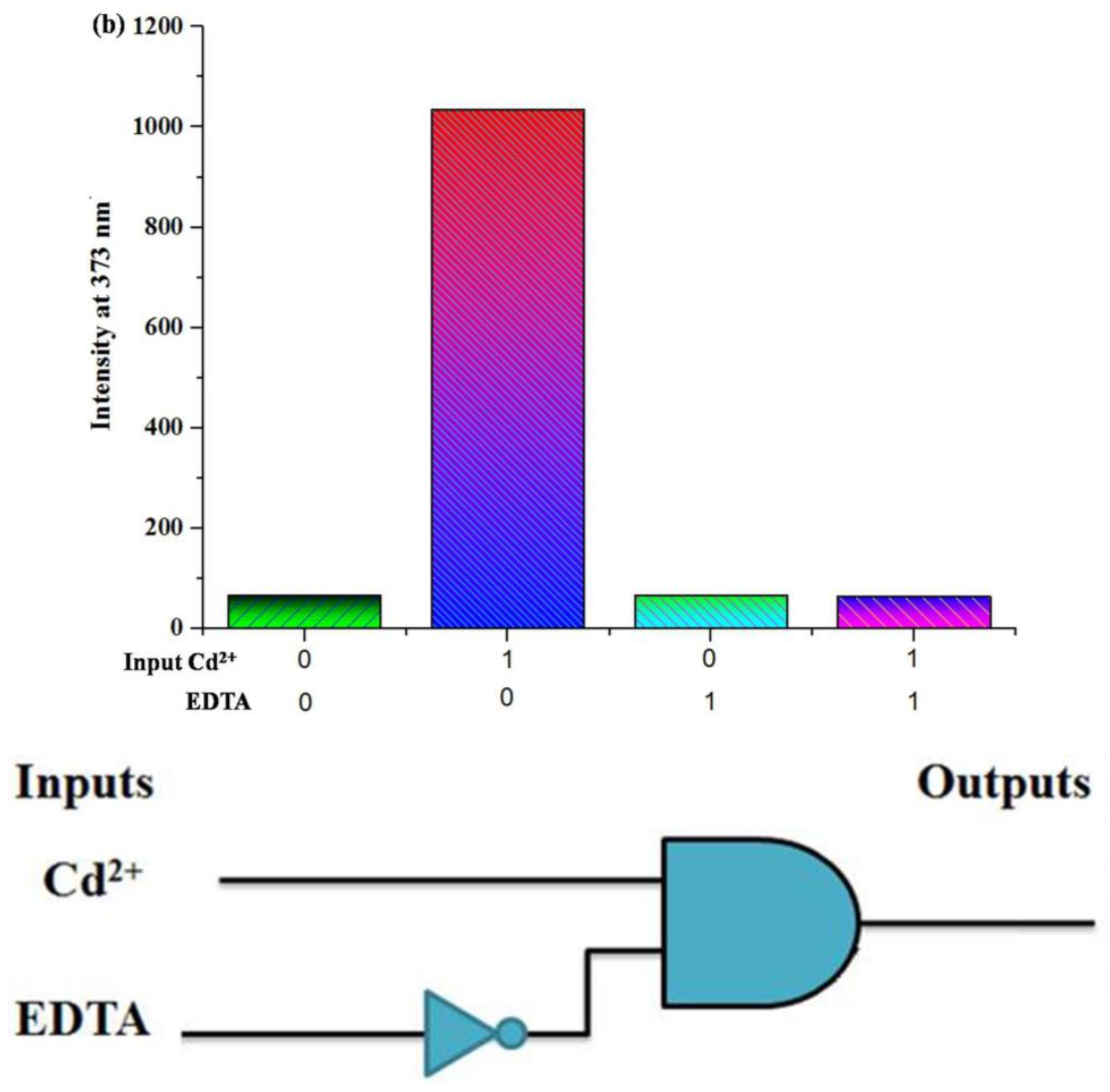

| Inputs | Outputs | ||
|---|---|---|---|
| Cd2+ | EDTA | (INHIBIT Logic Gate) Intensity λmax | |
| 0 | 0 | low flu. | 0 |
| 1 | 0 | high flu. | 1 |
| 0 | 1 | low flu. | 0 |
| 1 | 1 | low flu. | 0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, J.; Li, X.-Y.; Zhang, Y.; Dong, W.-K. A Reversible Bis(Salamo)-Based Fluorescence Sensor for Selective Detection of Cd2+ in Water-Containing Systems and Food Samples. Materials 2018, 11, 523. https://doi.org/10.3390/ma11040523
Hao J, Li X-Y, Zhang Y, Dong W-K. A Reversible Bis(Salamo)-Based Fluorescence Sensor for Selective Detection of Cd2+ in Water-Containing Systems and Food Samples. Materials. 2018; 11(4):523. https://doi.org/10.3390/ma11040523
Chicago/Turabian StyleHao, Jing, Xiao-Yan Li, Yang Zhang, and Wen-Kui Dong. 2018. "A Reversible Bis(Salamo)-Based Fluorescence Sensor for Selective Detection of Cd2+ in Water-Containing Systems and Food Samples" Materials 11, no. 4: 523. https://doi.org/10.3390/ma11040523
APA StyleHao, J., Li, X.-Y., Zhang, Y., & Dong, W.-K. (2018). A Reversible Bis(Salamo)-Based Fluorescence Sensor for Selective Detection of Cd2+ in Water-Containing Systems and Food Samples. Materials, 11(4), 523. https://doi.org/10.3390/ma11040523