Sulfide Stress Cracking Behavior of a Martensitic Steel Controlled by Tempering Temperature
Abstract
:1. Introduction
2. Results
2.1. Mechanical Property and KISSC Values
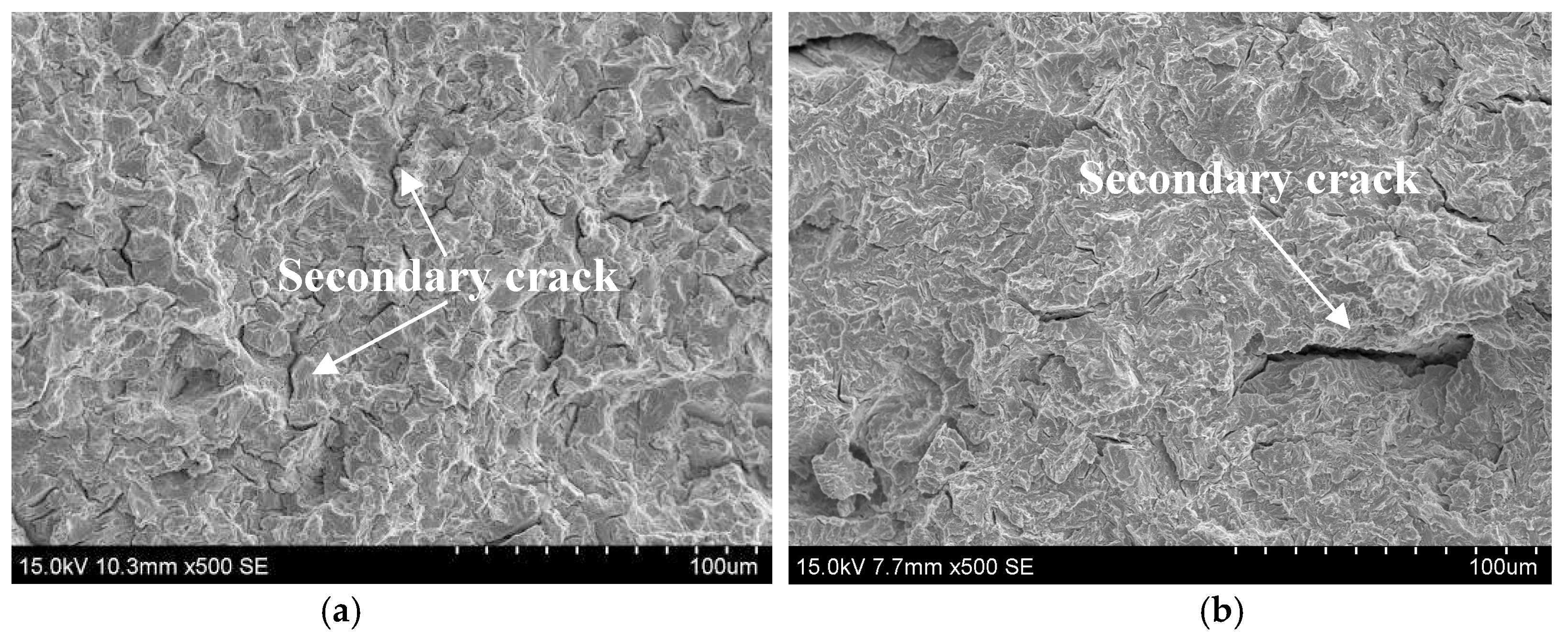
2.2. SSC Fracture Morphology of the DCB Specimen
2.3. Microstructure Observation
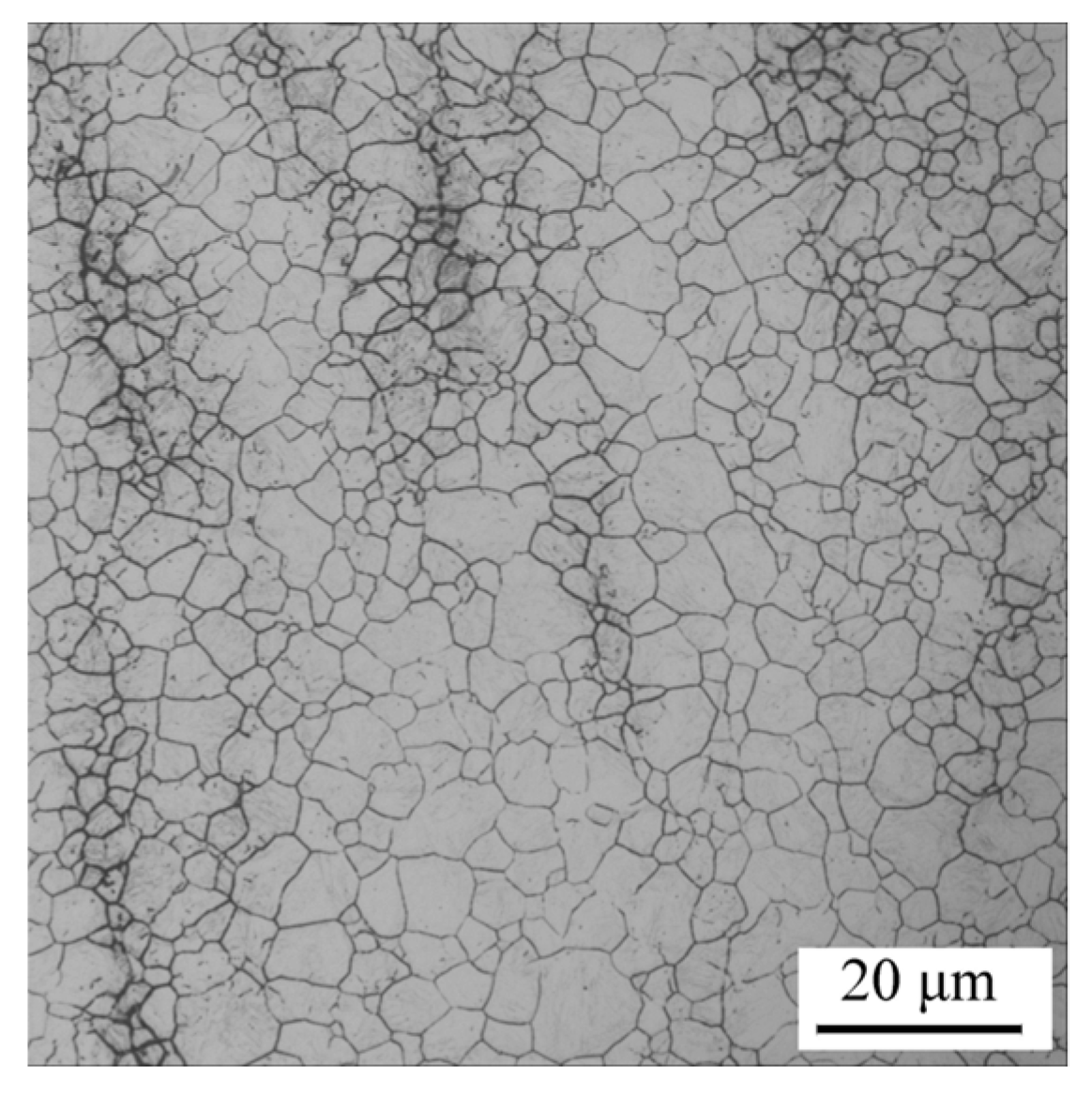
2.3.1. Prior Austenite Grain Observation
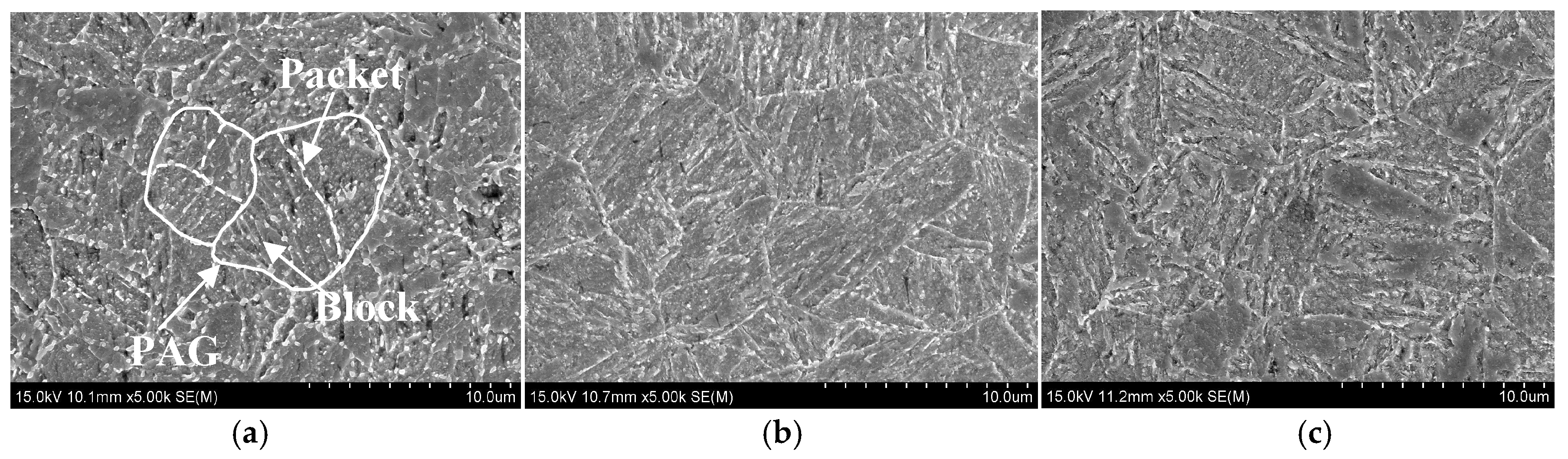
2.3.2. Martensitic Structure Observations
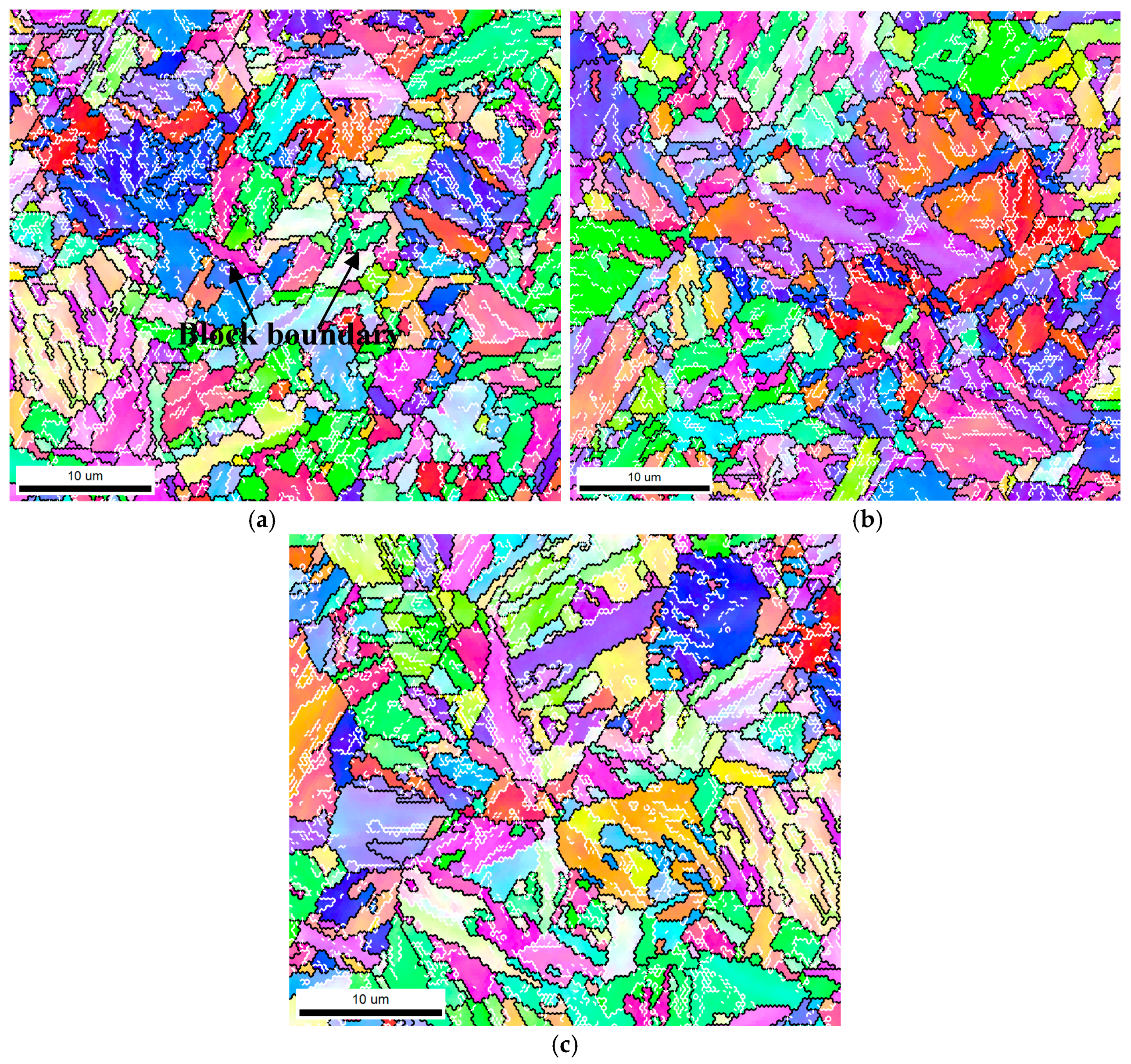
