A Heat-Stimulated Luminous Fiber Using Heat-Sensitive Green TF-G Pigment
Abstract
:1. Introduction
2. Experimental
2.1. Raw Materials
2.2. Preparation of Rare-Earth Luminescent Materials
2.3. Fabrication of the Heat-Stimulated Luminous Fiber
2.4. Characterization
3. Result and Discussion
3.1. Microstructure
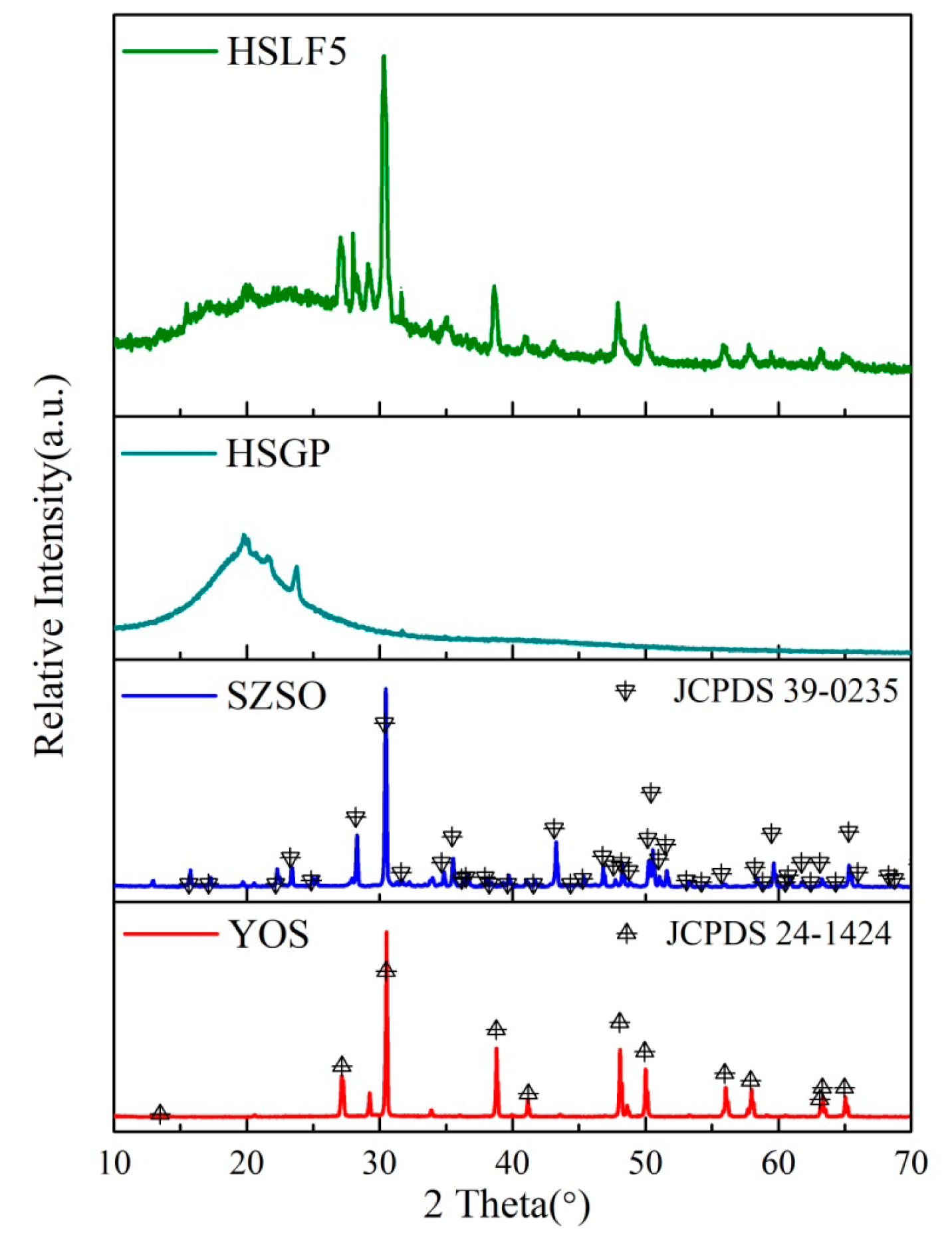
3.2. X-Ray Diffraction Analysis
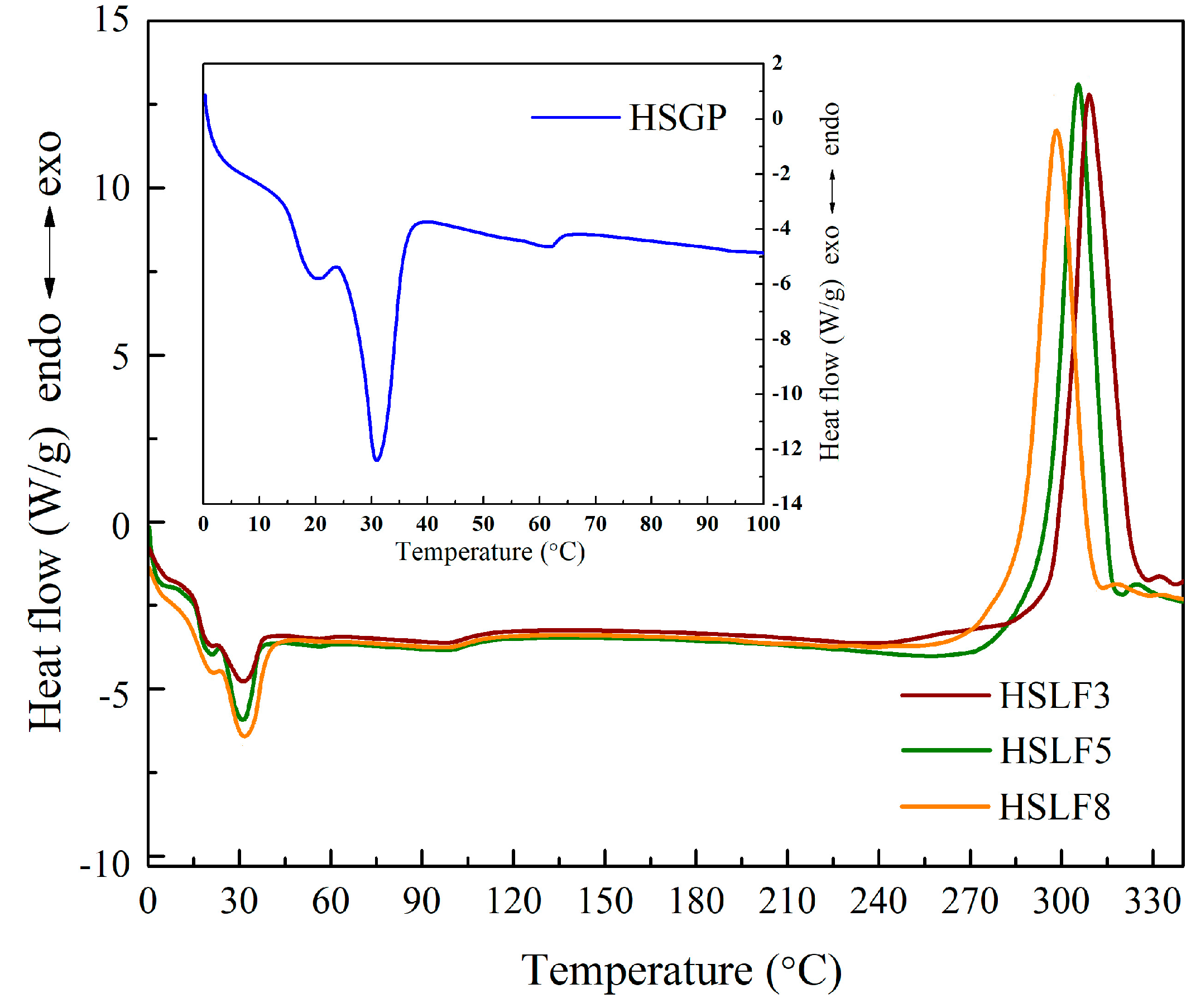
3.3. Differential Scanning Calorimetry Analysis
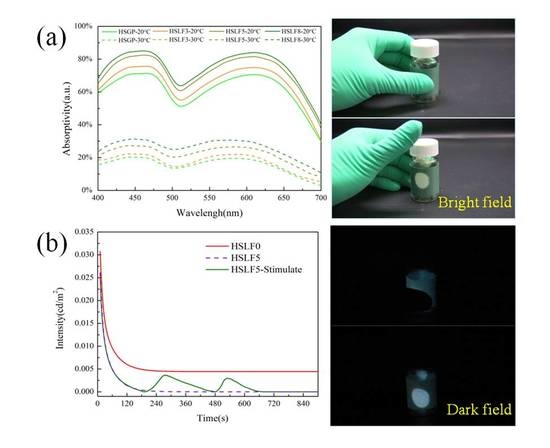
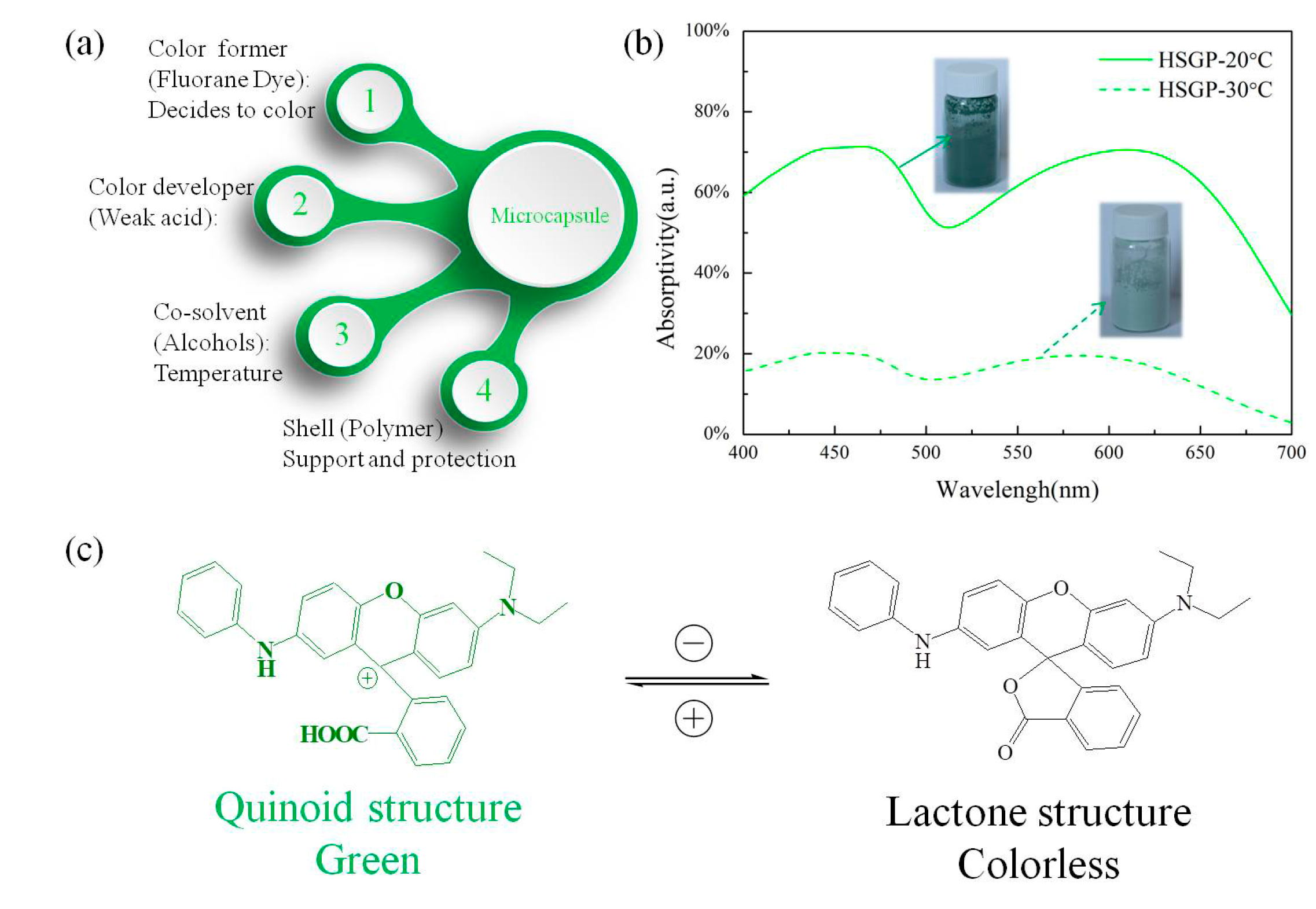
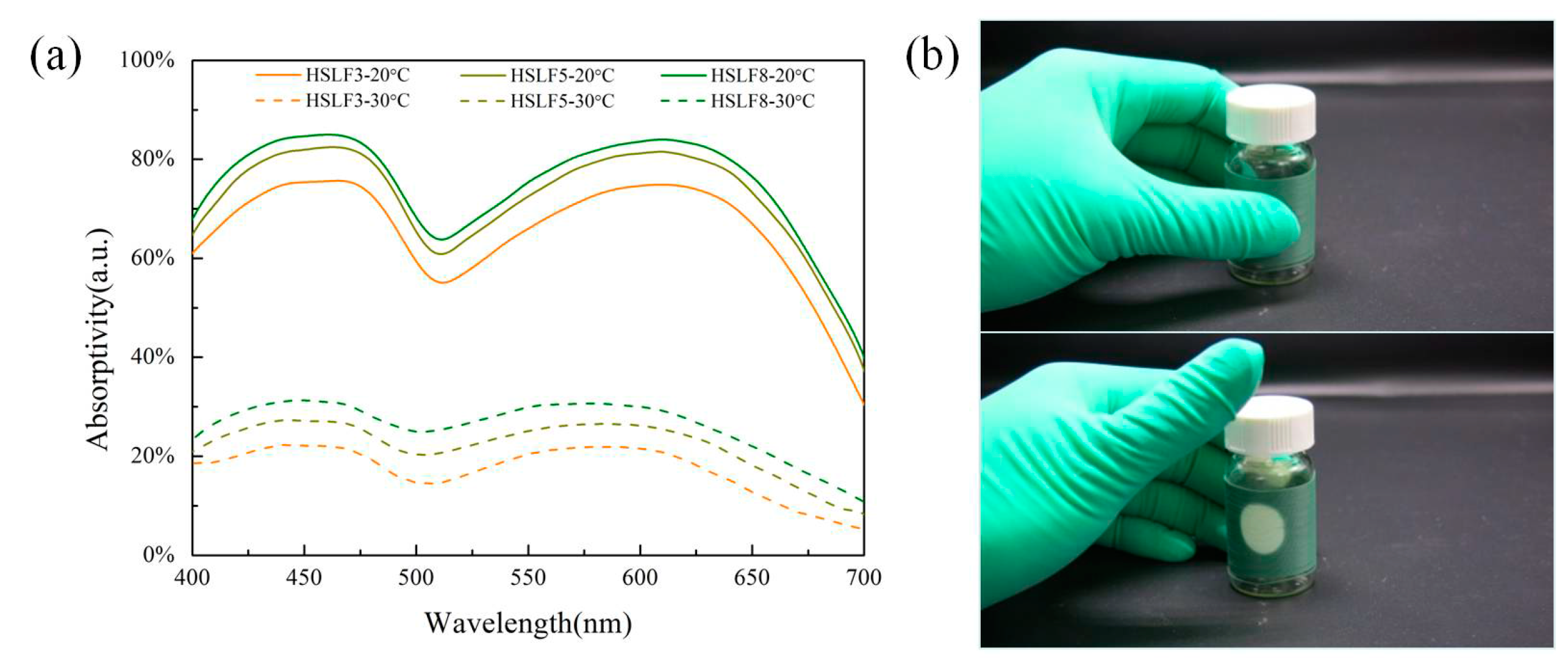
3.4. Absorptivity Analysis
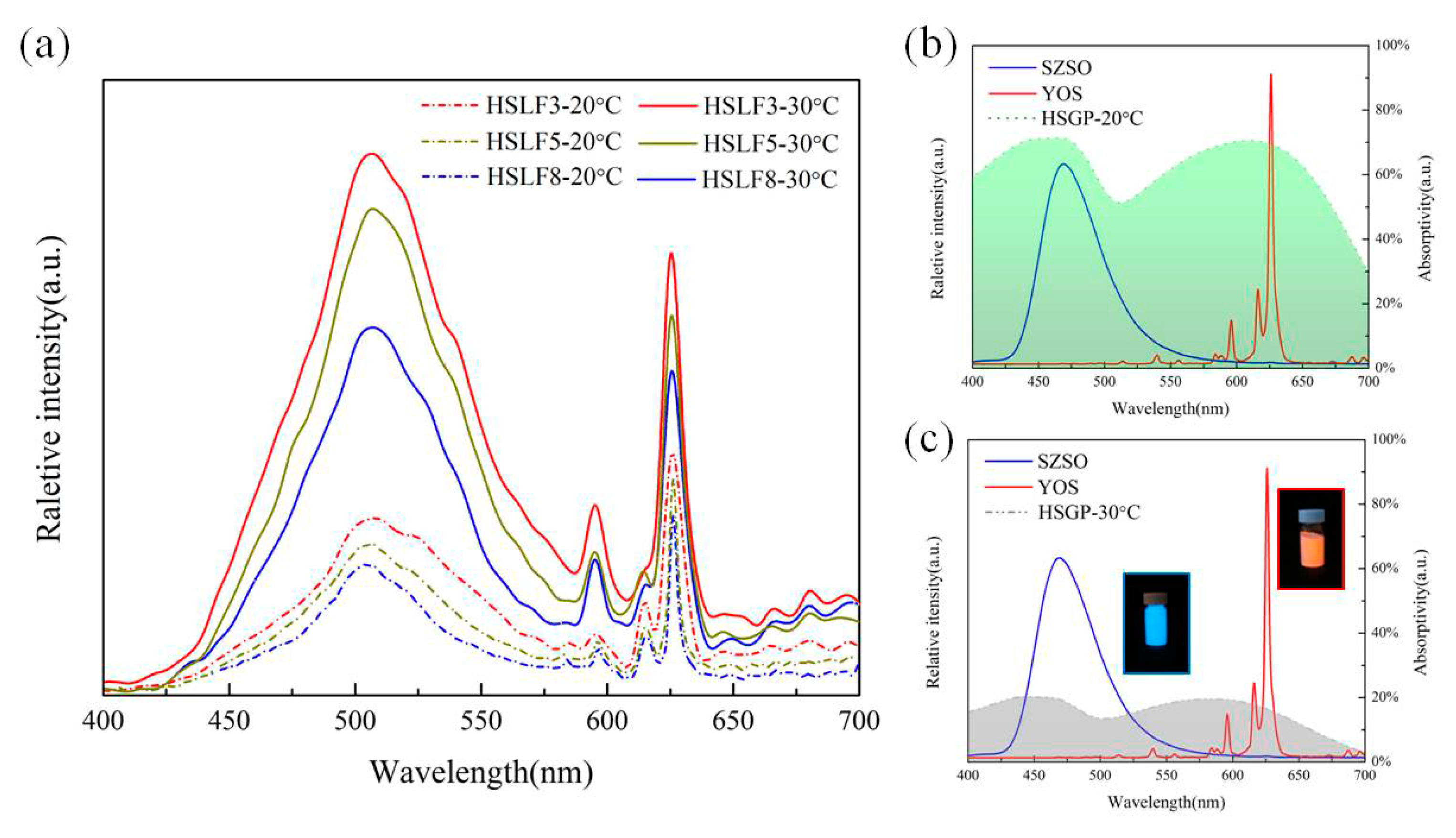
3.5. Emission Spectra Analysis
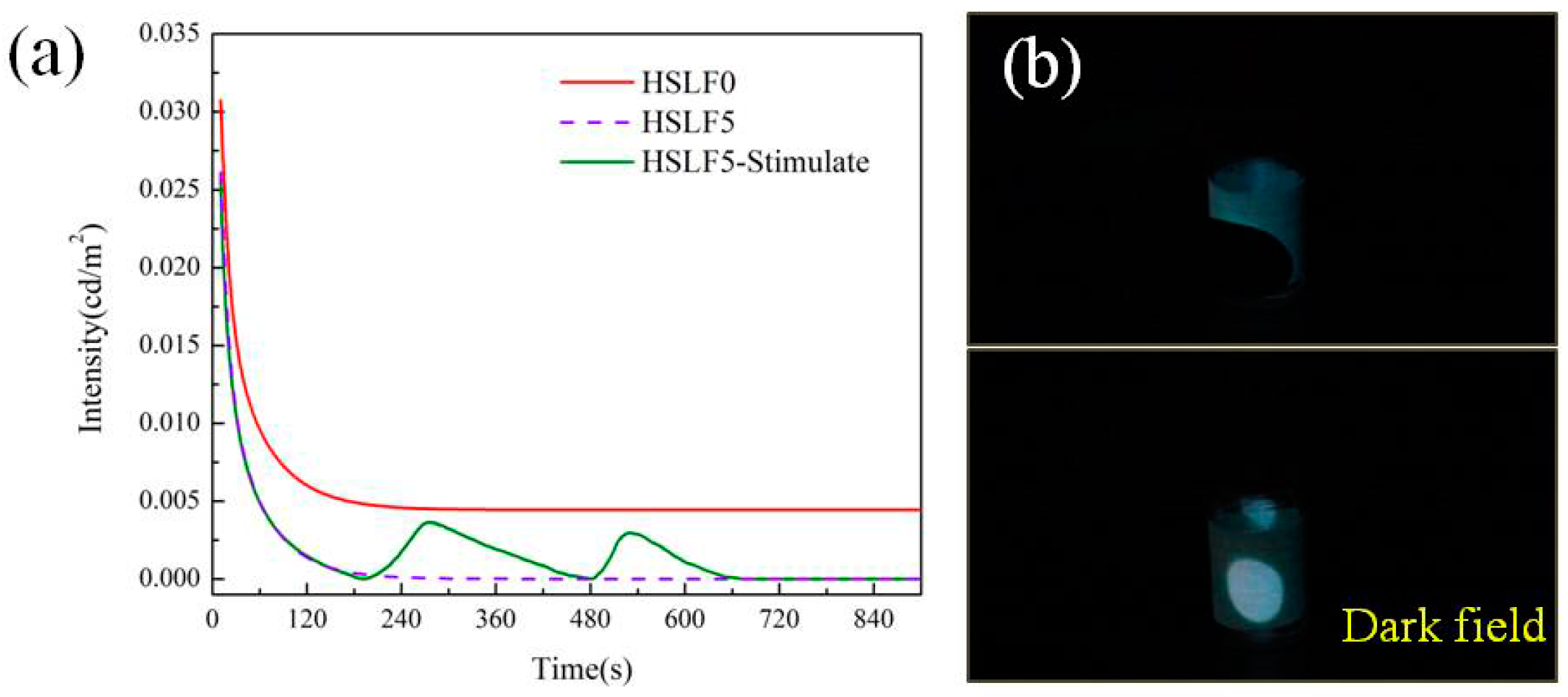
3.6. Heat-Stimulated Luminescence Property
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cui, C.E.; Liu, H.; Huang, P.; Wang, L. Influence of Eu3+ doping concentration on the luminescence properties of Y2O2S: Eu3+, Mg2+, Ti4+ nanoarrays via sol–gel template method. Opt. Mater. 2013, 36, 495–499. [Google Scholar] [CrossRef]
