Carbon-Based Nanomaterials/Allotropes: A Glimpse of Their Synthesis, Properties and Some Applications
Abstract
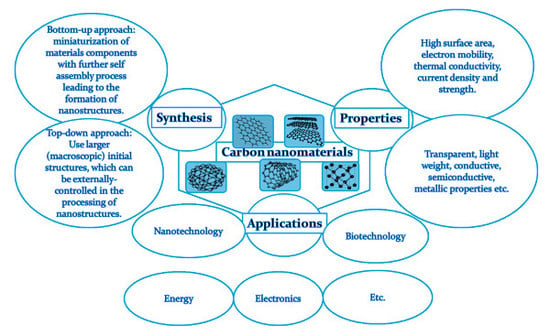
1. Introduction
1.1. Historical Chemical Background of Some Selected Carbon-Based Allotropes
1.2. Graphite
1.3. Diamond
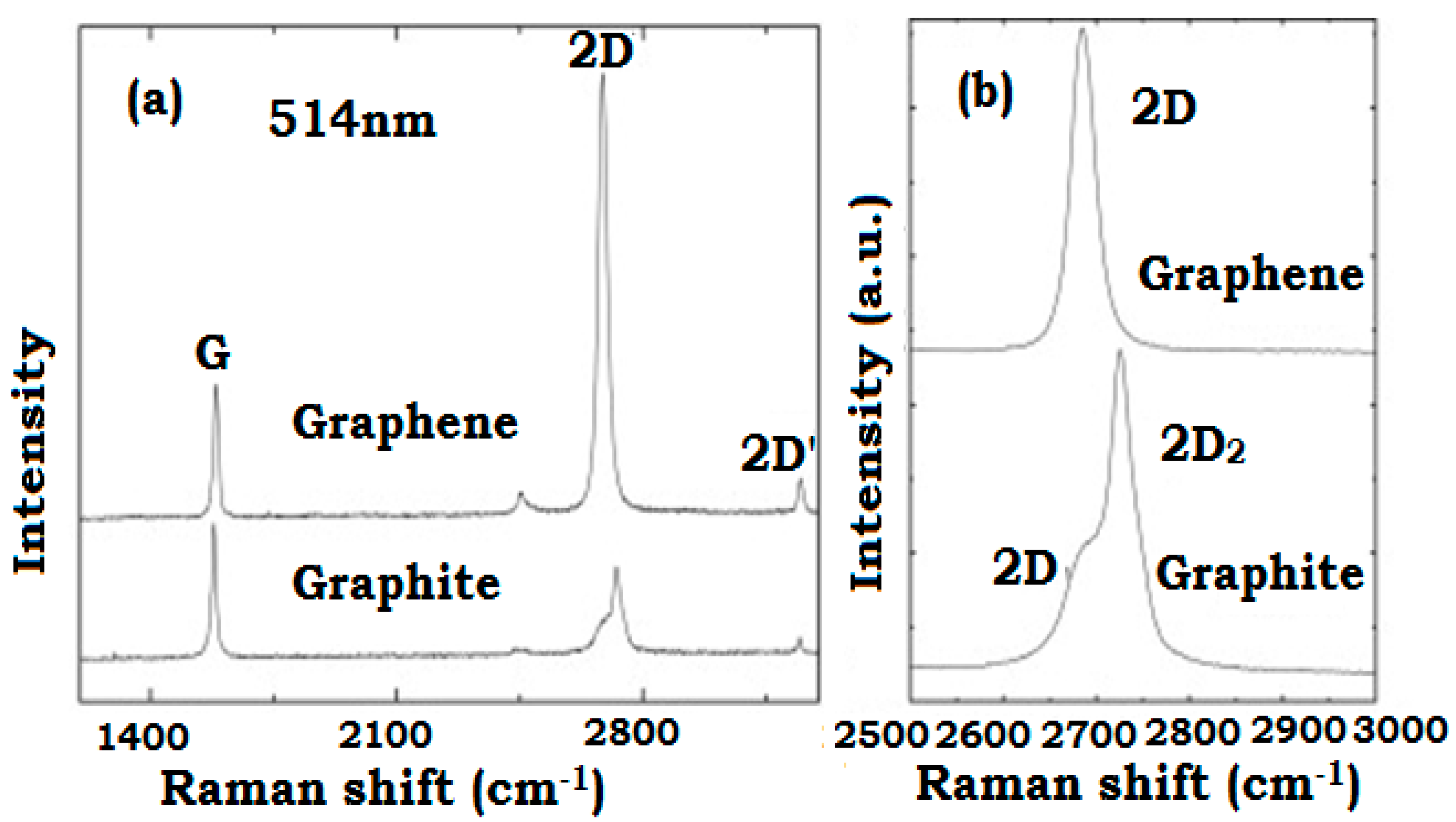
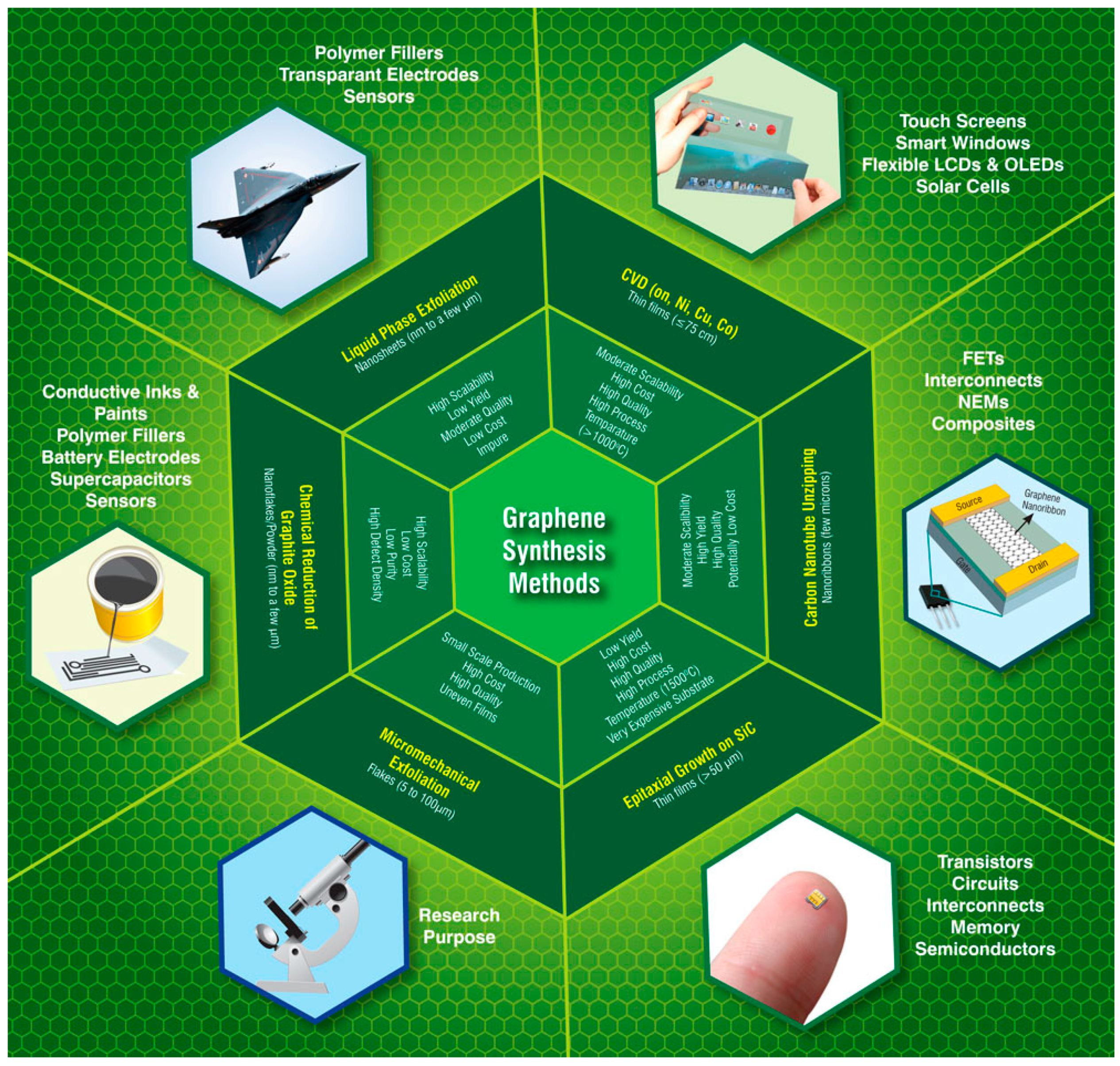
1.4. Graphene and Graphene-Derived Materials (‘Graphenoids’)
1.5. Activated Carbon
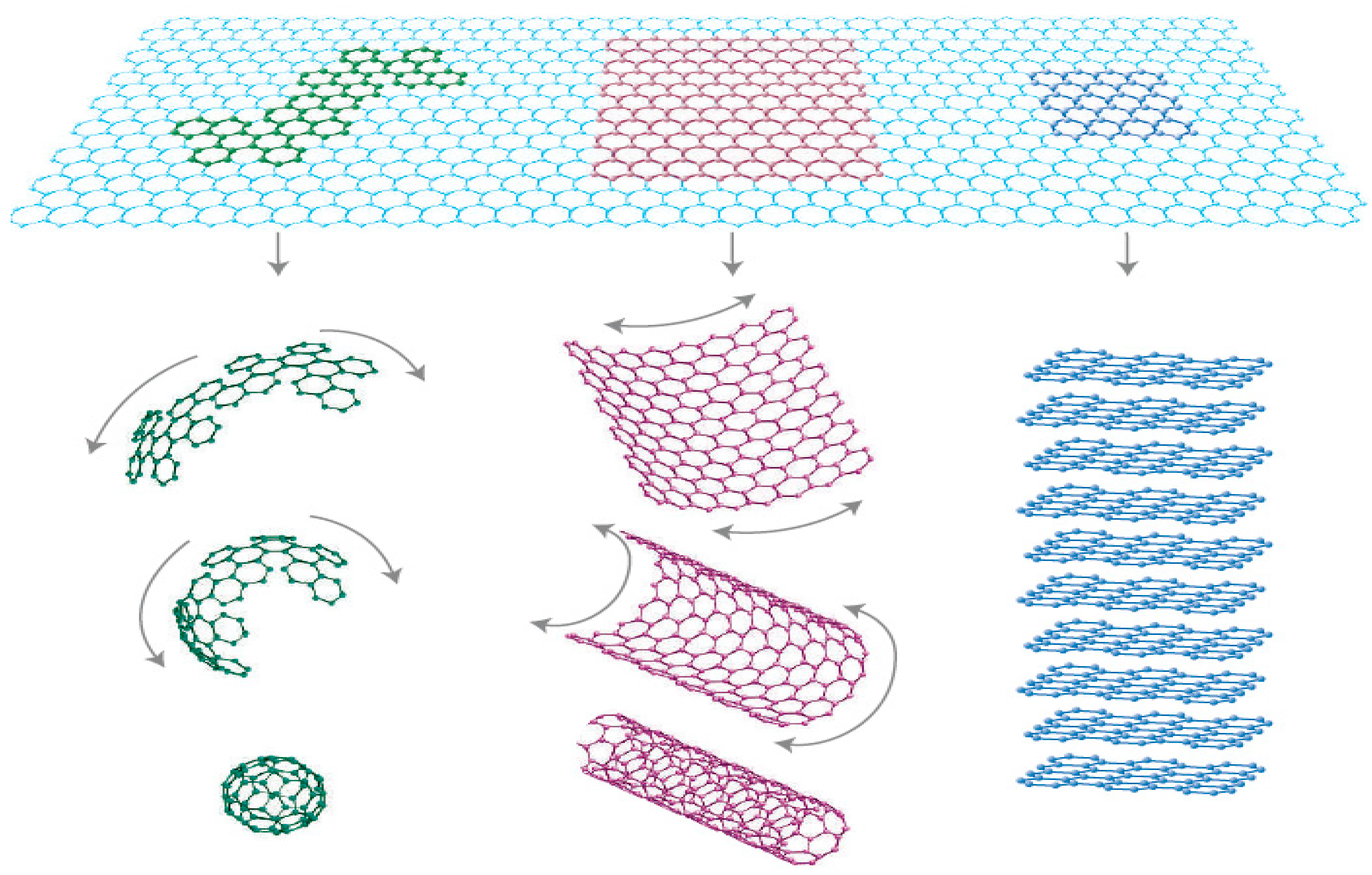
1.6. Fullerene, Buckyball and Carbon Nanotubes Family
2. Synthesis
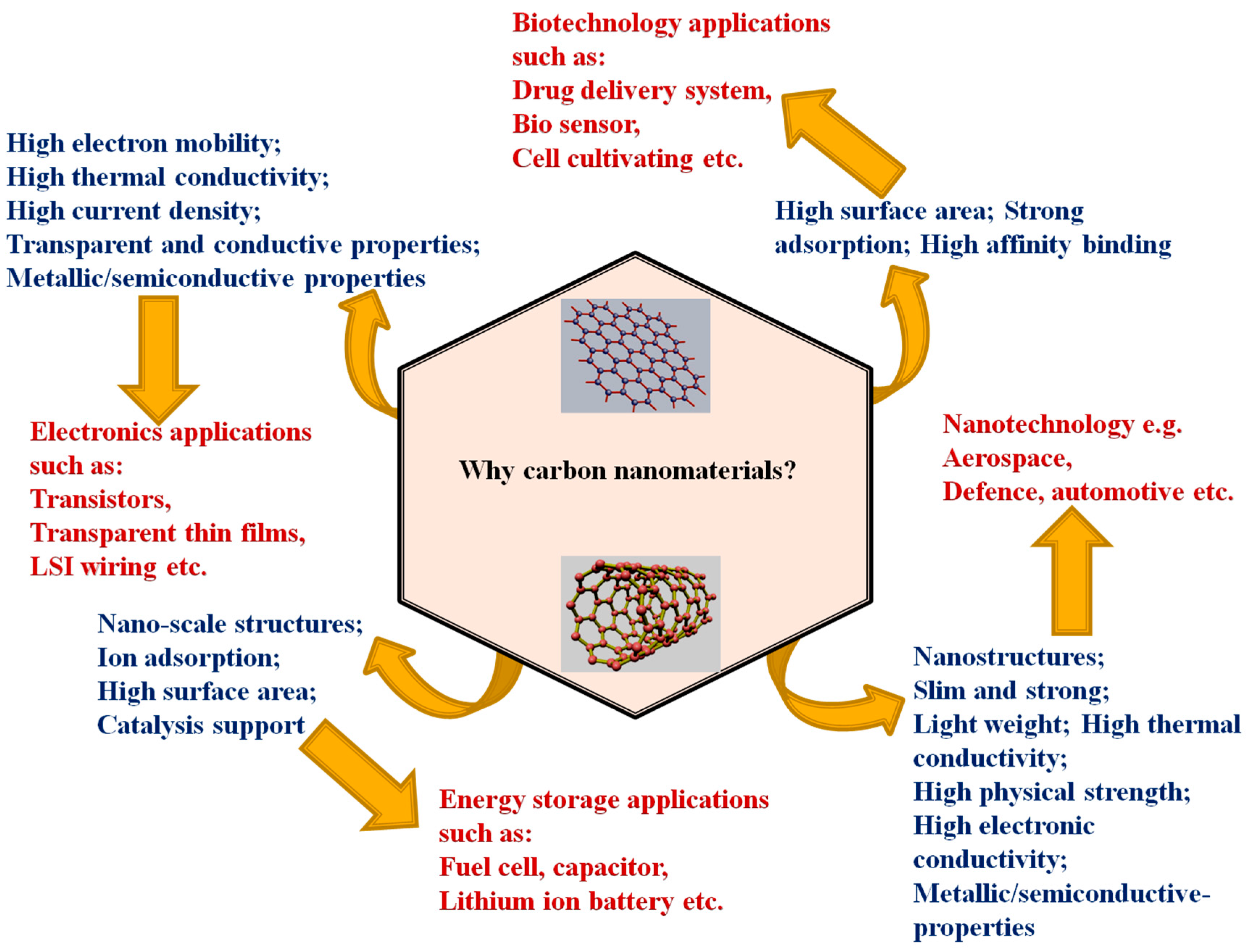
3. Properties
4. Applications
4.1. Electrochemical Energy Storage (Nanoenergy) Application
4.2. Biomedical Application
5. Conclusions
6. Outlook/Way Forward
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, Y.; Yin, Q.-Z. Carbon and other light element contents in the Earth’s core based on first-principles molecular dynamics. Proc. Natl. Acad. Sci. USA 2012, 109, 19579–19583. [Google Scholar] [CrossRef] [PubMed]
- Allègre, C.J.; Poirier, J.-P.; Humler, E.; Hofmann, A.W. The chemical composition of the Earth. Earth Planet. Sci. Lett. 1995, 134, 515–526. [Google Scholar] [CrossRef]
- Pace, N.R. The universal nature of biochemistry. Proc. Natl. Acad. Sci. USA 2001, 98, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Marty, B.; Alexander, C.M.O.; Raymond, S.N. Primordial origins of earth’s carbon. Rev. Mineral. Geochem. 2013, 75, 149–181. [Google Scholar] [CrossRef]
- Titirici, M.-M.; White, R.J.; Brun, N.; Budarin, V.L.; Su, D.S.; Del Monte, F.; Clark, J.H.; MacLachlan, M.J. Sustainable carbon materials. Chem. Soc. Rev. 2015, 44, 250–290. [Google Scholar] [CrossRef] [PubMed]
- Loos, M. Allotropes of Carbon and Carbon Nanotubes. In Carbon Nanotube Reinforced Composites; Elsevier: Amsterdam, The Netherlands, 2015; pp. 73–101. [Google Scholar]
