Factors Affecting the Power Conversion Efficiency in ZnO DSSCs: Nanowire vs. Nanoparticles
Abstract
1. Introduction
2. Methodology
2.1. ZnO Nanoparticle Films
2.2. ZnO Nanowire Arrays
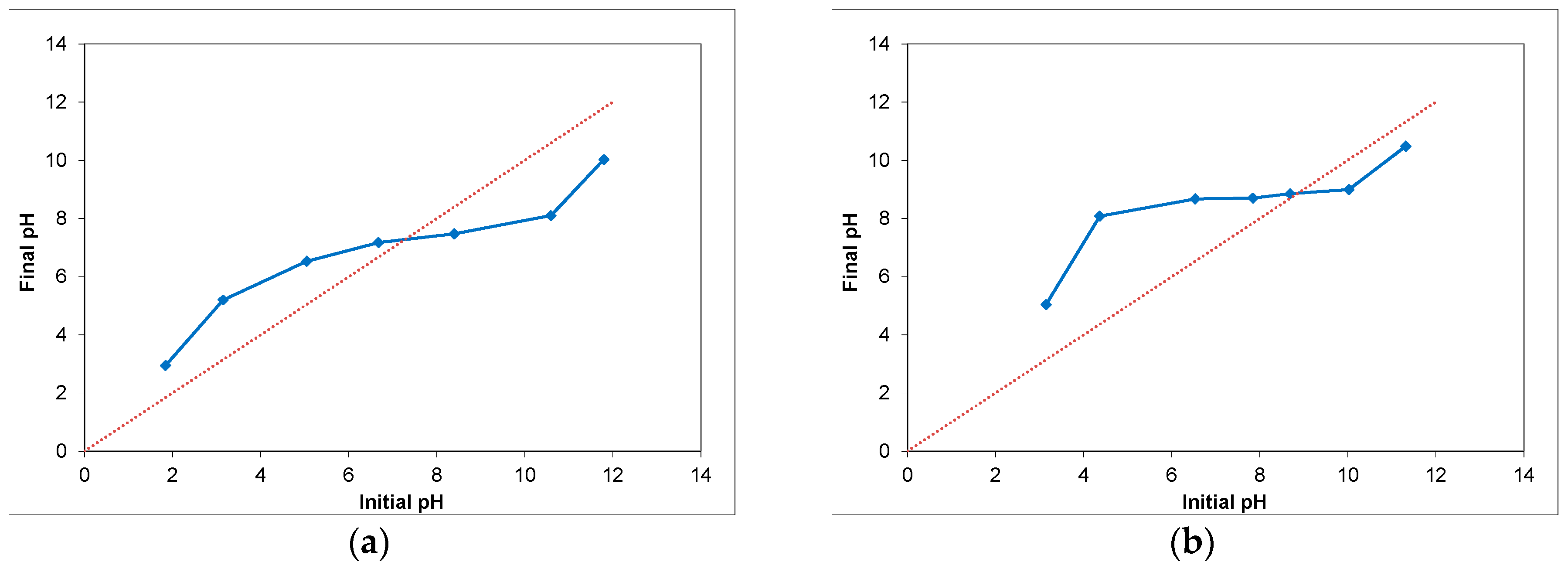
2.3. Film Sensitization-Assessment of Dye Loading and of the Point of Zero Charge
2.4. DSSC Fabrication
2.5. Device Characterization and Testing
3. Results and Discussion
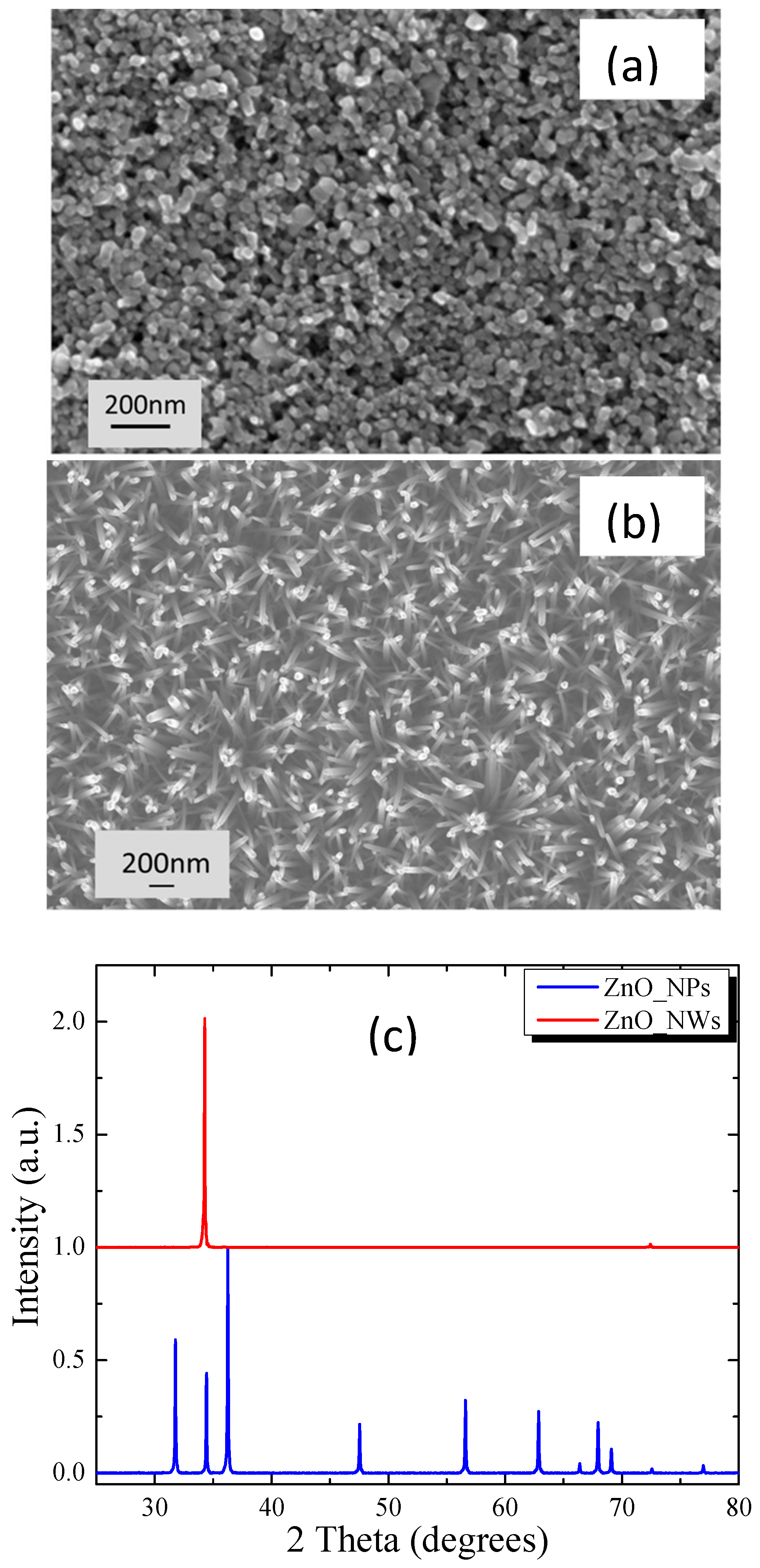
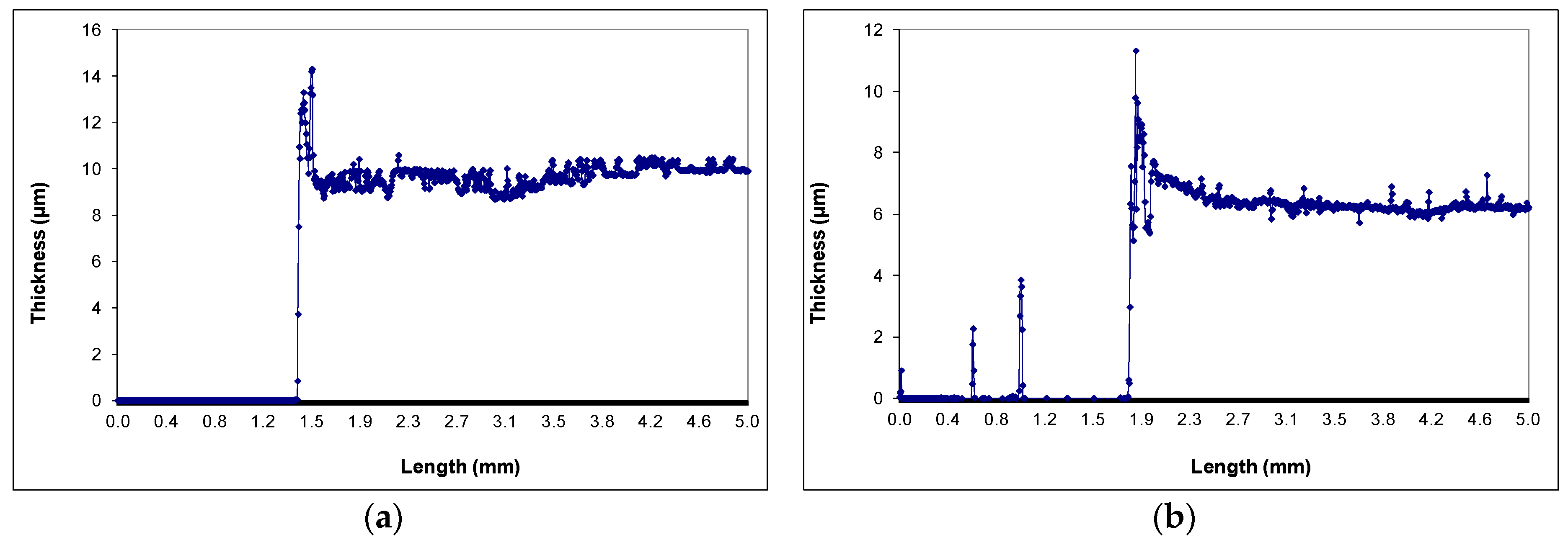
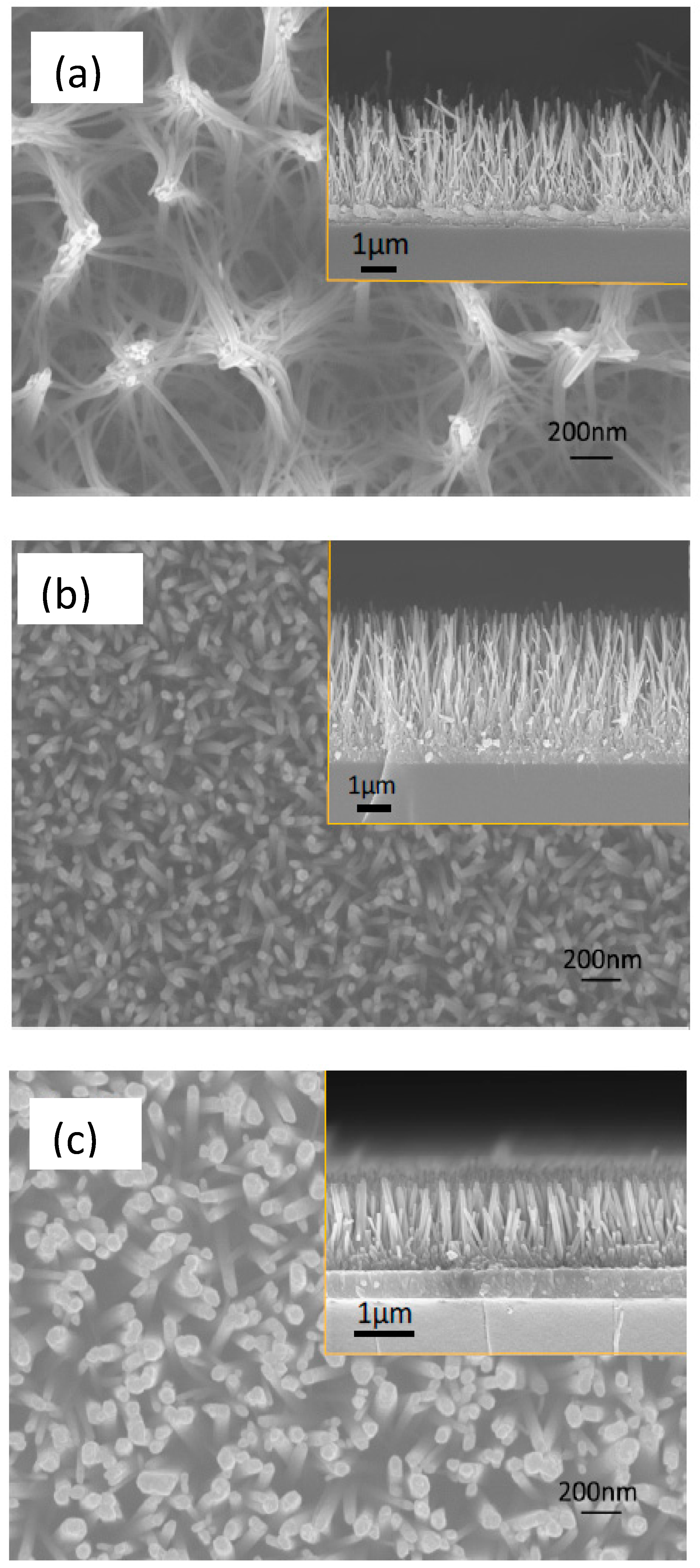
3.1. Film Morphology
3.2. Photovoltaic Characteristics
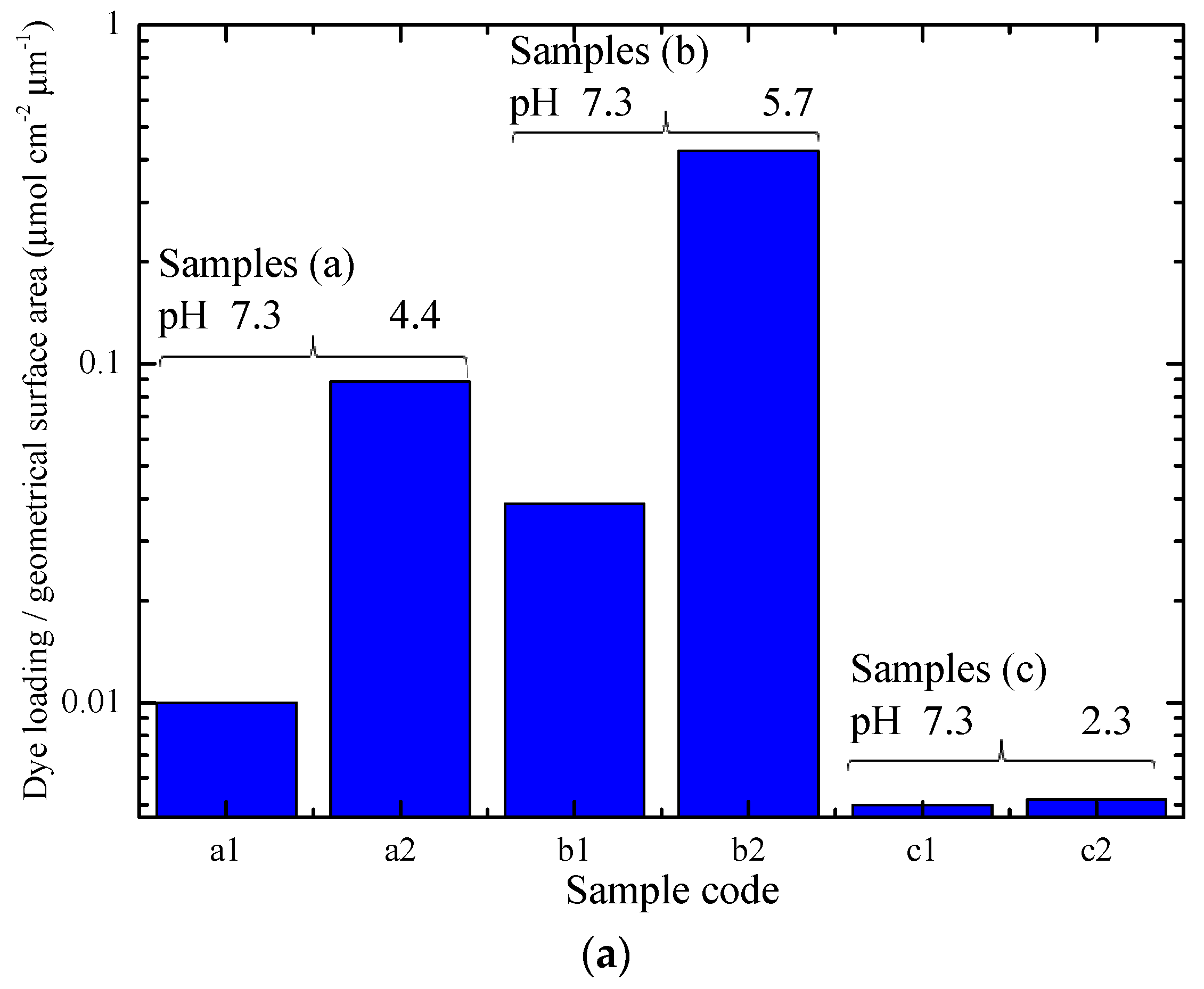
3.3. Dye Loading
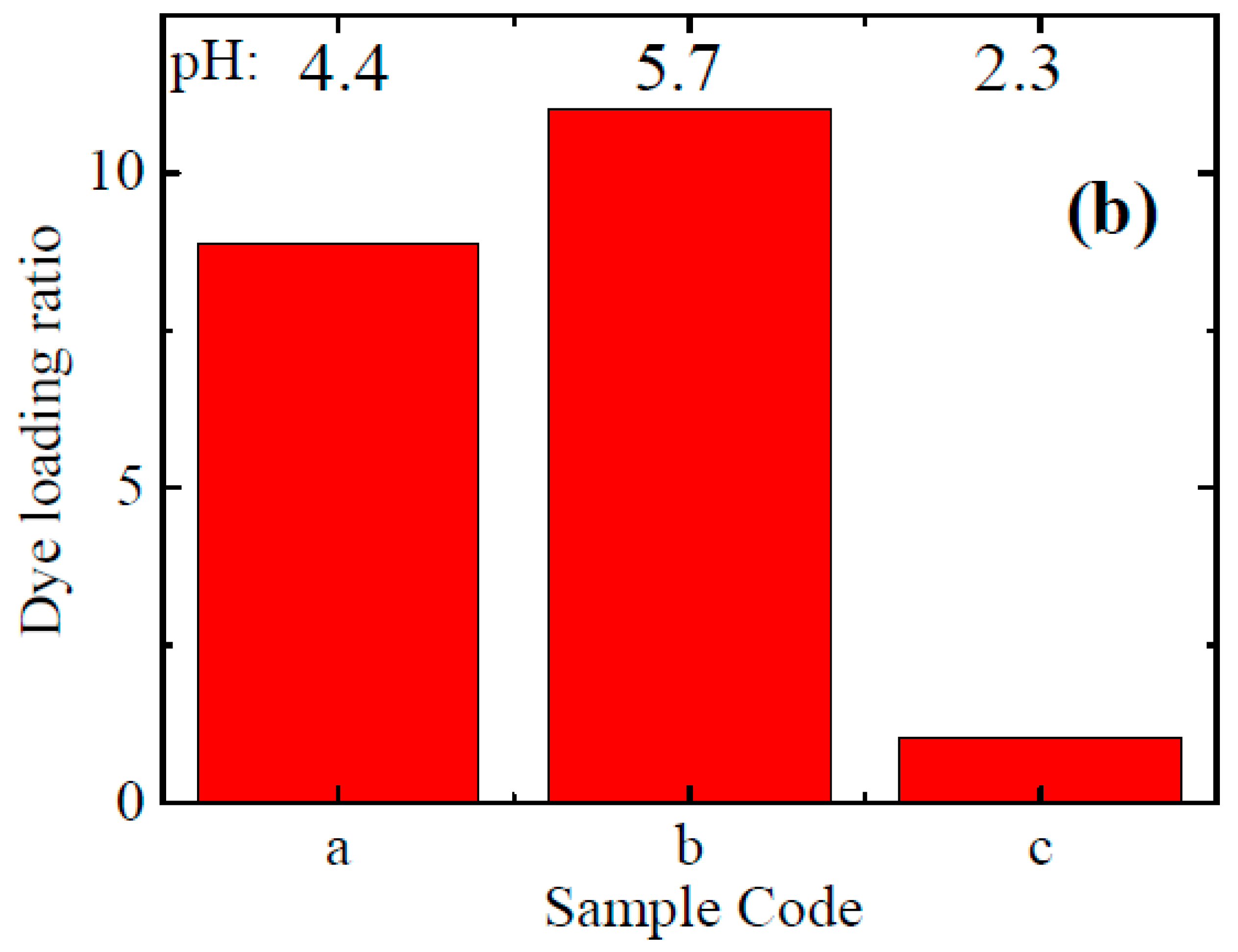
3.4. Nanowire DSSCs with Improved Dye Loading
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Green, M.A.; Hishikawa, Y.; Dunlop, E.D.; Levi, D.H.; Ebinger, J.H.; Ho-Baillie, A. Solar cell efficiency tables (version 51). Prog. Photovolt. 2018, 26, 3–12. [Google Scholar] [CrossRef]
- Komiya, R.; Fukui, A.; Murofushi, N.; Koide, N.; Yamanaka, R.; Katayama, H. Improvement of the conversion efficiency of a monolithic type dye-sensitized solar cell module. In Proceedings of the Technical Digest, 21st International Photovoltaic Science and Engineering Conference, Fukuoka, Japan, 28 November–2 December 2011; p. 2C-5O-08. [Google Scholar]
- Kanaparthi, R.K.; Kandhadi, J.; Giribabu, L. Metal-free organic dyes for dye-sensitized solar cells: Recent advances. Tetrahedron 2012, 68, 8383–8393. [Google Scholar] [CrossRef]
- Yella, A.; Lee, H.W.; Tsao, H.N.; Yi, C.; Chandiran, A.K.; Nazeeruddin, M.K.; Diau, E.W.; Yeh, C.Y.; Zakeeruddin, S.M.; Grätzel, M. Porphyrin-sensitized solar cells with cobalt (II/III)-based redox electrolyte exceed 12 percent efficiency. Science 2011, 4, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W.M.; Jolley, K.W.; Wagner, P.; Wagner, K.; Walsh, P.J.; Gordon, K.C.; Schmidt-Mende, L.; Nazeeruddin, M.K.; Wang, Q.; Grätzel, M.; et al. Highly Efficient Porphyrin Sensitizers for Dye-Sensitized Solar Cells. J. Phys. Chem. C 2007, 111, 11760–11762. [Google Scholar] [CrossRef]
- Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, R.; Curchod, B.F.; Ashari-Astani, N.; Tavernelli, I.; Rothlisberger, U.; Nazeeruddin, M.K.; Grätzel, M. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 2014, 6, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Grätzel, M.; O’Regan, B. A Low-Cost, High-Efficiency Solar Cell Based on Dye-Sensitized Colloidal TiO2 Films. Nature 1991, 352, 737–740. [Google Scholar]
- Wang, Y.; Tian, J.; Fei, C.; Lv, L.; Liu, X.; Zhao, Z.; Cao, G. Microwave-Assisted Synthesis of SnO2 Nanosheets Photoanodes for Dye-Sensitized Solar Cells. J. Phys. Chem. C 2014, 118, 25931–25938. [Google Scholar] [CrossRef]
- Farré, Y.; Zhang, L.; Pellegrin, Y.; Planchat, A.; Blart, E.; Boujtita, M.; Hammarström, L.; Jacquemin, D.; Odobel, F. Second Generation of Diketopyrrolopyrrole Dyes for NiO-Based Dye-Sensitized Solar Cells. J. Phys. Chem. C 2016, 120, 7923–7940. [Google Scholar] [CrossRef]
- Morkoc, H.; Ozgur, U. Zinc Oxide Fundamentals, Materials and Device Technology; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Schlur, L.; Carton, A.; Lévêque, P.; Guillon, D.; Pourroy, G. Optimization of a New ZnO Nanorods Hydrothermal Synthesis Method for Solid State Dye Sensitized Solar Cells Applications. J. Phys. Chem. C 2013, 117, 2993–3001. [Google Scholar] [CrossRef]
- Kao, M.C.; Chen, H.Z.; Young, S.L.; Lin, C.C.; Kung, C.Y. Structure and photovoltaic properties of ZnO nanowire for dye-sensitized solar cells. Nanoscale Res. Lett. 2012, 7, 260. [Google Scholar] [CrossRef] [PubMed]