- Cui, C.E.; Liu, H.; Huang, P.; Wang, L. Influence of Mg2+, Ti4+ co-doping concentration on the luminescence properties of Y2O2S: Eu3+, Mg2+, Ti4+ nanotube arrays. J. Lumin. 2014, 149, 196–199. [Google Scholar] [CrossRef]
- Song, Z.; Ding, X.; Yang, S.; Du, F.; Bian, L.; Duan, S.; Liu, Q.L. Thermochromic material Sr2SiO4: Eu2+ based on displacive transformation. J. Lumin. 2014, 152, 199–202. [Google Scholar] [CrossRef]
- Jin, Y.; Ge, M.; Gu, T. Effect of α/β ratio on color characteristics of SrAl2O4: αEu2+, βDy3+. Optoelectron. Adv. Mater. Rapid Commun. 2017, 11, 236–241. [Google Scholar]
- Zhu, Y.; Ge, M. Effect of light conversion agent on the luminous properties of rare earth strontium aluminate luminous fiber. J. Mater. Sci. Mater. Electron. 2015, 27, 580–586. [Google Scholar] [CrossRef]
- Jin, Y.; Bai, Y.; Zhu, Y.; Li, X.; Ge, M. Thermosensitive luminous fiber based on reversible thermochromic crystal violet lactone pigment. Dyes Pigment. 2017, 146, 567–575. [Google Scholar] [CrossRef]
- Jin, Y.; Zhu, Y.; Li, X.; Ge, M. Thermosensitive luminous fibers based on cresol red-boric acid reversible thermochromic pigments. J. Mater. Sci. Mater. Electron. 2018, 29, 138–145. [Google Scholar] [CrossRef]
- Yan, Y.; Ge, M.; Li, Y.; Kumar, D. Morphology and spectral characteristics of a luminous fiber containing a rare earth strontium aluminate. Text. Res. J. 2012, 82, 1819–1826. [Google Scholar] [CrossRef]
- Yan, Y.; Zhu, Y.; Guo, X.; Ge, M. The effects of inorganic pigments on the luminescent properties of colored luminous fiber. Text. Res. J. 2013, 84, 785–792. [Google Scholar] [CrossRef]
- Zhu, Y.; Pang, Z.; Wang, J.; Ge, M.; Sun, S.; Hu, Z.; Zhai, J.; Gao, J.; Jiang, F. Effect of light conversion agent on luminous properties of a new down-converting material SrAl2O4: Eu2+, Dy3+/light conversion agent. J. Rare Earths 2016, 34, 483–488. [Google Scholar] [CrossRef]