2.3.3. Martensitic Lath and Precipitate Observations
2.3.4. Dislocation Estimations
2.4. Hydrogen Permeation
3. Discussion
3.1. Relationships among Tempering Temperature, H Mobility and SSC Initiation
3.1.1. Effect of Tempering Temperature on H Permeability
3.1.2. Effect of H Permeability on SSC Initiation
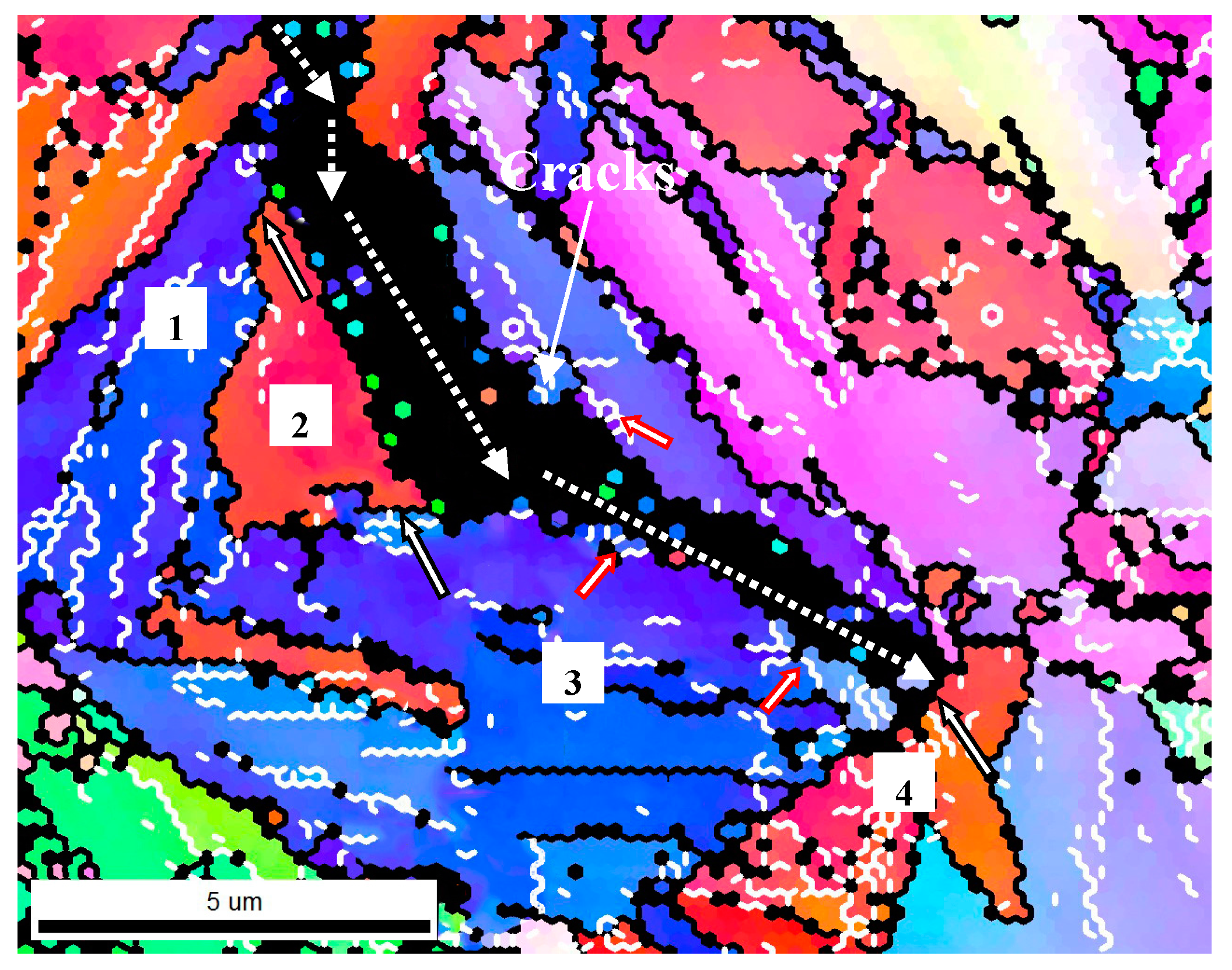
3.2. Effect of GBs on SSC Propagation
4. Materials and Methods
4.1. Materials and Heat Treatment
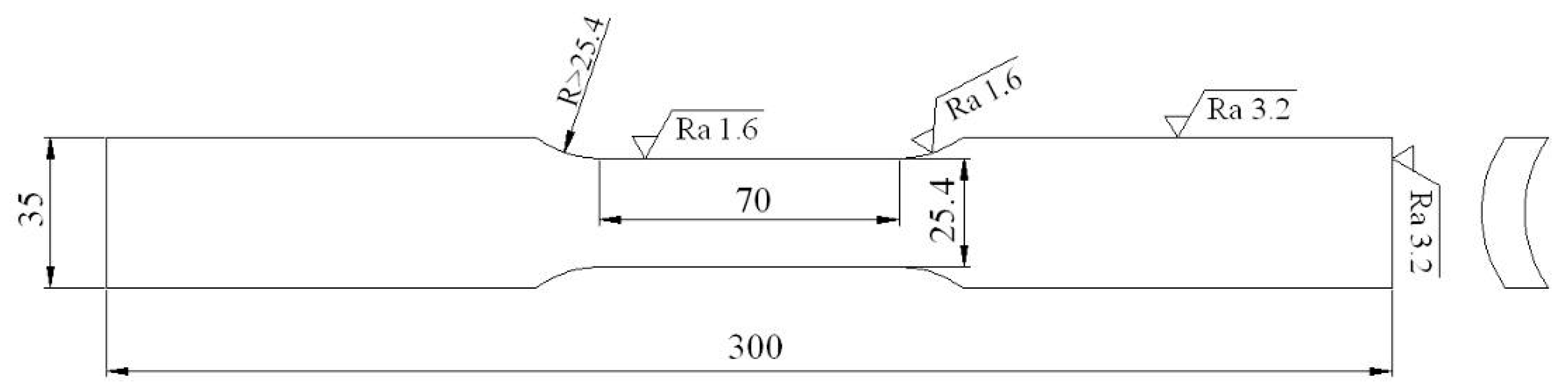
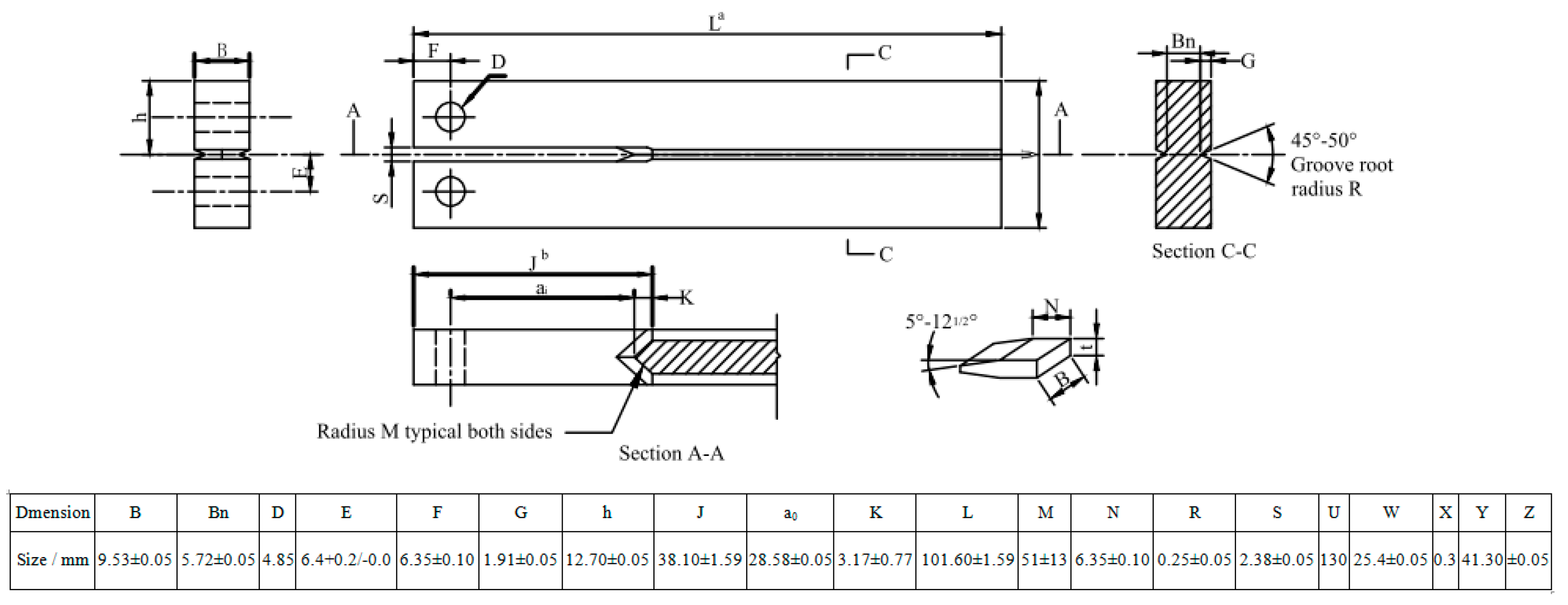
4.2. Estimations of Mechanical Properties and SSC Susceptibility
4.3. Electrochemical H Permeation Test
4.4. Microstructure Observation
5. Conclusions
- KISSC and the overall resistance to SSC of this martensitic steel were obviously enhanced by increasing Tt from 650 to 720 °C.
- Increased Tt led to decreases in overall and local H concentration around the inclusions, owing to martensitic lath polygonization and widening and resulted in a lowered susceptibility to SSC initiation.