- Law, M.; Greene, L.; Johnson, J.; Saykally, R.; Yang, P. Nanowire dye-sensitized solar cells. Nat. Mater. 2005, 4, 455. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.M.; Chiu, W.H.; Lee, C.H.; Tsai, S.Y.; Hsieh, W.F. Formation of Branched ZnO Nanowires from Solvothermal Method and Dye-Sensitized Solar Cells Applications. J. Phys. Chem. C 2008, 112, 16359–16364. [Google Scholar] [CrossRef]
- Bai, Y.; Yu, H.; Li, Z.; Amal, R.; Lu, G.Q.; Wang, L. In situ growth of a ZnO nanowire network within a TiO2 nanoparticle film for enhanced dye-sensitized solar cell performance. Adv. Mater. 2012, 24, 5850–5856. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xu, T.; Ito, Y.; Welp, U.; Kwok, W.K. Enhanced Electron Transport in Dye-Sensitized Solar Cells Using Short ZnO Nanotips on A Rough Metal Anode. J. Phys. Chem. C 2009, 113, 20521–20526. [Google Scholar] [CrossRef]
- Xu, C.K.; Shin, P.; Cao, L.L.; Gao, D. Preferential Growth of Long ZnO Nanowire Array and Its Application in Dye-Sensitized Solar Cells. J. Phys. Chem. C 2010, 114, 125–129. [Google Scholar] [CrossRef]
- Guerin, V.M.; Rathousky, J.; Pauporte, T. Electrochemical design of ZnO hierarchical structures for dye-sensitized solar cells. Sol. Energy Mater. Sol. Cells 2012, 102, 8–14. [Google Scholar] [CrossRef]
- Giannouli, M. Nanostructured ZnO, TiO2, and Composite ZnO/TiO2 Films for Application in Dye-Sensitized Solar Cells. Int. J. Photoenergy 2013, 2013, 612095. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Kay, A.; Rodicio, I.; Humpbry-Baker, R.; Miiller, E.; Liska, P.; Vlachopoulos, N.; Grätzel, M. Conversion of light to electricity by cis-X2bis(2,2′-bipyridyl-4,4′-dicarboxylate)ruthenium(II) charge-transfer sensitizers (X = Cl-, Br-, I-, CN-, and SCN-) on nanocrystalline titanium dioxide electrodes. J. Am. Chem. Soc. 1993, 115, 6382–6390. [Google Scholar] [CrossRef]