- Tang, H.; Meng, G.; Chen, Z.; Wang, K.; Zhou, Q.; Wang, Z. Warm white light-emitting diodes based on a novel orange cationic iridium(iii) complex. Materials 2017, 10, 657. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Mohamed, M.G.; Qian, C.; Fan, X.; Zhang, G.; Pecht, M. Color shift failure prediction for phosphor-converted white leds by modeling features of spectral power distribution with a nonlinear filter approach. Materials 2017, 10, 819. [Google Scholar] [CrossRef] [PubMed]
- Bellina, F.; Manzini, C.; Marianetti, G.; Pezzetta, C.; Fanizza, E.; Lessi, M.; Minei, P.; Barone, V.; Pucci, A. Colourless p-phenylene-spaced bis-azoles for luminescent concentrators. Dyes Pigment. 2016, 134, 118–128. [Google Scholar] [CrossRef]
- Cegielski, R.; Niedbalska, M. Effect of substituents on the thermochromism of stilbazolium merocyanines incorporated in epoxy resin. J. Mol. Struct. 2013, 1034, 95–100. [Google Scholar] [CrossRef]
- Panák, O.; Držková, M.; Kaplanová, M.; Novak, U.; Klanjšek Gunde, M. The relation between colour and structural changes in thermochromic systems comprising crystal violet lactone, bisphenol a, and tetradecanol. Dyes Pigment. 2017, 136, 382–389. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Wu, L.C. Fluorescent and electrochromic polymers from 2,8-di(carbazol-9-yl) dibenzothiophene and its s,s-dioxide derivative. Dyes Pigment. 2016, 134, 51–63. [Google Scholar] [CrossRef]
- Jin, Y.; Bai, Y.M.; Ge, M.Q. Preparation and luminescence properties of a white emitting long afterglow luminous fiber based on fret. J. Rare Earths 2016, 34, 374–380. [Google Scholar] [CrossRef]
- Jin, Y.; Long, X.Y.; Zhu, Y.N.; Ge, M.Q. Optical performance study of sr2znsi2o7:Eu2+,dy3+, SrAl2O4:Eu2+, Dy3+ and Y2O2S: Eu3+, Mg2+, Ti4+ ternary luminous fiber. J. Rare Earths 2016, 34, 1206–1212. [Google Scholar] [CrossRef]