- Deng, J.; You, Y.; Sahajwalla, V.; Joshi, R.K. Transforming waste into carbon-based nanomaterials. Carbon 2016, 96, 105–115. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F. The role of carbon materials in heterogeneous catalysis. Carbon 1998, 36, 159–175. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb carbon: A review of graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Fal, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Carbon-based supercapacitors produced by activation of graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- Gadipelli, S.; Guo, Z.X. Graphene-based materials: Synthesis and gas sorption, storage and separation. Prog. Mater. Sci. 2015, 69, 1–60. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Colombo, L.; Yu, G.; Stoller, M.; Tozzini, V.; Ferrari, A.C.; Ruoff, R.S.; Pellegrini, V. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science 2015, 347, 1246501–1246509. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.J.; Cao, X.; Cao, Z. Si(C≡C)4-Based Single-Crystalline Semiconductor: Diamond-like superlight and superflexible wide-bandgap material for the UV photoconductive device. ACS Appl. Mater. Interfaces 2016, 8, 16551–16554. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fu, K.; Zhu, S.; Luo, W.; Wang, Y.; Li, Y.; Hitz, E.; Yao, Y.; Dai, J.; Wan, J.; et al. Reduced graphene oxide films with ultrahigh conductivity as Li-ion battery current collectors. Nano Lett. 2016, 16, 3616–3623. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent functionalization of graphene and graphene oxide for energy materials, biosensing, catalytic, and biomedical applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Liu, S.; Sun, H.; Yao, Y.; Zhi, L.; Wang, S. Synthesis of porous reduced graphene oxide as metal-free carbon for adsorption and catalytic oxidation of organics in water. J. Mater. Chem. A 2013, 1, 5854–5859. [Google Scholar] [CrossRef]