- Increased Tt brought about a higher fraction of HAGBs and resulted in enhanced resistance to SSC propagation by more frequently diverting the propagating direction and lowering the propagation rate.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Motoda, K.; Masuda, T. Development of 110-ksi grade OCTG with good resistance to sulfide-stress-corrosion cracking. J. Pet. Technol. 1988, 40, 1232–1236. [Google Scholar] [CrossRef]
- Garrison, B.; Urband, B.E.; Prideco, G.; Morey, S. Production and Fit for Service Attributes of C125 High Strength Casing; NACE International: Houston, TX, USA, 2005. [Google Scholar]
- Zhang, C.; Wang, Q.; Ren, J.; Li, R.; Wang, M.; Zhang, F.; Sun, K. Effect of martensitic morphology on mechanical properties of an as-quenched and tempered 25CrMo48V Steel. Mater. Sci. Eng. A 2012, 534, 339–346. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Q.; Ren, J.; Li, R.; Wang, M.; Zhang, F.; Yan, Z. Effect of microstructure on the strength of 25CrMo48V martensitic steel tempered at different temperature and time. Mater. Des. 2012, 36, 220–226. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, C.; Li, R.; Gao, J.; Wang, M.; Zhang, F. Characterization of the microstructures and mechanical properties of 25CrMo48V martensitic steel tempered at different times. Mater. Sci. Eng. A 2013, 559, 130–134. [Google Scholar] [CrossRef]
- Ming, L.; Wang, Q.; Wang, H.; Zhang, C.; Wei, Z.; Guo, A. A remarkable role of niobium precipitation in refining microstructure and improving toughness of A QT-treated 20CrMo47NbV steel with ultrahigh strength. Mater. Sci. Eng. A 2014, 613, 240–249. [Google Scholar] [CrossRef]
- Perez, T.E.; Echaniz, G.; Morales, C. The effect of microstructure on the Kissc low alloy carbon steels. In Corrosion 98; NACE International: Houston, TX, USA, 1998. [Google Scholar]
- Turconi, G.L.; Gluseppe, C.; Anelli, E.; Scoppio, L.; Perez, T.E.; Morales, C.; Echaniz, G. Improvement of resistance to SSC initiation and propagation of high strength OCTG through microstructure and precipitation control. In CORROSION 2001; NACE International: Houston, TX, USA, 2001. [Google Scholar]
- Kaneko, T.; Okada, Y.; Ikeda, A. Influence of microstructure on SSC susceptibility of low-alloy, high-strength oil country tubular goods. Corrosion 1989, 45, 2–6. [Google Scholar] [CrossRef]
- Huang, H.; Shaw, W.J.D. Cold work effects on sulfide stress cracking of pipeline steel exposed to sour environments. Corros. Sci. 1993, 34, 61–78. [Google Scholar] [CrossRef]