- Kulčar, R.; Friškovec, M.; Hauptman, N.; Vesel, A.; Gunde, M.K. Colorimetric properties of reversible thermochromic printing inks. Dyes Pigment. 2010, 86, 271–277. [Google Scholar] [CrossRef]
- Panák, O.; Držková, M.; Kaplanová, M. Insight into the evaluation of colour changes of leuco dye based thermochromic systems as a function of temperature. Dyes Pigment. 2015, 120, 279–287. [Google Scholar] [CrossRef]
- Raditoiu, A.; Raditoiu, V.; Nicolae, C.A.; Raduly, M.F.; Amariutei, V.; Wagner, L.E. Optical and structural dynamical behavior of crystal violet lactone—Phenolphthalein binary thermochromic systems. Dyes Pigment. 2016, 134, 69–76. [Google Scholar] [CrossRef]
- Zhu, C.F.; Wu, A.B. Studies on the synthesis and thermochromic properties of crystal violet lactone and its reversible thermochromic complexes. Thermochim. Acta 2005, 425, 7–12. [Google Scholar] [CrossRef]
- Chatterjee, N.; Basu, S.; Palit, S.K.; Maiti, M.M. An XRD characterization of the thermal degradation of polyacrylonitrile. J. Polym. Sci. A Polym. Chem. 1995, 33, 1705–1712. [Google Scholar] [CrossRef]
- Ji, B.H. Effect of Solvent Residuals on the Structure and Properties of Polyacrylonitrile Fibers. Appl. Mech. Mater. 2011, 79, 254–257. [Google Scholar] [CrossRef]
- Jin, Y.; Shi, C.; Li, X.Q.; Wang, Y.W.; Wang, F.F.; Ge, M.Q. Preparation and luminescence studies of thermosensitive pan luminous fiber based on the heat sensitive rose red TF-R1 thermochromic pigment. Dyes Pigment. 2017, 139, 693–700. [Google Scholar] [CrossRef]

| Sample | Integral Area | Ratio(30 °C/20 °C) | Increment | |
|---|---|---|---|---|
| 20 °C | 30 °C | |||
| HSLF3 | 90,716.70 | 246,481.86 | 2.72 | 171.71% |
| HSLF5 | 67,544.30 | 204,112.11 | 3.02 | 202.19% |
| HSLF8 | 52,807.11 | 161,821.93 | 3.06 | 206.44% |
| Sample | A0 | A1 | A2 | A3 | λ1 | λ2 | λ3 | R2 |