- Liu, J.; Cui, L.; Losic, D. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 2013, 9, 9243–9257. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Kanchi, S.; Sabela, M.I.; Bisetty, K. Insight into the biosensing of graphene oxide: Present and future prospects. Arab. J. Chem. 2015, 9, 238–261. [Google Scholar] [CrossRef]
- Usman, M.S.; Hussein, M.Z.; Fakurazi, S.; Ahmad Saad, F.F. Gadolinium-based layered double hydroxide and graphene oxide nano-carriers for magnetic resonance imaging and drug delivery. Chem. Cent. J. 2017, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Han, J.W.; Kim, E.; Kim, J.; Han, J.W.; Kim, J. Reduction of graphene oxide by resveratrol: A novel and simple biological method for the synthesis of an effective anticancer nanotherapeutic molecule. Int. J. Nanomed. 2015, 10, 2951–2969. [Google Scholar] [CrossRef]
- Khadiran, T.; Hussein, M.Z.; Zainal, Z.; Rusli, R. Activated carbon derived from peat soil as a framework for the preparation of shape-stabilized phase change material. Energy 2015, 82, 468–478. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, G.; Lin, K.; Zhang, Q.; Di, H. Application of latent heat thermal energy storage in buildings: State-of-the-art and outlook. Build. Environ. 2007, 42, 2197–2209. [Google Scholar] [CrossRef]
- Baetens, R.; Jelle, B.P.; Gustavsen, A. Phase change materials for building applications: A state-of-the-art review. Energy Build. 2010, 42, 1361–1368. [Google Scholar] [CrossRef]
- Simen, E.K.; Jelle, B.P. Phase change materials and products for building applications: A state-of-the-art review and future research opportunities. Energy Build. 2015, 94, 150–176. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Luther, W. Application of Nano-Technologies in the Energy Sector; Hessen-Nanotech of the Hessian Ministry of Economy, Transport, Urban and Regional Development: Wiesbaden, Germany, 2008; p. 88.