- Ogundele, G.I.; White, W.E. Some Observations on the corrosion of carbon steel in sour gas environments: Effects of H2S and H2S/CO2/CH4/C3H8 mixtures. Corrosion 1986, 42, 398–408. [Google Scholar] [CrossRef]
- Hirth, J.P. Effects of hydrogen on the properties of iron and steel. Metall. Trans. A 1980, 11, 861–890. [Google Scholar] [CrossRef]
- Takai, K.; Watanuki, R. Hydrogen in trapping states innocuous to environmental degradation of high-strength steels. ISIJ Int. 2003, 43, 520–526. [Google Scholar] [CrossRef]
- Nagumo, M. Function of hydrogen in embrittlement of high-strength steels. ISIJ Int. 2001, 41, 590–598. [Google Scholar] [CrossRef]
- Nagumo, M.; Takai, K.; Okuda, N. Nature of hydrogen trapping sites in steels induced by plastic deformation. J. Alloy. Compd. 1999, 293, 310–316. [Google Scholar] [CrossRef]
- Akhurst, K.N.; Baker, T.J. The threshold stress intensity for hydrogen-induced crack growth. Metall. Trans. A 1981, 12, 1059–1070. [Google Scholar] [CrossRef]
- Schiapparelli, E.; Prado, S.; Tiebas, J.J.; Garibaldi, J. Relation between different inclusion-matrix interfaces in steels and the susceptibility to hydrogen embrittlement. J. Mater. Sci. 1992, 27, 2053–2060. [Google Scholar] [CrossRef]
- Fujita, S.; Murakami, Y. A new nonmetallic inclusion rating method by positive use of hydrogen embrittlement phenomenon. Metall. Mater. Trans. A 2013, 44, 303–322. [Google Scholar] [CrossRef]
- Todoshchenko, O.; Yagodzinskyy, Y.; Yagodzinska, V.; Saukkonen, T.; Hänninen, H. Hydrogen effects on fracture of high-strength steels with different micro-alloying. Corros. Rev. 2015, 33, 515–527. [Google Scholar] [CrossRef]
- Masoumi, M.; Silva, C.C.; de Abreu, H.F.G. Effect of crystallographic orientations on the hydrogen-induced cracking resistance improvement of API 5L X70 pipeline steel under various thermomechanical processing. Corros. Sci. 2016, 111, 121–131. [Google Scholar] [CrossRef]
- Masoumi, M.; Tavares, S.S.M.; Pardal, J.M.; Martins, T.R.B.; Gomes da Silva, M.J.; de Abreu, H.F.G. The role of microstructure and grain orientations on intergranular cracking susceptibility of UNS 17400 martensitic stainless steel. Eng. Fail. Anal. 2017, 79, 198–207. [Google Scholar] [CrossRef]
- Di Schino, A.; Porcu, G.; Scoppio, L.; Longobardo, M.; Turconi, G.L. Metallurgical Design and Development of C125 Grade for Mild Sour Service Application; NACE Corrosion Paper 06125; NACE International: Houston, TX, USA, 2006; pp. 1–14. [Google Scholar]
- Lee, J.-Y.; Lee, S.M. Hydrogen trapping phenomena in metals with B.C.C. and F.C.C. crystals structures by the desorption thermal analysis technique. Surf. Coat. Technol. 1986, 28, 301–314. [Google Scholar] [CrossRef]