|---|---|---|---|---|---|---|---|---|
| HSLF0 | 0.00443 | 0.01276 | 0.00248 | 0.01361 | 10.25226 | 2.25055 | 51.60385 | 0.9996 |
| HSLF5 | 0.0000103 | 0.00225 | 0.01304 | 0.01496 | 8.66898 | 1.45401 | 46.29428 | 0.99881 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; An, X.; Ge, M. A Heat-Stimulated Luminous Fiber Using Heat-Sensitive Green TF-G Pigment. Materials 2018, 11, 425. https://doi.org/10.3390/ma11030425
Jin Y, An X, Ge M. A Heat-Stimulated Luminous Fiber Using Heat-Sensitive Green TF-G Pigment. Materials. 2018; 11(3):425. https://doi.org/10.3390/ma11030425
Chicago/Turabian StyleJin, Yang, Xiaolong An, and Mingqiao Ge. 2018. "A Heat-Stimulated Luminous Fiber Using Heat-Sensitive Green TF-G Pigment" Materials 11, no. 3: 425. https://doi.org/10.3390/ma11030425
APA StyleJin, Y., An, X., & Ge, M. (2018). A Heat-Stimulated Luminous Fiber Using Heat-Sensitive Green TF-G Pigment. Materials, 11(3), 425. https://doi.org/10.3390/ma11030425