- Smestad, G. Education and solar conversion: Demonstrating electron transfer. Sol. Energy Mater. Sol. Cells 1998, 55, 157–178. [Google Scholar] [CrossRef]
- Pichot, F.; Pitts, R.; Gregg, B. Low-Temperature Sintering of TiO2 Colloids: Application to Flexible Dye-Sensitized Solar Cells. Langmuir 2000, 16, 5626–5630. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Shen, H.; Chen, W. The 3-dimensional dye-sensitized solar cell and module based on all titanium substrates. Appl. Energy 2010, 87, 436–441. [Google Scholar] [CrossRef]
- Van der Zanden, B.; Goossens, A. The Nature of Electron Migration in Dye-Sensitized Nanostructured TiO2. J. Phys. Chem. B 2000, 104, 7171–7178. [Google Scholar] [CrossRef]
- Shklover, V.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Barbe, C.; Kay, A.; Haibach, T.; Steurer, W.; Hermann, R.; Nissen, H.U.; Grätzel, M. Structure of Nanocrystalline TiO2 Powders and Precursor to Their Highly Efficient Photosensitizer. Chem. Mater. 1997, 9, 430–439. [Google Scholar] [CrossRef]
- Giannouli, M.; Spiliopoulou, F. Effects of the morphology of nanostructured ZnO films on the efficiency of dye-sensitized solar cells. Renew. Energy 2012, 41, 115–122. [Google Scholar] [CrossRef]
- Lee, W.; Okada, H.; Wakahara, A.; Yoshida, A. Structural and photoelectrochemical characteristics of nanocrystalline ZnO electrode with Eosin-Y. Ceram. Int. 2006, 32, 495–498. [Google Scholar] [CrossRef]
- Syrrokostas, G.; Govatsi, K.; Yannopoulos, S.N. High-Quality, Reproducible ZnO Nanowire Arrays Obtained by a Multiparameter Optimization of Chemical Bath Deposition Growth. Cryst. Growth Des. 2016, 16, 2140–2150. [Google Scholar] [CrossRef]