- Wu, Y.; Lin, Y.; Bol, A.A.; Jenkins, K.A.; Xia, F.; Farmer, D.B.; Zhu, Y.; Avouris, P. High-frequency, scaled graphene transistors on diamond-like carbon. Nature 2011, 472, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Li, M.; Wang, Y. Biomass-derived carbon: Synthesis and applications in energy storage and conversion. Green Chem. 2016, 18, 4824–4854. [Google Scholar] [CrossRef]
- Ferrari, A.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Wei, L.; Kuo, P.K.; Thomas, R.L. Thermal conductivity of isotopically modified single crystal diamond. Phys. Rev. Lett. 1993, 70, 3764–3767. [Google Scholar] [CrossRef] [PubMed]
- Hodkiewicz, J.; Scientific, T.F. Characterizing carbon materials with Raman spectroscopy. Prog. Mater. Sci. 2005, 50, 929–961. [Google Scholar] [CrossRef]
- Titirici, M. Sustainable Carbon Materials from Hydrothermal Processes; John Wiley & Sons, Ltd.: Chichester, UK, 2013. [Google Scholar]
- Dai, L.; Chang, D.W.; Baek, J.-B.; Lu, W. Carbon nanomaterials for advanced energy conversion and storage. Small 2012, 8, 1130–1166. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, B.; Neel, P.; Varadarajan, T. Methods of Activation and Specific Applications of Carbon Materials; Viswanathan, B., Ed.; NCCR IIT Madras: Chennai, India, 2009. [Google Scholar]
- Kaneko, K.; Ishii, C.; Ruike, M.; kuwabara, H. Origin of superhigh surface area and microcrystalline graphitic structures of activated carbons. Carbon 1992, 30, 1075–1088. [Google Scholar] [CrossRef]
- Pang, J.; Bachmatiuk, A.; Ibrahim, I.; Fu, L.; Placha, D. CVD growth of 1D and 2D sp2 carbon nanomaterials. J. Mater. Sci. 2016, 51, 640–667. [Google Scholar] [CrossRef]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Smalley, R.E. Great balls of carbon: The story of Buckminsterfullerene. Sciences 1991, 31, 22–28. [Google Scholar] [CrossRef]
- Iijima, S. Carbon nanotubes: Past, present, and future. Physica B 2002, 323, 1–5. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Tripathi, A.C.; Saraf, S.A.; Saraf, S.K. Carbon nanotropes: A contemporary paradigm in drug delivery. Materials 2015, 8, 3068–3100. [Google Scholar] [CrossRef]
- Conly, A. Mining carbon to decrease the carbon footprint. Scientia 2017, 112, 17–20. [Google Scholar]
- Bowers, B. History of Electric Light and Power; Peter Peregrinus Ltd.: London, UK, 1982; Volume 55, pp. 71–72. [Google Scholar]
- Brandt, N.B.; Chudinov, S.M.; Ponomarev, Y.G. (Eds.) Semimetals Graphite and Its Compounds; Modern Problem in Condensed Matter Sciences Series 20; Elsevier: Amsterdam, The Netherlands, 1988. [Google Scholar]
- Sugihara, K.; Sato, H. Electrical conductivity of graphite. J. Phys. Soc. Jpn. 1963, 18, 332–341. [Google Scholar] [CrossRef]
- Deprez, N.; McLachlan, D.S. The analysis of the electrical conductivity of graphite conductivity of graphite powders during compaction. J. Phys. D Appl. Phys. 1988, 21, 101–107. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Weng, W.; Wu, C. Exfoliation of graphite flake and its nanocomposites. Carbon 2003, 41, 619–621. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron-phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Budde, H.; Coca Lopez, N.; Shi, X.; Ciesielski, R.; Lombardo, A.; Yoon, D.; Ferrari, A.C.; Hartschuh, A. Raman radiation patterns of graphene. ACS Nano 2016, 10, 1756–1763. [Google Scholar] [CrossRef] [PubMed]
- Tuinstra, F.; Koenig, L. Raman spectrum of graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Kataura, H.; Kumazawa, Y.; Maniwa, Y.; Umezu, I.; Suzuki, S.; Ohtsuka, Y.; Achiba, Y. Optical properties of single-wall carbon nanotubes. Synth. Met. 1999, 103, 2555–2558. [Google Scholar] [CrossRef]
- Raman, C.V. A new radiation. Indian J. Phys. 1928, 2, 387–398. [Google Scholar] [CrossRef]
- Krishnan, R.S.; Shankar, R.K. Raman effect: History of the discovery. J. Raman Spectrosc. 1981, 10, 1–8. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Resonant Raman spectroscopy of disordered, amorphous, and diamondlike carbon. Phys. Rev. B 2001, 64, 075414. [Google Scholar] [CrossRef]
- Ianoul, A.; Thomas Coleman, A.; Asher, S.A. UV Resonance Raman spectroscopic detection of nitrate and nitrite in wastewater treatment processes. Anal. Chem. 2002, 74, 1458–1461. [Google Scholar] [CrossRef]
- Merlen, A.; Buijnsters, J.; Pardanaud, C. A guide to and review of the use of multiwavelength Raman spectroscopy for characterizing defective aromatic carbon solids: From graphene to amorphous carbons. Coatings 2017, 7, 153. [Google Scholar] [CrossRef]
- Ekimov, E.A.; Suetin, N.V.; Popovich, A.F.; Ralchenko, V.G. Thermal conductivity of diamond composites sintered under high pressures. Diam. Relat. Mater. 2008, 17, 838–843. [Google Scholar] [CrossRef]
- Kidalov, S.V.; Shakhov, F.M. Thermal conductivity of diamond composites. Materials 2009, 2, 2467–2495. [Google Scholar] [CrossRef]
- Mildren, R.P. Intrinsic optical properties of diamond. In Optical Engineering of Diamond; Mildren, R.P., Rabeau, J.R., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2001; pp. 1–34. [Google Scholar]
- Deneuville, A. Electronic properties, devices and applications of diamond thin films. Acad. Sci. 2000, 1, 81–90. [Google Scholar] [CrossRef]
- Hausmann, B.J.M.; Khan, M.; Zhang, Y.; Babinec, T.M.; Martinick, K.; Mccutcheon, M.; Hemmer, P.R.; Loncar, M. Fabrication of diamond nanowires for quantum information processing applications. Diam. Relat. Mater. 2010, 19, 621–629. [Google Scholar] [CrossRef]
- Pierson, H.O. Handbook of Carbon, Graphite, Diamond and Fullerenes; Noyes Publications: Park Ridge, NJ, USA, 1993. [Google Scholar]