- Pressouyre, G.M.; Bernstein, I.M. A quantitative analysis of hydrogen trapping. Metall. Mater. Trans. A 1978, 9, 1571–1580. [Google Scholar] [CrossRef]
- Villalba, E.; Atrens, A. SCC of commercial steels exposed to high hydrogen fugacity. Eng. Fail. Anal. 2008, 15, 617–641. [Google Scholar] [CrossRef]
- Echaniz, G.P.; Perez, T.E.; Pampillo, C.; Newman, R.C.; Procter, R.P.M.; Lorimer, G.W. The Effect of Microstructure on SSC Resistance of Low Alloy Carbon Steels; NACE International: Houston, TX, USA, 1997. [Google Scholar]
- Omura, T.; Ohe, T.; Abe, T.; Ueda, M.; Nice, P.I.; Martin, J.W. Sulfide stress cracking (SSC) resistance limits for a 125 Ksi grade high strength low alloy steel OCTG developed for mildly sour service. In CORROSION 2010; NACE International: Houston, TX, USA, 2010. [Google Scholar]
- Dadfarnia, M.; Nagao, A.; Wang, S.; Martin, M.L.; Somerday, B.P.; Sofronis, P. Recent advances on hydrogen embrittlement of structural materials. Int. J. Fract. 2015, 196, 223–243. [Google Scholar] [CrossRef]
- Frappart, S.; Feaugas, X.; Creus, J.; Thebault, F.; Delattre, L.; Marchebois, H. Hydrogen solubility, diffusivity and trapping in a tempered Fe–C–Cr martensitic steel under various mechanical stress states. Mater. Sci. Eng. A 2012, 534, 384–393. [Google Scholar] [CrossRef]
- Saha, S.K.; Hilinski, E.J. Study of C110 critical stress intensity factor (KISSC) in various weak H2S solutions. In Corrosion 2013, March 17, 2013–March 21, 2013; NACE—International Corrosion Conference Series; NACE International: Houston, TX, USA, 2013. [Google Scholar]
- NACE Standard. TM0177-2016 Labortory Testing of Metals for Resistance to Sulfide Stress Cracking and Stress Corrosion Cracking in H2S Environments; NACE International: Houston, TX, USA, 2016. [Google Scholar]
- Morito, S.; Tanaka, H.; Konishi, R.; Furuhara, T.; Maki, T. The morphology and crystallography of lath martensite in Fe-C alloys. Acta Mater. 2003, 51, 1789–1799. [Google Scholar] [CrossRef]
- Qi, J.; Li, Y.; Zhou, H. The retained austenite, twinned substructure and auto-tempered carbide in as-quenched low-carbon martensite. Trans. Met. Heat Treat. 1984, 5, 42–51. [Google Scholar]
- Koyama, M.; Yamasaki, D.; Nagashima, T.; Tasan, C.C.; Tsuzaki, K. In situ observations of silver-decoration evolution under hydrogen permeation: Effects of grain boundary misorientation on hydrogen flux in pure iron. Scr. Mater. 2017, 129, 48–51. [Google Scholar] [CrossRef]
- Caron, R.N.; Krauss, G. The tempering of Fe-C lath martensite. Metall. Trans. 1972, 3, 2381–2389. [Google Scholar] [CrossRef]