- Han, J.; Fan, F.; Xu, C.; Lin, S.; Wei, M.; Duan, X.; Wang, Z.L. ZnO nanotube-based dye-sensitized solar cell and its application in self-powered devices. Nanotechnology 2010, 21, 405203. [Google Scholar] [CrossRef] [PubMed]
- Benhebal, H.; Chaib, M.; Salmon, T.; Geens, J.; Leonard, A.; Lambert, S.D.; Crine, M.; Heinrichs, B. Photocatalytic degradation of phenol and benzoic acid using zinc oxide powders prepared by the sol–gel process. Alex. Eng. J. 2013, 52, 517–523. [Google Scholar] [CrossRef]
- Lopez-Ramon, M.V.; Stoeckli, F.; Moreno-Castilla, C.; Carrasco-Marin, F. On the characterization of acidic and basic surface sites on carbons by various techniques. Carbon 1999, 37, 1215–1221. [Google Scholar] [CrossRef]
- Giannouli, M.; Fakis, M. Interfacial Electron Transfer Dynamics and Photovoltaic Performance of TiO2 and ZnO Solar Cells Sensitized with Coumarin 343. J. Photochem. Photobiol. A 2011, 226, 42–50. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, S.; Zhao, H.; Will, G.; Liu, P. An Efficient and Low-Cost TiO2 Compact Layer for Performance Improvement of Dye-Sensitized Solar Cells. Electrochim. Acta 2009, 54, 1319–1324. [Google Scholar] [CrossRef]
- Xu, W.; Dai, S.; Hu, L.; Zhang, C.; Xiao, S.; Luo, X.; Jing, W.; Wang, K. Influence of Different Surface Modifications on the Photovoltaic Performance and Dark Current of Dye-Sensitized Solar Cells. Plasma Sci. Technol. 2007, 9, 554–559. [Google Scholar]
- Jasim, K.H. Dye Sensitized Solar Cells—Working Principles, Challenges and Opportunities; InTech: London, UK, 2011; ISBN 978-953-307-735-2. [Google Scholar] [CrossRef]