- Hausmann, B.J.M.; Bulu, I.; Venkataraman, V.; Deotare, P.; Lonc, M. Diamond Nonlinear Photonics. Nat. Photonics 2014, 8, 369–374. [Google Scholar] [CrossRef]
- Walkert, J. Optical absorption and luminescence in diamond. Rep. Prog. Phys. 1979, 42, 1606–1659. [Google Scholar]
- Zaitsev, A.M. Optical Properties of Diamond, 1st ed.; Springer-Verlag Berlin Heidelberg GmbH: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Shatskiy, A.; Yamazaki, D.; Morard, G.; Cooray, T.; Matsuzaki, T.; Higo, Y.; Sumiya, H.; Ito, E.; Katsura, T.; Shatskiy, A.; et al. Boron-doped diamond heater and its application to large-volume, high-pressure, and high-temperature experiments. Rev. Sci. Instrum. 2009, 80, 023907. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Soldano, C.; Mahmood, A.; Dujardin, E. Production, properties and potential of graphene. Carbon 2010, 48, 2127–2150. [Google Scholar] [CrossRef]
- Botas, C.; Álvarez, P.; Blanco, P.; Granda, M.; Blanco, C.; Santamaría, R.; Romasanta, L.J.; Verdejo, R.; López-Manchado, M.A.; Menéndez, R. Graphene materials with different structures prepared from the same graphite by the Hummers and Brodie methods. Carbon 2013, 65, 156–164. [Google Scholar] [CrossRef]
- Mattevi, B.C.; Eda, G.; Agnoli, S.; Miller, S.; Mkhoyan, K.A.; Celik, O.; Mastrogiovanni, D.; Granozzi, G.; Garfunkel, E.; Chhowalla, M. Evolution of electrical, chemical, and structural properties of transparent and conducting chemically derived graphene thin films. Adv. Funct. Mater. 2009, 8854, 2577–2583. [Google Scholar] [CrossRef]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.; Kim, P.; Choi, J.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2008, 457, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Yang, M.; Cho, K.M.; Jun, Y.-S.; Lee, S.B.; Jung, H.-T. High quality reduced graphene oxide through repairing with multi-layered graphene ball nanostructures. Sci. Rep. 2013, 3, 3251. [Google Scholar] [CrossRef] [PubMed]
- Kosidlo, U.; Arias Ruiz de Larramendi, M.; Tonner, F.; Park, H.J.; Glanz, C.; Skakalova, V.; Roth, S.; Kolaric, I. Production methods of graphene and resulting material properties. In Proceedings of the 7th NanoEurope Symposium 2009, Rapperswil, Switzerland, 25–26 November 2009. [Google Scholar]
- Ji, L.; Meduri, P.; Agubra, V.; Xiao, X.; Alcoutlabi, M. Graphene-based nanocomposites for energy storage. Adv. Energy Mater. 2016, 6, 7–16. [Google Scholar] [CrossRef]
- Yu, C.; Wu, Y.; Liu, X.; Yao, B.; Fu, F.; Gong, Y.; Rao, Y.; Chen, Y. graphene oxide deposited microfiber knot resonator for gas sensing. Opt. Mater. Express 2016, 6, 727–733. [Google Scholar] [CrossRef]
- Shams, S.S.; Zhang, L.S.; Hu, R.; Zhang, R.; Zhu, J. Synthesis of graphene from biomass: A green chemistry approach. Mater. Lett. 2015, 161, 476–479. [Google Scholar] [CrossRef]
- Akhavan, O.; Bijanzad, K.; Mirsepah, A. Synthesis of Graphene from natural and industrial carbonaceous Wastes. RSC Adv. 2014, 4, 20441–20448. [Google Scholar] [CrossRef]
- Somanathan, T.; Prasad, K.; Ostrikov, K.; Saravanan, A.; Krishna, V. Graphene oxide synthesis from agro waste. Nanomaterials 2015, 5, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Ruan, G.; Sun, Z.; Peng, Z.; Tour, J.M. Growth of graphene from food, insects, and waste. ACS Nano 2011, 5, 7601–7607. [Google Scholar] [CrossRef] [PubMed]
- Future Markets. The Global Market for Graphene 2010-2017; Future Markets, Inc.: Edinburgh, UK, 2012. [Google Scholar]
- Marsh, H.; Rodríguez-Reinoso, F. Activated Carbon, 1st ed.; Marsh, H., Rodríguez-Reinoso, F., Eds.; Elsevier Science & Technology Books: London, UK, 2006. [Google Scholar]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Preparation of highly microporous carbons from fir wood by KOH activation for adsorption of dyes and phenols from water. Sep. Purif. Technol. 2005, 47, 10–19. [Google Scholar] [CrossRef]
- Mahmoudi, K.; Hamdi, N. preparation and characterization of activated carbon from date pits by chemical activation with zinc chloride for methyl orange adsorption. J. Mater. Environ. Sci. 2014, 5, 1758–1769. [Google Scholar] [CrossRef]
- Peláez-Cid, A.A.; Teutli-León, M.M.M. Lignocellulosic Precursors Used in the Synthesis of Activated Carbon: Characterization Techniques and Applications in the Wastewater Treatment, 1st ed.; Montoya, V.H., Bonilla-Petriciolet, A., Eds.; InTech: Rijeka, Crotia, 2012. [Google Scholar]
- Pereira, R.G.; Veloso, C.M.; Da Silva, N.M.; De Sousa, L.F.; Bonomo, R.C.F.; De Souza, A.O.; Da Guarda Souza, M.O.; Da Costa Ilhéu Fontan, R. Preparation of activated carbons from cocoa shells and siriguela seeds using H3PO4 and ZnCL2 as activating agents for BSA and α-lactalbumin adsorption. Fuel Process. Technol. 2014, 126, 476–486. [Google Scholar] [CrossRef]
- Teo, E.Y.L.; Muniandy, L.; Ng, E.-P.; Adam, F.; Mohamed, A.R.; Jose, R.; Chong, K.F. High surface area activated carbon from rice husk as a high performance supercapacitor electrode. Electrochim. Acta 2016, 192, 110–119. [Google Scholar] [CrossRef]
- Abdel-Ghani, N.T.; El-Chaghaby, G.A.; ElGammal, M.H.; Rawash, E.A. optimizing the preparation conditions of activated carbons from olive cake using KOH activation. New Carbon Mater. 2016, 31, 2–10. [Google Scholar] [CrossRef]
- Yahya, M.A.; Al-Qodah, Z.; Ngah, C.W.Z. Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: A review. Renew. Sustain. Energy Rev. 2015, 46, 218–235. [Google Scholar] [CrossRef]
- Rafatullah, M.; Ahmad, T.; Ghazali, A.; Sulaiman, O.; Danish, M.; Hashim, R. Oil palm biomass as a precursor of activated carbons: A review. Crit. Rev. Environ. Sci. Technol. 2013, 43, 1117–1161. [Google Scholar] [CrossRef]