- Speich, G.R.; Leslie, W.C. Tempering of steel. Metall. Trans. 1972, 3, 1043–1054. [Google Scholar] [CrossRef]
- Masakatsu, U.; Nakamura, S.; Abe, T.; Nakamura, K.; Omura, T.T. Development of 125ksi Grade Hsla Steel Octg For Mildly Sour Environments; NACE International: Houston, TX, USA, 2005. [Google Scholar]
- Pressouyre, G.M. Trap theory of Hydrogen embrittlement. Acta Metall. 1980, 28, 895–911. [Google Scholar] [CrossRef]
- Tiwari, G.P.; Bose, A.; Chakravartty, J.K.; Wadekar, S.L.; Totlani, M.K.; Arya, R.N.; Fotedar, R.K. A study of internal hydrogen embrittlement of steels. Mater. Sci. Eng. A 2000, 286, 269–281. [Google Scholar] [CrossRef]
- Michler, T.; Naumann, J. Microstructural aspects upon hydrogen environment embrittlement of various bcc steels. Int. J. Hydrogen Energy 2010, 35, 821–832. [Google Scholar] [CrossRef]
- Solheim, K.G.; Solberg, J.K.; Walmsley, J.; Rosenqvist, F.; Bjørnå, T.H. The role of retained austenite in hydrogen embrittlement of supermartensitic stainless steel. Eng. Fail. Anal. 2013, 34, 140–149. [Google Scholar] [CrossRef]
- Maroef, I.; Olson, D.L.; Eberhart, M.; Edwards, G.R. Hydrogen trapping in ferritic steel weld metal. Int. Mater. Rev. 2002, 47, 191–223. [Google Scholar] [CrossRef]
- Inoue, A.; Masumoto, T. Carbide reactions (M3C→M7C3→M23C6→M6C) during tempering of rapidly solidified high carbon Cr-W and Cr-Mo steels. Metall. Trans. A 1980, 11, 739–747. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Liu, G. Hydrogen trapping in high strength martensitic steel after austenitized at different temperatures. Int. J. Hydrogen Energy 2013, 38, 14364–14368. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Shi, J.; Hui, W.; Dong, H. Effect of microstructural refinement on the toughness of low carbon martensitic steel. Scr. Mater. 2008, 58, 492–495. [Google Scholar] [CrossRef]
- Hajilou, T.; Deng, Y.; Rogne, B.R.; Kheradmand, N.; Barnoush, A. In situ electrochemical microcantilever bending test: A new insight into hydrogen enhanced cracking. Scr. Mater. 2017, 132, 17–21. [Google Scholar] [CrossRef]
- Cui, H.B.; Xie, G.M.; Luo, Z.A.; Ma, J.; Wang, G.D.; Misra, R.D.K. The microstructural evolution and impact toughness of nugget zone in friction stir welded X100 pipeline steel. J. Alloy. Compd. 2016, 681, 426–433. [Google Scholar] [CrossRef]
- Zhong, Y.; Xiao, F.; Zhang, J.; Shan, Y.; Wang, W.; Yang, K. In situ TEM study of the effect of M/A films at grain boundaries on crack propagation in an ultra-fine acicular ferrite pipeline steel. Acta Mater. 2006, 54, 435–443. [Google Scholar] [CrossRef]