- Zaban, A.; Greenshtein, M.; Bisquert, J. Determination of the electron lifetime in nanocrystalline dye solar cells by photovoltage decay measurements. ChemPhysChem 2003, 4, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Chiba, Y.; Islam, A.; Watanabe, Y.; Komiya, R.; Koide, N.; Han, L. Dye-Sensitized Solar Cells with Conversion Efficiency of 11.1%. Jpn. J. Appl. Phys. 2006, 45, 638–640. [Google Scholar] [CrossRef]
- Hara, K.; Tachiban, Y.; Ohga, Y.; Shinpo, A.; Suga, S.; Sayama, K.; Sugihara, H.; Arakawa, H. Dye-sensitized nanocrystalline TiO2 solar cells based on novel coumarin dyes. Sol. Energy Mater. Sol. Cells 2003, 77, 89–103. [Google Scholar] [CrossRef]
- Rani, S.; Shishodia, P.K.; Mehra, R.M. Development of a dye with broadband absorbance in visible spectrum for an efficient dye-sensitized solar cell. J. Renew. Sustain. Energy 2010, 2, 043103. [Google Scholar] [CrossRef]
- Keis, K.; Magnusson, E.; Lindström, H.; Lindquist, S.; Hagfeldt, A. A 5% efficient photoelectrochemical solar cell based on nanostructured ZnO electrodes. Sol. Energy Mater. Sol. Cells 2002, 73, 51–58. [Google Scholar] [CrossRef]
- Chang, W.; Lee, C.; Yu, W.; Lin, C. Optimization of dye adsorption time and film thickness for efficient ZnO dye-sensitized solar cells with high at-rest stability. Nanoscale Res. Lett. 2012, 7, 688. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hu, J.; Xie, Y. High-efficiency dye-sensitized solar cells of up to 8.03% by air plasma treatment of ZnO nanostructures. Chem. Commun. 2015, 51, 16229–16232. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Sun, X.; Lo, G.; Kwong, D. Improved dye-sensitized solar cells with a ZnO-nanoflower photoanode. Appl. Phys. Lett. 2007, 90, 263501. [Google Scholar] [CrossRef]
- Ito, S.; Liska, P.; Comte, P.; Charvet, R.; Pechy, P.; Bach, U.; Schmidt-Mende, L.; Zakeeruddin, S.M.; Kay, A.; Nazeeruddin, M.K.; et al. Control of dark current in photoelectrochemical (TiO2/I--I3-)) and dye-sensitized solar cells. Chem. Commun. 2005, 34, 4351–4353. [Google Scholar] [CrossRef] [PubMed]