- Wirasnita, R.; Hadibarata, T.; Yusoff, A.R.M.; Mat Lazim, Z. Preparation and characterization of activated carbon from oil palm empty fruit bunch wastes using zinc chloride. J. Teknol. 2015, 74, 77–81. [Google Scholar] [CrossRef]
- Demiral, H.; Demiral, I.; Karabacakoĝlu, B.; Tümsek, F. Production of activated carbon from olive bagasse by physical activation. Chem. Eng. Res. Des. 2011, 89, 206–213. [Google Scholar] [CrossRef]
- Hussein, M.Z.; Abdul Rahman, M.B.; Yahaya, A.H.; Taufiq-Yap, Y.H.; Ahmad, N. Oil palm trunk as a raw material for activated carbon production. J. Porous Mater. 2001, 8, 327–334. [Google Scholar] [CrossRef]
- Yang, T.; Lua, A.C. Textural and chemical properties of zinc chloride activated carbons prepared from pistachio-nut shells. Mater. Chem. Phys. 2006, 100, 438–444. [Google Scholar] [CrossRef]
- Gao, Y.; Yue, Q.Y.; Sun, Y.Y.; Xiao, J.N.; Gao, B.Y.; Zhao, P.; Yu, H. Optimization of high surface area activated carbon production from enteromorpha prolifra with low-dose activating agent. Fuel Process. Technol. 2015, 132, 180–187. [Google Scholar] [CrossRef]
- Benaddi, H.; Legras, D.; Rouzaud, J.N.; Beguin, F. Influence of the atmosphere in the chemical activation of wood by phosphoric acid. Carbon 1998, 36, 306–309. [Google Scholar] [CrossRef]
- Herawan, S.G.; Hadi, M.S.; Ayob, M.R.; Putra, A. Characterization of activated carbons from oil-palm shell by CO2 activation with no holding carbonization temperature. Sci. World J. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Molina-Sabio, M.; Rodríguez-Reinoso, F. Role of chemical activation in the development of carbon porosity. Colloids Surf. A Physicochem. Eng. Asp. 2004, 241, 15–25. [Google Scholar] [CrossRef]
- Dolas, H.; Sahin, O.; Saka, C.; Demir, H. A new method on producing high surface area activated carbon: The effect of salt on the surface area and the pore size distribution of activated carbon prepared from pistachio shell. Chem. Eng. J. 2011, 166, 191–197. [Google Scholar] [CrossRef]
- Jagtoyen, M.; Derbyshire, F. Activated carbons from yellow poplar and white oak by H3PO4 activation. Carbon 1998, 36, 1085–1097. [Google Scholar] [CrossRef]
- Endo, M.; Iijima, S.; Dresselhaus, M.S. Carbon Nanotubes, 1st ed.; Endo, M., Iijima, S., Dresselhaus, M.S., Eds.; BPC Wheatons Ltd.: Exeter, UK, 1996. [Google Scholar]
- Sun, H.; She, P.; Lu, G. Recent advances in the development of functionalized carbon nanotubes: A versatile vector for drug delivery. J. Mater. Sci. 2014, 49, 6845–6854. [Google Scholar] [CrossRef]
- Sambasivudu, K.; Mahajan, Y.R. Challenges and opportunities for the mass production of high quality graphene: An analysis of worldwide patents. Nanotech Insights 2012, 3, 1–16. [Google Scholar]
- Lu, X.; Yu, M.; Huang, H.; Ruoff, R.S. Tailoring graphite with the goal of achieving single sheets. Nanotechnology 1999, 10, 269–272. [Google Scholar] [CrossRef]
- Ohta, T. Controlling the electronic structure of bilayer graphene. Science 2006, 313, 951–954. [Google Scholar] [CrossRef] [PubMed]
- Eda, G.; Fanchini, G.; Chhowalla, M. Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nat. Nanotechnol. 2008, 3, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Juang, Z.Y.; Wu, C.Y.; Lu, A.Y.; Su, C.Y.; Leou, K.C.; Chen, F.R.; Tsai, C.H. Graphene synthesis by chemical vapor deposition and transfer by a roll-to-roll process. Carbon 2010, 48, 3169–3174. [Google Scholar] [CrossRef]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquidphase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Panchakarla, L.S.; Rao, C.N.R. Laser-Induced unzipping of carbon nanotubes to yield graphene nanoribbons. Nanoscale 2011, 3, 2127–2129. [Google Scholar] [CrossRef] [PubMed]
- Nasir, S.; Hussein, M.Z.; Yusof, N.A.; Zainal, Z. Oil palm waste-based precursors as a renewable and economical carbon sources for the preparation of reduced graphene oxide from graphene oxide. Nanomaterials 2017, 7, 182. [Google Scholar] [CrossRef] [PubMed]
- Brodie, B.C.; Trans, P.; Lond, R.S. On the atomic weight of graphite. Philos. Trans. R. Soc. Lond. 1859, 149, 249–259. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Park, S.; Bielawski, W.; Ruoff, R.S. The Chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Todd, A.D.; Bielawski, C.W. Harnessing the chemistry of graphene oxide. Chem. Soc. Rev. 2014, 43, 5288–5301. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Cheng, H. The Reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Chem, J.M.; Bai, H.; Sheng, K.; Zhang, P.; Li, C.; Shi, G. Graphene oxide/conducting polymer composite hydrogels. J. Mater. Chem. 2011, 21, 18653–18658. [Google Scholar] [CrossRef]
- Fryczkowska, B.; Janicki, J.; Fryczkowski, R.; Gorczowska, M.; Czesław, S. The possibility of obtaining graphene/polymer composites from graphene oxide by a one step process. Compos. Sci. Technol. 2013, 80, 87–92. [Google Scholar] [CrossRef]
- Liao, L.; Lin, Y.; Bao, M.; Cheng, R.; Bai, J.; Liu, Y.; Qu, Y.; Wang, K.L. High-speed graphene transistors with a self-aligned nanowire gate. Nature 2010, 467, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Salimian, M.; Ivanov, M.; Deepak, L.; Petrovykh, D.Y.; Bdikin, I.; Ferro, M.; Kholkin, A.; Goncalves, G. Synthesis and characterization of reduced graphene oxide/spiky nickel nanocomposite for nanoelectronic applications. J. Mater. Chem. C 2015, 3, 11516–11523. [Google Scholar] [CrossRef]