- ASTM. A370-10 Standard Test Methods and Definitions for Mechanical Testing of Steel Products; ASTM International: West Conshohocken, PA, USA, 2010. [Google Scholar] [CrossRef]
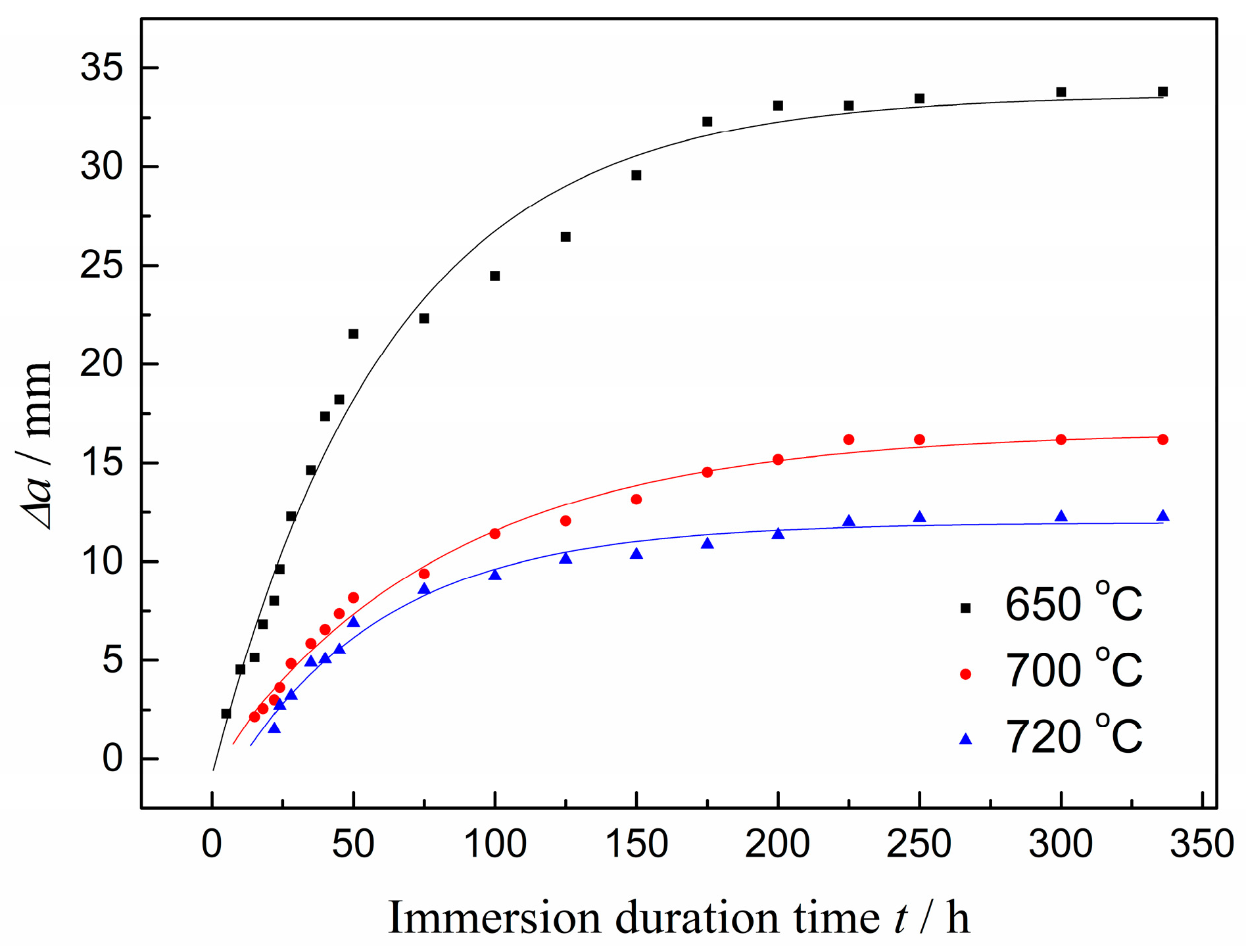
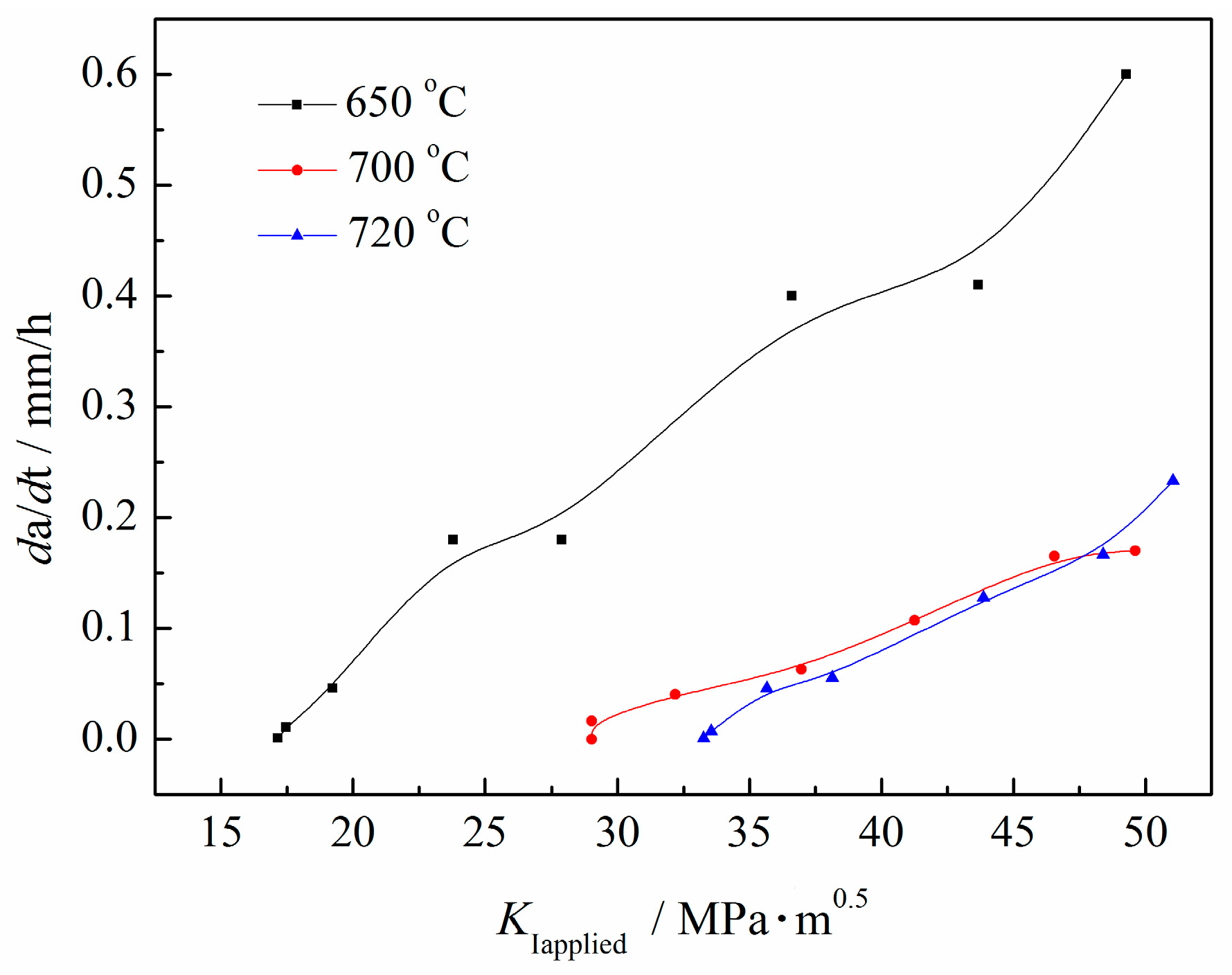
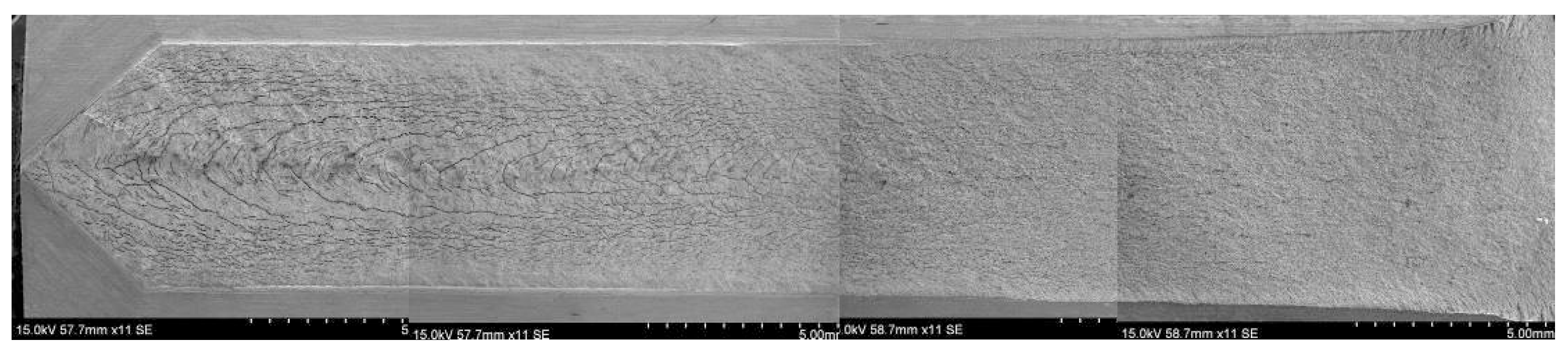
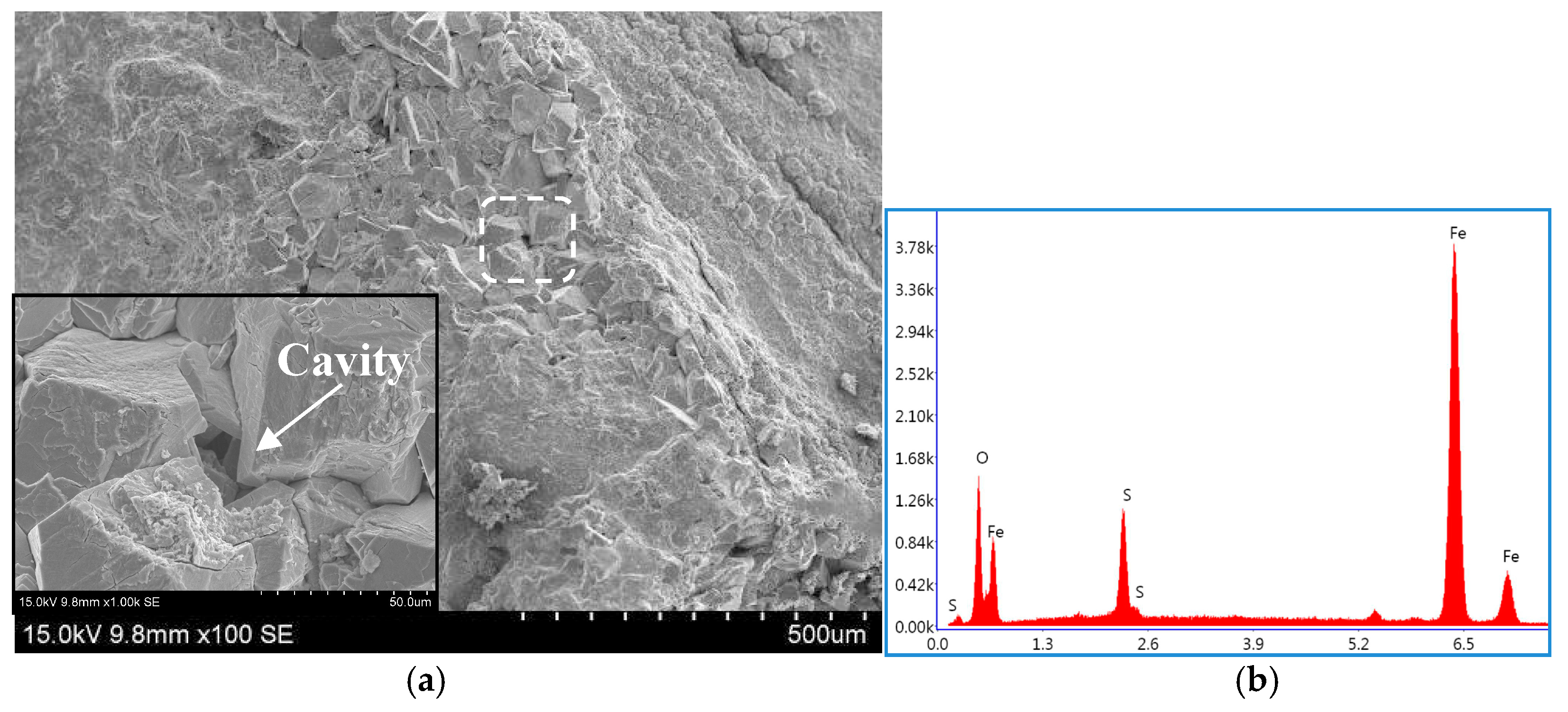

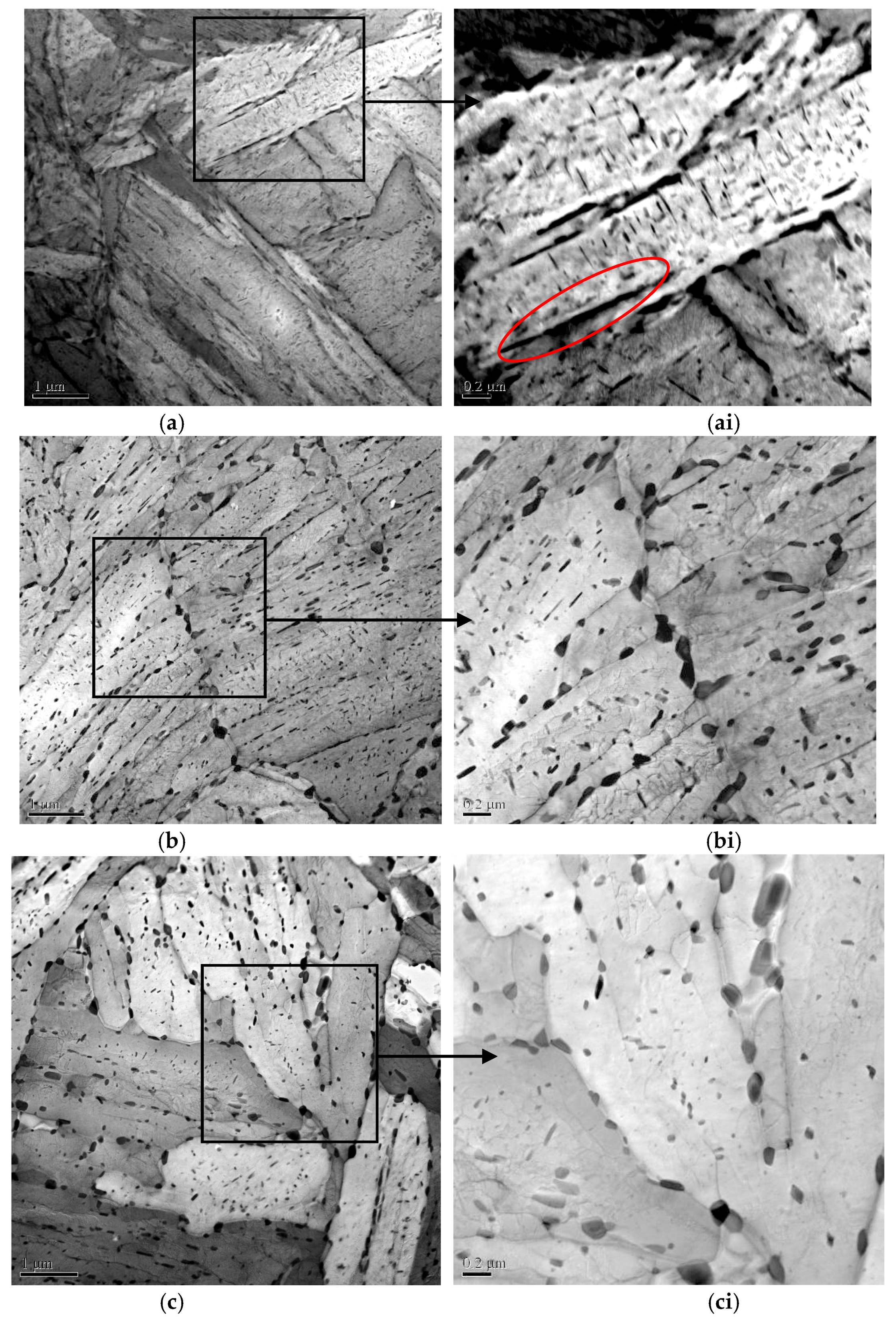
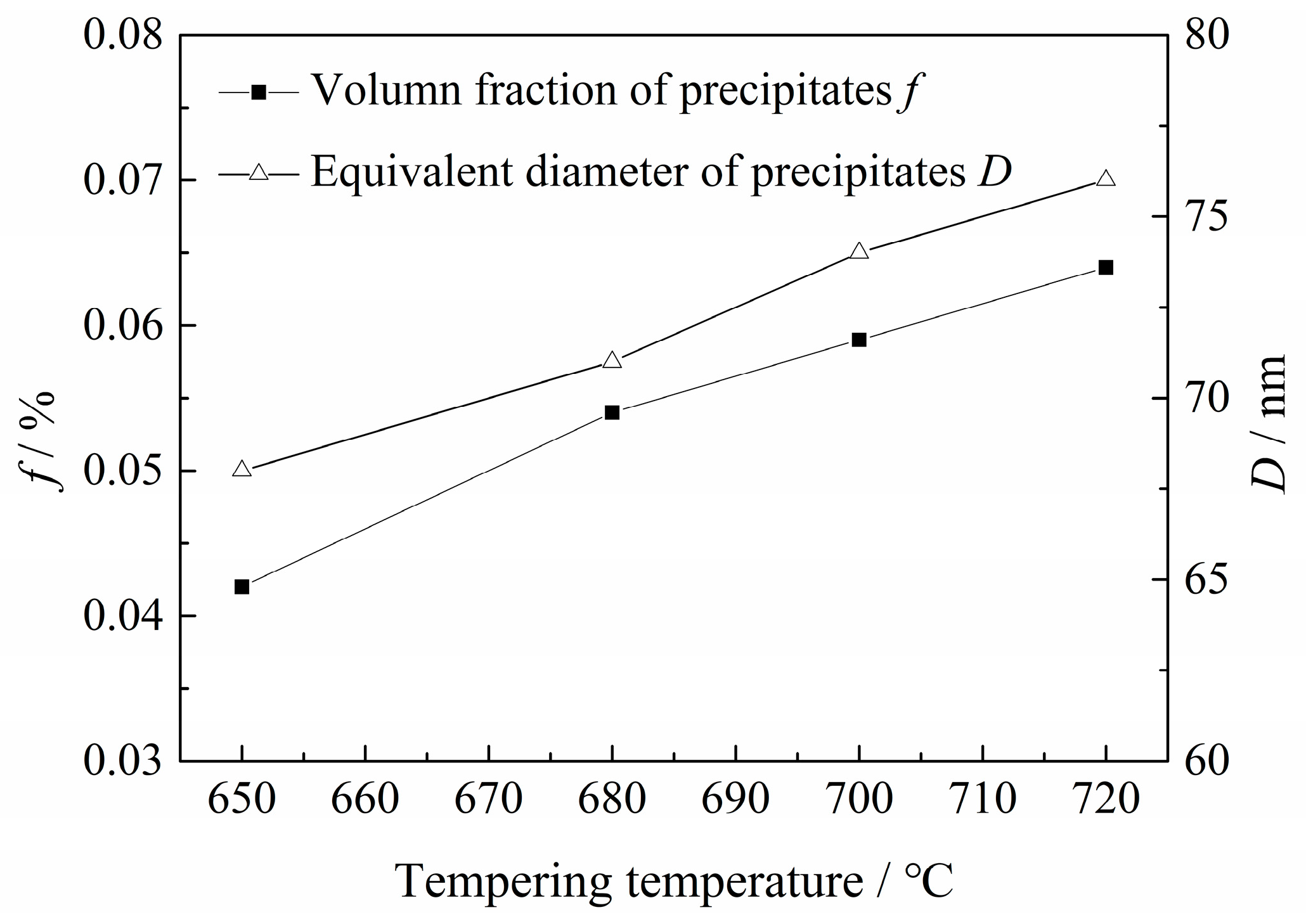
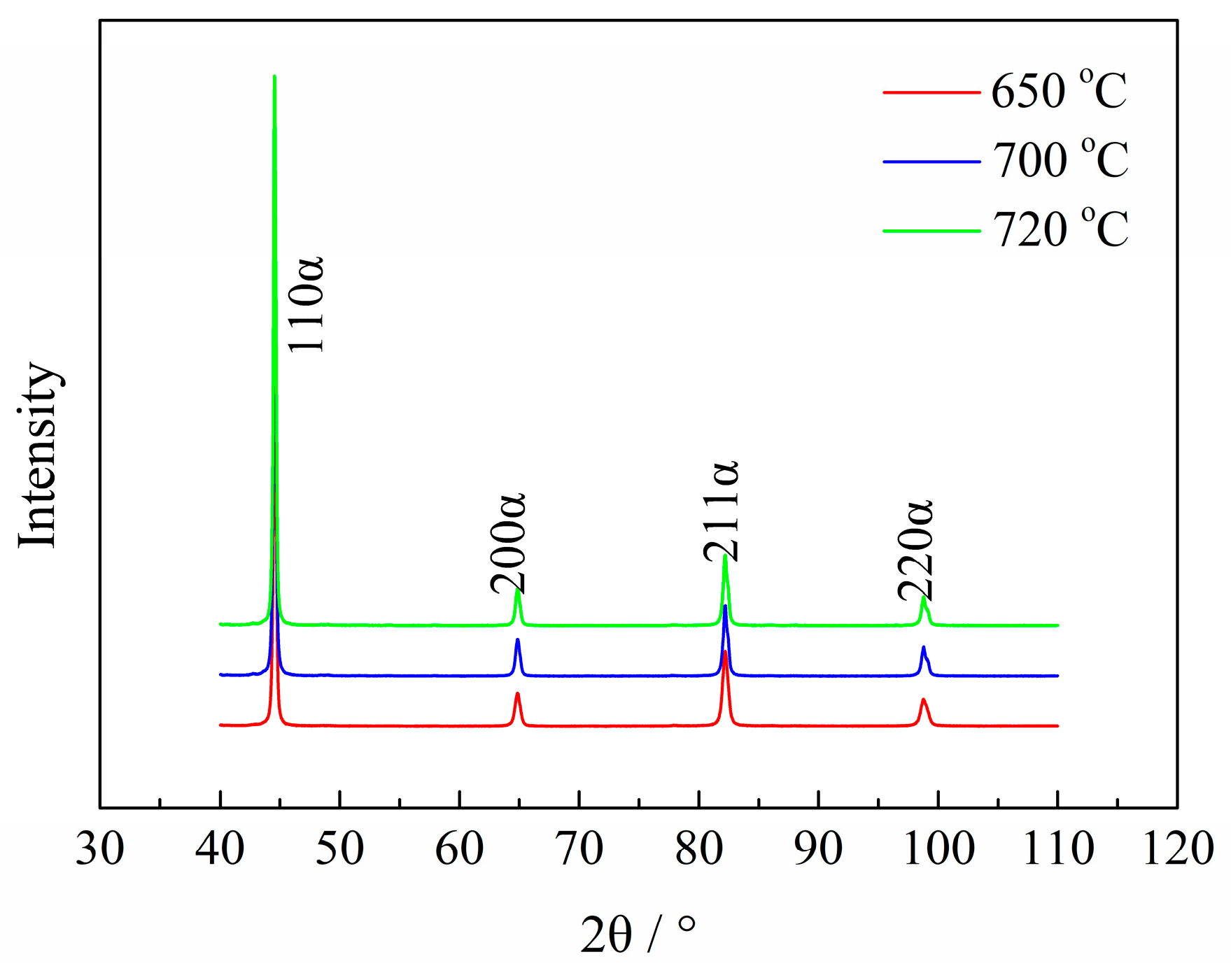

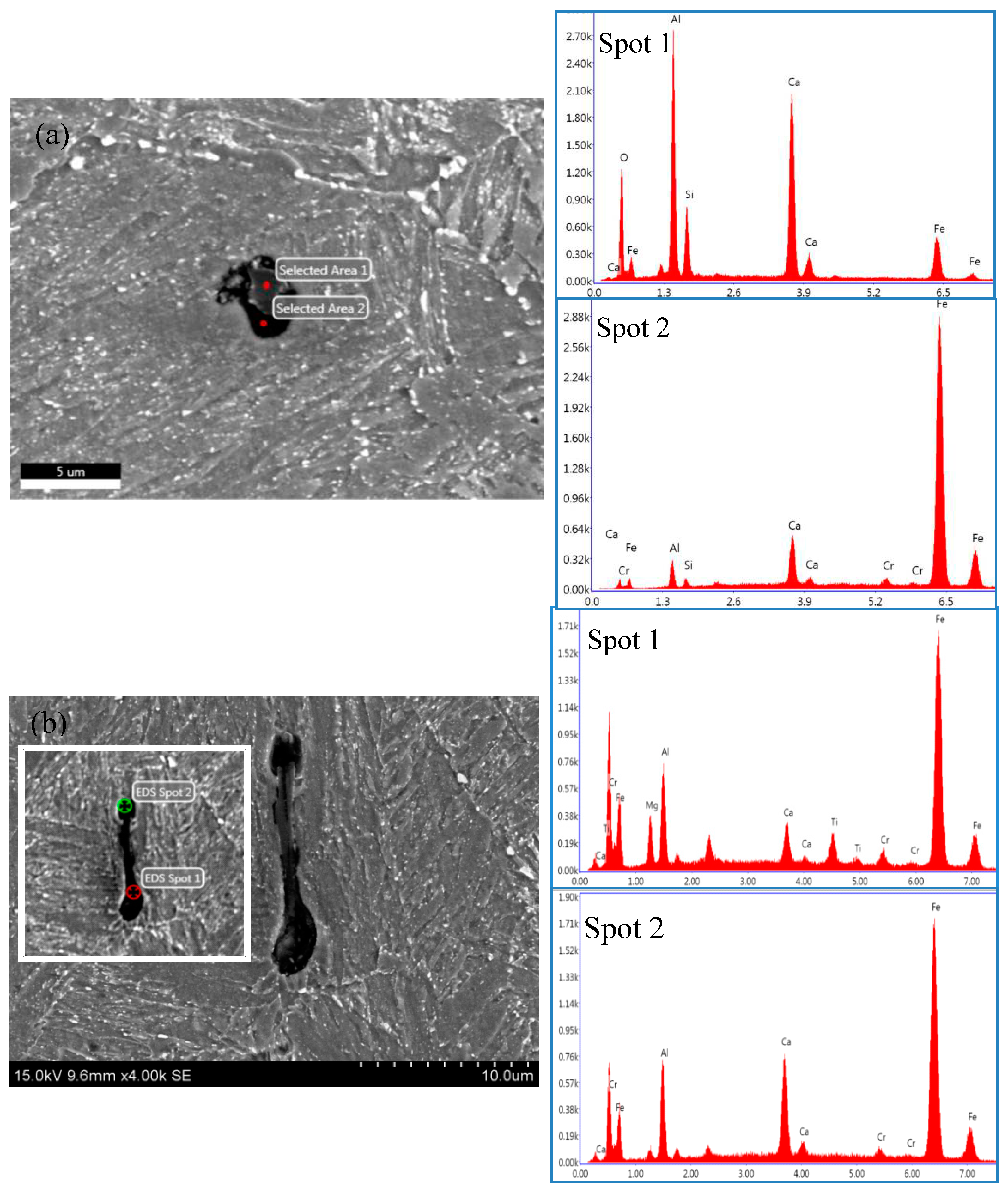
| Sample | Tt/°C | YS/MPa | TS/MPa | EL/% | HRC | KISSC/MPa·mm0.5 |
|---|---|---|---|---|---|---|
| 1 | 650 | 1007 | 1062 | 18 | 35.4 | 17.16 |
| 2 | 700 | 776 | 857 | 23 | 25.1 | 29.02 |
| 3 | 720 | 728 | 805 | 25 | 23.4 | 33.26 |
| Tt/°C | Dγ/μm | Dp/μm | Db/μm | Wl/μm | fGBMA≥15°/% | ρ/1014 m−2 |
|---|---|---|---|---|---|---|
| 650 | 7.6 | 3.73 | 1.15 | 0.32 | 66.8 | 1.41 |
| 700 | 7.5 | 4.01 | 1.32 | 0.53 | 69.8 | 0.84 |
| 720 | 7.1 | 4.20 | 1.35 | 0.58 | 70.5 | 0.62 |
| Tt/°C | Do/10−7cm2/s | Co/ppm |
|---|---|---|
| 650 | 0.48 | 6.45 |
| 700 | 1.78 | 1.87 |
| 720 | 2.20 | 0.86 |
| Tt/°C | 650 | 700 | 720 |
|---|---|---|---|
| NT-PAG/1025 m−3 | 5.8 | 5.9 | 6.2 |
| NT-MP/1025 m−3 | 11.9 | 11.1 | 10.6 |
| NT-MB/1025 m−3 | 38.6 | 33.6 | 32.9 |
| NT-ML/1025 m−3 | 138.7 | 83.7 | 76.5 |
| NT-GB/1025 m−3 | 92.6 | 58.4 | 53.9 |
| NT-dis/1025 m−3 | 0.27 | 0.16 | 0.12 |
| Grain Boundary | Misorientation Angle (°) | Cracking Behavior |
|---|---|---|
| 1 and 2 | 49.3 | Deviated |
| 2 and 3 | 35.6 | Deviated |
| 3 and 4 | 59.5 | Arrested |
| C | Si | Mn | P | S | Cr | Mo | V | Ti | B |
|---|---|---|---|---|---|---|---|---|---|
| 0.28 | 0.25 | 0.40 | 0.008 | 0.002 | 1.00 | 0.80 | 0.15 | 0.015 | trace |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Wang, Q.; Gu, S.; He, Z.; Wang, Q.; Zhang, F. Sulfide Stress Cracking Behavior of a Martensitic Steel Controlled by Tempering Temperature. Materials 2018, 11, 412. https://doi.org/10.3390/ma11030412
Sun Y, Wang Q, Gu S, He Z, Wang Q, Zhang F. Sulfide Stress Cracking Behavior of a Martensitic Steel Controlled by Tempering Temperature. Materials. 2018; 11(3):412. https://doi.org/10.3390/ma11030412
Chicago/Turabian StyleSun, Yu, Qian Wang, Shunjie Gu, Zaoneng He, Qingfeng Wang, and Fucheng Zhang. 2018. "Sulfide Stress Cracking Behavior of a Martensitic Steel Controlled by Tempering Temperature" Materials 11, no. 3: 412. https://doi.org/10.3390/ma11030412
APA StyleSun, Y., Wang, Q., Gu, S., He, Z., Wang, Q., & Zhang, F. (2018). Sulfide Stress Cracking Behavior of a Martensitic Steel Controlled by Tempering Temperature. Materials, 11(3), 412. https://doi.org/10.3390/ma11030412