- Bisquert, A.; Zaban, M.; Greenshtein, M.; Mora-Seró, I. Determination of Rate Constants for Charge Transfer and the Distribution of Semiconductor and Electrolyte Electronic Energy Levels in Dye-Sensitized Solar Cells by Open-Circuit Photovoltage Decay Method. J. Am. Chem. Soc. 2004, 126, 13550–13559. [Google Scholar] [CrossRef] [PubMed]
- Fabregat-Santiago, F.; Garcia-Canadas, J.; Palomares, E.; Clifford, J.N.; Haque, S.A.; Durrant, J.R.; Garcia-Belmonte, G.; Bisquert, J. The origin of slow electron recombination processes in dye-sensitized solar cells with alumina barrier coatings. J. Appl. Phys. 2004, 96, 6903–6907. [Google Scholar] [CrossRef]
- Azpiroz, J.M.; De Angelis, F. DFT/TDDFT Study of the Adsorption of N3 and N719 Dyes on ZnO(101̅0) Surfaces. J. Phys. Chem. A 2014, 118, 5885–5893. [Google Scholar] [CrossRef] [PubMed]
- Schiffmann, F.; Van deVondel, J.; Hutter, J.; Wirz, R.; Urakawa, A.; Baiker, A. Protonation-Dependent Binding of Ruthenium Bipyridyl Complexes to the Anatase(101) Surface. J. Phys. Chem. C 2010, 114, 8398–8404. [Google Scholar] [CrossRef]
- Bahnemann, D.W. Ultrasmall Metal Oxide Particles: Preparation, Photophysical Characterization, and Photocatalytic Properties. Isr. J. Chem. 1993, 33, 115–136. [Google Scholar] [CrossRef]
- Keis, K.; Bauer, C.; Boschloo, G.; Hagfeldt, A.; Westermark, K.; Rensmob, H.; Siegbahn, H. Nanostructured ZnO electrodes for dye-sensitized solar cell applications. J. Photochem. Photobiol. A 2002, 148, 57–64. [Google Scholar] [CrossRef]
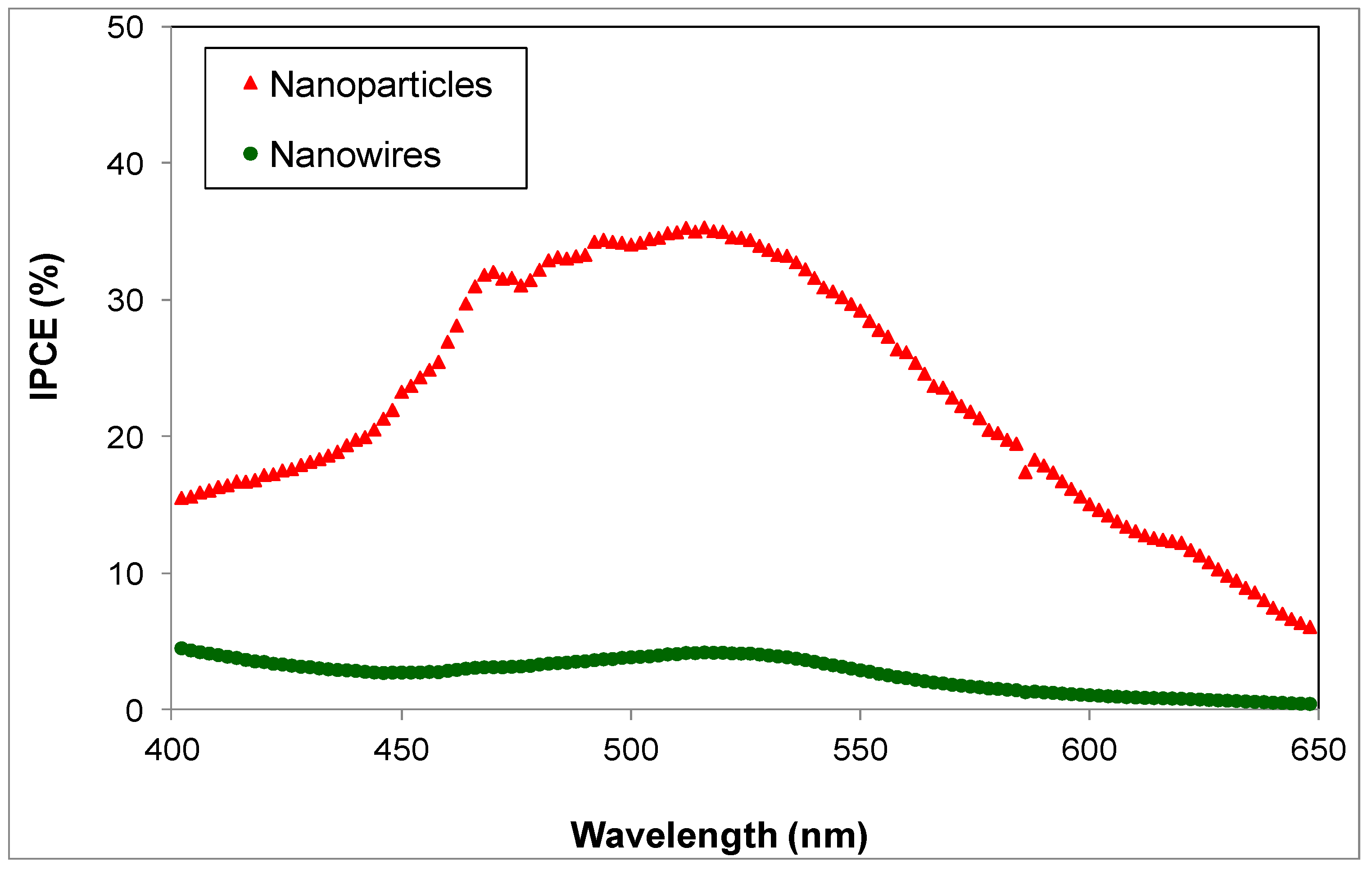
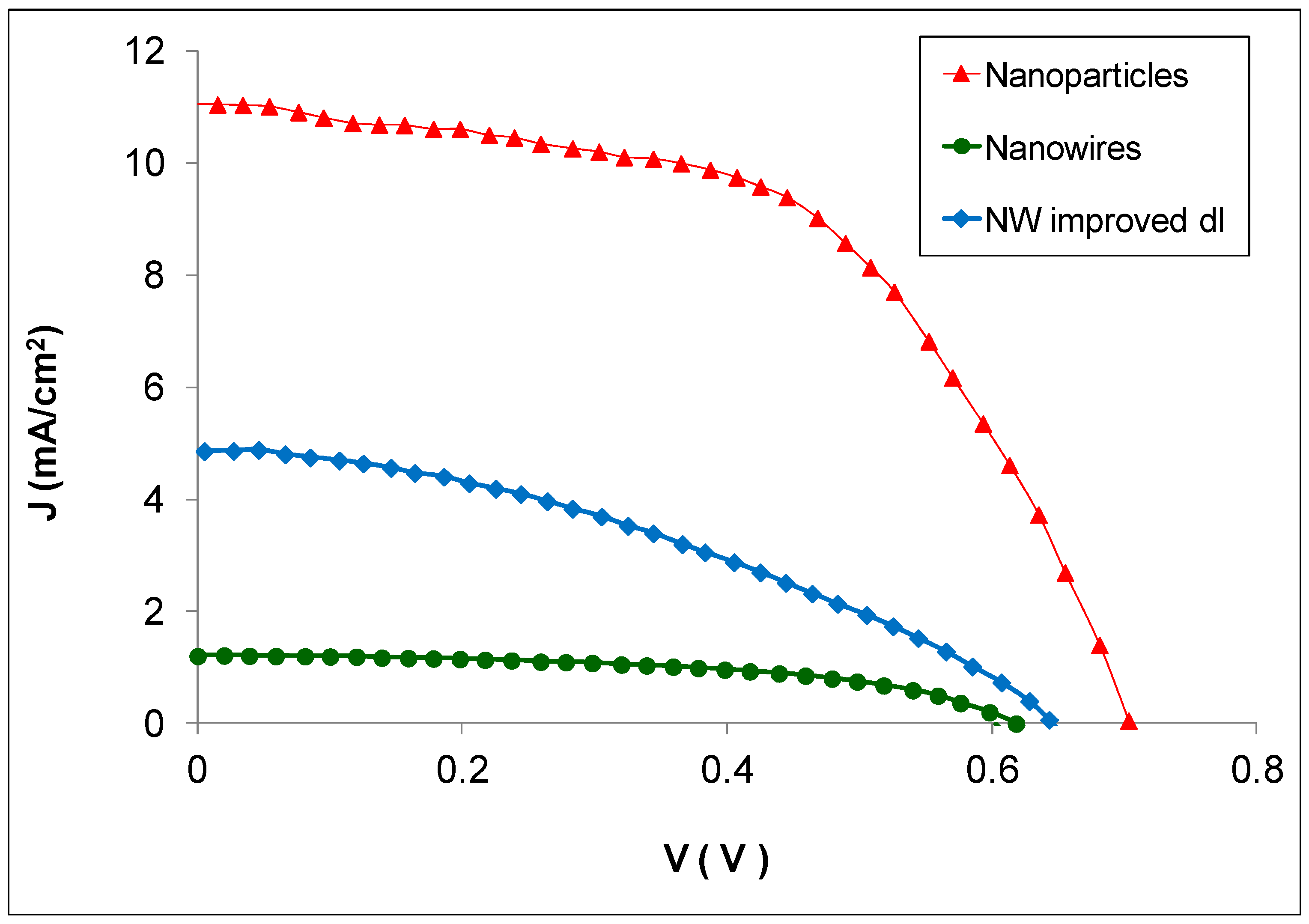
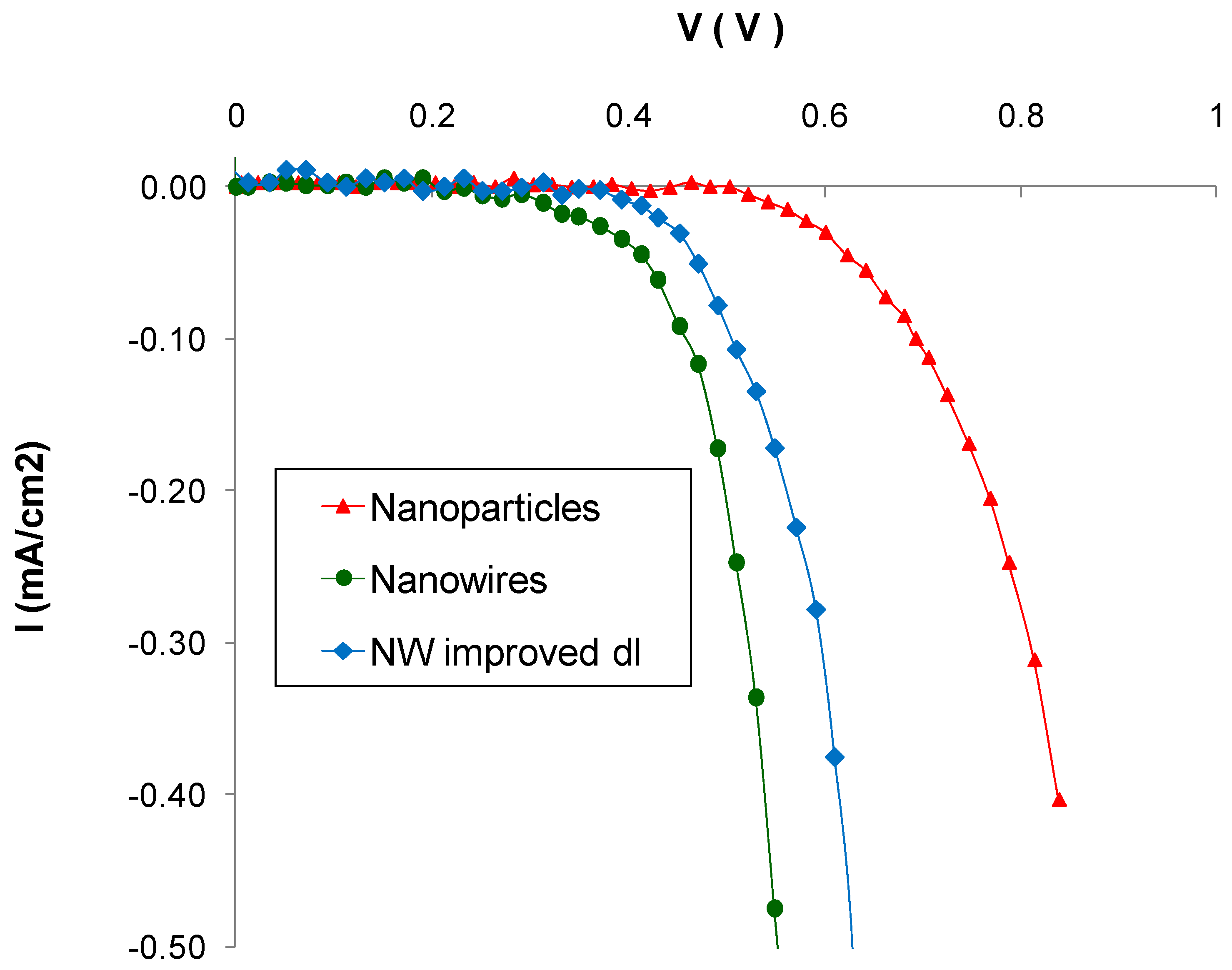
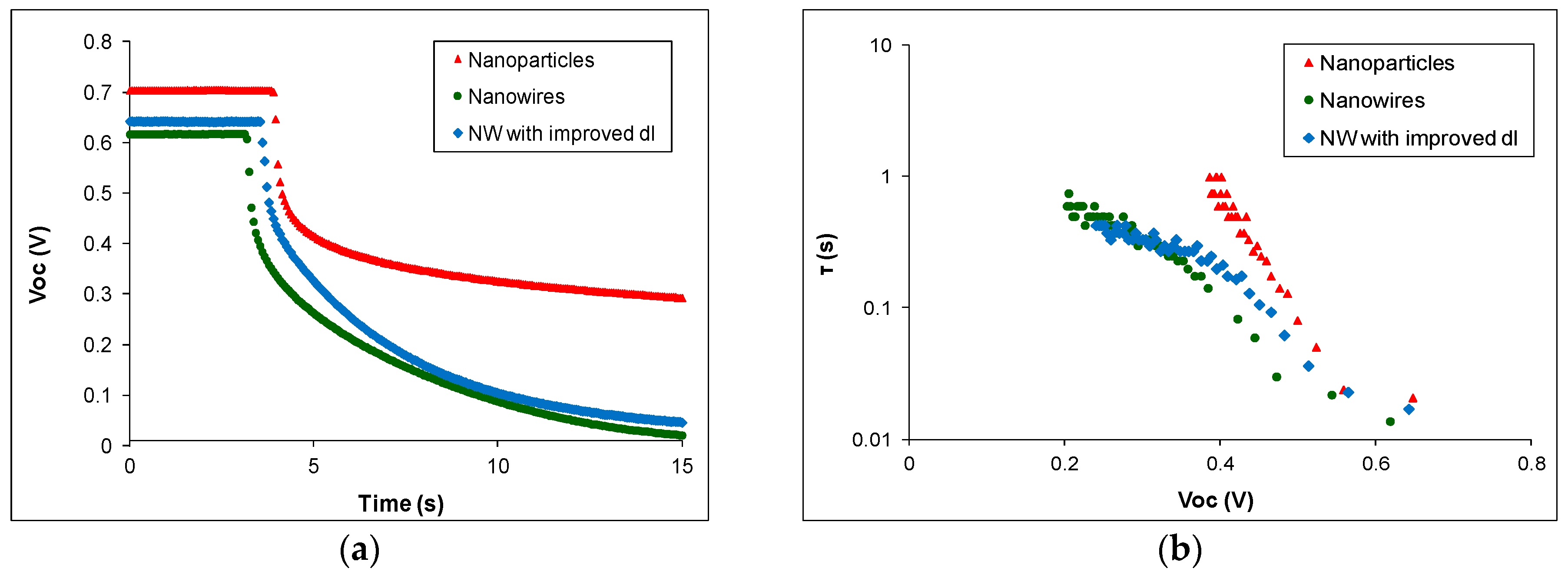