- Primo, A.; Atienzar, P.; Sanchez, E. From biomass wastes to large-area, high-quality, n-doped graphene: Catalyst-free carbonization of chitosan coatings on arbitrary substrates. Chem. Commun. 2012, 48, 9254–9256. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Tian, C.; Li, M.; Meng, X.; Wang, L.; Wang, R.; Yin, J.; Fu, H. From coconut shell to porous graphene-like nanosheets for highpower supercapacitors supercapacitors. J. Mater. Chem. A 2013, 1, 6462–6470. [Google Scholar] [CrossRef]
- Suryawanshi, A.; Biswal, M.; Mhamane, D.; Gokhale, R.; Patil, S.; Guin, D.; Ogale, S. Large scale synthesis of graphene quantum dots (GQDs) from waste biomass and their use as an efficient and selective photoluminescence on-off-on probe for Ag+ ions. Nanoscale 2014, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Manukyan, K.V.; Rouvimov, S.; Wolf, E.E.; Mukasyan, A.S. Combustion synthesis of graphene materials. Carbon 2013, 62, 302–311. [Google Scholar] [CrossRef]
- Takami, T.; Seino, R.; Yamazaki, K.; Ogino, T. Graphene film formation on insulating substrates using polymer films as carbon. J. Phys. D Appl. Phys. 2014, 47, 094015. [Google Scholar] [CrossRef]
- Sharma, S.; Kalita, G.; Hirano, R.; Shinde, S.M.; Papon, R.; Ohtani, H.; Tanemura, M. Synthesis of graphene crystals from solid waste plastic by chemical vapor deposition. Carbon 2014, 72, 66–73. [Google Scholar] [CrossRef]
- Hu, Z.; Srinivasan, M.P.; Ni, Y. Novel activation process for preparing highly microporous and mesoporous activated carbons. Carbon 2001, 39, 877–886. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Preparation and characterization of activated carbon from sunflower seed oil residue via microwave assisted K2CO3 activation. Bioresour. Technol. 2011, 102, 9794–9799. [Google Scholar] [CrossRef] [PubMed]
- Tongpoothorn, W.; Sriuttha, M.; Homchan, P.; Chanthai, S.; Ruangviriyachai, C. Chemical engineering research and design preparation of activated carbon derived from Jatropha curcas fruit shell by simple thermo-chemical activation and characterization of their physico-chemical properties. Chem. Eng. Res. Des. 2010, 89, 335–340. [Google Scholar] [CrossRef]
- Gan, J.; Encinar, J.M.; Sabio, E.; Roma, S. Almond residues gasification plant for generation of electric power. Preliminary study. Fuel Process. Technol. 2006, 87, 149–155. [Google Scholar] [CrossRef]
- Boonpoke, A.; Chiarakorn, S.; Laosiripojana, N.; Towprayoon, S.; Chidthaisong, A. Investigation of CO2 adsorption by bagasse-based activated carbon. Korean J. Chem. Eng. 2012, 29, 89–94. [Google Scholar] [CrossRef]
- Ademiluyi, F.T.; Amadi, S.A.; Amakama, N.J. Adsorption and treatment of organic contaminants using activated carbon from waste nigerian bamboo. J. Appl. Sci. Environ. Manag. 2009, 133, 39–47. [Google Scholar] [CrossRef]
- Olowoyo, D.N.; Orere, E.E. Preparation and characterization of activated carbon made from palm-kernel shell, coconut shell, groundnut shell and obeche wood (investigation of apparent density, total ash content, moisture content, particle size distribution parameters). Int. J. Res. Chem. Environ. 2012, 2, 32–35. [Google Scholar]
- Cagnon, B.; Py, X.; Guillot, A.; Stoeckli, F. The effect of the carbonization/activation procedure on the microporous texture of the subsequent chars and active carbons. Microporous Mesoporous Mater. 2003, 57, 273–282. [Google Scholar] [CrossRef]
- Daud, W.M.; Ali, W.S. Comparison on pore development of activated carbon produced from palm shell and coconut shell. Bioresour. Technol. 2004, 93, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, L.; Moreno-Pirajan, J.C. Synthesis of activated carbon mesoporous from coffee waste and its application in adsorption zinc and mercury ions from aqueous solution. Eur. J. Chem. 2012, 9, 938–949. [Google Scholar] [CrossRef]
- Al-swaidan, H.M.; Ahmad, A. Synthesis and characterization of activated carbon from Saudi Arabian dates tree’s fronds wastes. In 2011 3rd International Conference on Chemical, Biological and Environmental Engineering (IPCBEE); IACSIT Press: Singapore, 2011; Volume 20, pp. 25–31. [Google Scholar]
- Gimba, C.E.; Salihu, A.A.; Kagbu, J.A.; Itodo, M.T.A.U.; Sariyya, A.I. Study of pesticide (dichlorvos) removal from aqueous medium by arachis hypogaea (groundnut) shell using GC/MS. World Rural Obs. 2010, 2, 1–9. [Google Scholar]
- Demiral, H.; Demiral, I.; Tumsek, F.; Karabecakoglu, B. Pore structure of activated carbon prepared from hazelnut bagasse by chemical activation pore structure of activated carbon prepared from hazelnut bagasse by chemical activation. Surf. Interface Anal. 2008, 40, 616–619. [Google Scholar] [CrossRef]
- Chowdhury, Z.Z.; Zain, S.M.; Khan, R.A.; Islam, S. preparation and characterizations of activated carbon from kenaf fiber for equilibrium adsorption studies of copper from wastewater. Korean J. Chem. Eng. 2012, 29, 1187–1195. [Google Scholar] [CrossRef]
- Akpen, G.D.; Leton, T.G.; Harcourt, P. Column studies on the removal of chromium from waste water by mango seed shell activated carbon. J. Sci. Technol. 2015, 35, 1–12. [Google Scholar] [CrossRef]
- Alau, K.K.; Gimba, C.E.; Kagbu, J.A. Removal of dyes from aqueous solution using neem (Azadirachta indica) husk as activated carbon. Arch. Appl. Sci. Res. 2010, 2, 456–461. [Google Scholar]
- Baccar, R.; Bouzid, J.; Feki, M.; Montiel, A. Preparation of activated carbon from tunisian olive-waste cakes and its application for adsorption of heavy metal ions. J. Hazard. Mater. J. 2009, 162, 1522–1529. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.Z.; Tarmizi, R.S.H.; Zainal, Z.; Ibrahim, R. Preparation and characterization of active carbons from oil palm shells. Carbon 1996, 34, 1447–1449. [Google Scholar] [CrossRef]