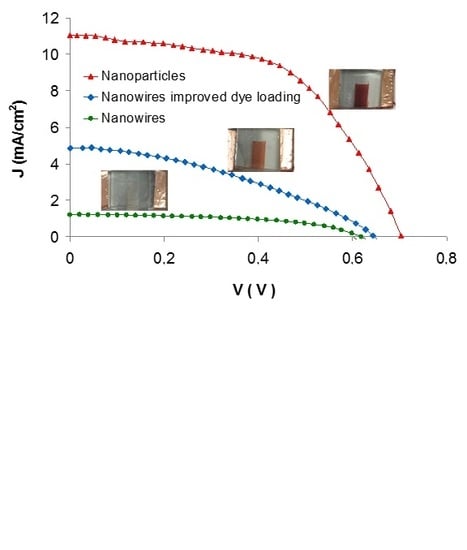
| Sample Type Header | Voc (V) | Jsc (mA/cm2) | FF | η (%) |
|---|---|---|---|---|
| Nanoparticles | 0.70 ± 0.02 | 11.2 ± 1.0 | 0.54 ± 0.06 | 6.19 ± 0.60 |
| Nanowires | 0.62 ± 0.03 | 1.2 ± 0.4 | 0.53 ± 0.04 | 0.63 ± 0.09 |
| Nanowires with improved dl | 0.64 ± 0.02 | 4.8 ± 0.4 | 0.41 ± 0.03 | 1.80 ± 0.20 |
| Sample Code | Seed Layer | Growth Conditions | |
|---|---|---|---|
| a | 0.05 M Zinc acetate in ethanol | 0.04 M Zn(NO3)2 0.02 M HMTA 0.16 M PEI 0.04 M NH4OH | Without renewal |
| b | 0.04 M Zn(NO3)2 0.02 M HMTA 0.16 M PEI 0.04 M NH4OH | With renewal | |
| c | 0.005 M Zinc acetate in ethanol | 0.05 M Zn(NO3)2 0.025 M HMTA 0.08 gr PEI 0.7 M NH4OH | Without renewal |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannouli, M.; Govatsi, Κ.; Syrrokostas, G.; Yannopoulos, S.N.; Leftheriotis, G. Factors Affecting the Power Conversion Efficiency in ZnO DSSCs: Nanowire vs. Nanoparticles. Materials 2018, 11, 411. https://doi.org/10.3390/ma11030411
Giannouli M, Govatsi Κ, Syrrokostas G, Yannopoulos SN, Leftheriotis G. Factors Affecting the Power Conversion Efficiency in ZnO DSSCs: Nanowire vs. Nanoparticles. Materials. 2018; 11(3):411. https://doi.org/10.3390/ma11030411
Chicago/Turabian StyleGiannouli, Myrsini, Κaterina Govatsi, George Syrrokostas, Spyros N. Yannopoulos, and George Leftheriotis. 2018. "Factors Affecting the Power Conversion Efficiency in ZnO DSSCs: Nanowire vs. Nanoparticles" Materials 11, no. 3: 411. https://doi.org/10.3390/ma11030411
APA StyleGiannouli, M., Govatsi, Κ., Syrrokostas, G., Yannopoulos, S. N., & Leftheriotis, G. (2018). Factors Affecting the Power Conversion Efficiency in ZnO DSSCs: Nanowire vs. Nanoparticles. Materials, 11(3), 411. https://doi.org/10.3390/ma11030411