- Abechi, S.E.; Gimba, C.E.; Uzairu, A.; Dallatu, Y.A. Preparation and characterization of activated carbon from palm kernel shell by chemical activation. Res. J. Chem. Sci. 2013, 3, 54–61. [Google Scholar]
- Allwar, A.B.M.N.; Nawi, M.A.B.M. Textural characteristics of activated carbons prepared from oil palm shells activated with ZnCl2 and pyrolysis under nitrogen and carbon dioxide. J. Phys. Sci. 2008, 19, 93–104. [Google Scholar]
- Boonpoke, A.; Chiarakorn, S.; Laosiripojana, N.; Towprayoon, S.; Chidthaisong, A. Synthesis of activated carbon and MCM-41 from bagasse and rice husk and their carbon dioxide adsorption capacity. J. Sustain. Energy Environ. 2011, 2, 77–81. [Google Scholar]
- Gonzalez, J.F.; Roman, S.; Encinar, J.M.; Martinz, G. Pyrolysis of various biomass residues and char utilization for the production of activated carbons. J. Anal. Appl. Pyrolysis 2009, 85, 134–141. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Blake, P.; Hill, E.W.; Castro Neto, A.H.; Novoselov, K.S.; Jiang, D.; Yang, R.; Booth, T.J.; Geim, A.K. Making graphene visible. Appl. Phys. Lett. 2007, 91, 2007–2009. [Google Scholar] [CrossRef]
- Li, N.; Huang, X.; Zhang, H.; Li, Y.; Wang, C. Transparent and self-supporting graphene films with wrinkled-graphene-wall-assembled opening polyhedron building blocks for high performance flexible/transparent supercapacitors. Appl. Mater. Interfaces 2017, 9, 9763–9771. [Google Scholar] [CrossRef] [PubMed]
- Staudenmaier, L. Verfahren zur darstellung der graphitsäure. Eur. J. Inorg. Chem. 1898, 31, 1481–1487. (In German) [Google Scholar] [CrossRef]
- Dimiev, A.; Eigler, S. Graphene Oxide-Fundamentals and Applications, 1st ed.; Dimiev, A.M., Eigler, S., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2017. [Google Scholar]
- Chua, C.K.; Pumera, M. Chemical reduction of graphene oxide: A synthetic chemistry viewpoint. Chem. Soc. Rev. 2014, 43, 291–312. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Raccichini, R.; Varzi, A.; Passerini, S.; Scrosati, B. The role of graphene for electrochemical energy storage. Nat. Mater. 2014, 14, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Andre, V.P.; Han, D.; Shih, W.M.; Yan, H. Challenges and Opportunities for structural DNA nanotechnology. Nat. Nanotechnol. 2011, 6, 763–772. [Google Scholar] [CrossRef]
- Park, B. Current and Future Applications of Nanotechnology. Issues Environ. Sci. Technol. 2007, 24, 1–19. [Google Scholar] [CrossRef]
- Presting, H.; Ko, U. Future nanotechnology developments for automotive applications. Mater. Sci. Eng. C 2003, 23, 737–741. [Google Scholar] [CrossRef]
- Lackner, K.S. Comparative impacts of fossil fuels and alternative energy sources. In Issues in Environmental Science and Technology; Harrison, R.M., Hester, R.E., Eds.; Royal Society of Chemistry: London, UK, 2010; Volume 29, pp. 1–41. [Google Scholar]
- Kalyani, P.; Anitha, A. Biomass carbon & its prospects in electrochemical energy systems. Int. J. Hydrog. Energy 2013, 38, 4034–4045. [Google Scholar] [CrossRef]
- Candelaria, S.L.; Shao, Y.; Zhou, W.; Li, X.; Xiao, J.; Zhang, J.G.; Wang, Y.; Liu, J.; Li, J.; Cao, G. Nanostructured carbon for energy storage and conversion. Nano Energy 2012, 1, 195–220. [Google Scholar] [CrossRef]
- Adzic, R.R.; Zhang, J.; Sasaki, K.; Vukmirovic, M.B.; Shao, M.; Wang, J.X.; Nilekar, A.U.; Mavrikakis, M.; Valerio, J.A.; Uribe, F. Platinum monolayer fuel cell electrocatalysts. Top. Catal. 2007, 46, 249–262. [Google Scholar] [CrossRef]
- Fei, H.; Ye, R.; Ye, G.; Gong, Y.; Peng, Z.; Fan, X.; Samuel, E.L.G.; Ajayan, P.M.; Tour, J.M. Boron- and nitrogen-doped graphene quantum dots/graphene hybrid nanoplatelets as efficient electrocatalysts for oxygen reduction. ACS Nano 2014, 10, 10837–10843. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Amade, R.; Jover, E. Nitrogen plasma functionalization of carbon nanotubes for supercapacitor applications. J. Mater. Sci. 2013. [Google Scholar] [CrossRef]
- Fam, D.W.H.; Azoubel, S.; Liu, L.; Huang, J.; Mandler, D.; Magdassi, S.; Tok, A.I.Y. novel felt pseudocapacitor based on carbon nanotube/metal oxides. J. Mater. Sci. 2015, 50, 6578–6585. [Google Scholar] [CrossRef]
- Abbas, S.M.; Hussain, S.T.; Ali, S.; Ahmad, N.; Ali, N.; Abbas, S. Structure and electrochemical performance of ZnO/CNT composite as anode material for lithium-ion batteries. J. Mater. Sci. 2013, 48, 5429–5436. [Google Scholar] [CrossRef]
- Ambrosi, A.; Chua, C.K.; Bonanni, A.; Pumera, M. Electrochemistry of graphene and related materials. Chem. Rev. 2014, 114, 7150–7188. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Joshua, T.R.; Xiaoming, S.; Hongjie, D. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, X.; Chen, P. Biological and chemical sensors based on graphene materials. Chem. Soc. Rev. 2012, 41, 2283–2307. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Riechers, S.L.; Mellen, M.C.; Lin, Y. Sensitive electrochemical detection of enzymatically generated thiocholine at carbon nanotube modified glassy carbon electrode. Electrochem. Commun. 2005, 7, 1163–1169. [Google Scholar] [CrossRef]
- Kang, X.; Wang, J.; Wu, H.; Aksay, I.A.; Liu, J.; Lin, Y. Glucose oxidase-graphene-chitosan modified electrode for direct electrochemistry and glucose sensing. Biosens. Bioelectron. 2009, 25, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Zou, Z.; Shin, Y.; Wang, J.; Wu, H.; Engelhard, M.H.; Liu, J.; Aksay, L.A.; Lin, Y. Sensitive immunosensor for cancer biomarker based on dual signal amplification strategy of graphene sheets and multienzyme functionalized carbon nanospheres. Anal. Chem. 2010, 82, 2989–2995. [Google Scholar] [CrossRef] [PubMed]


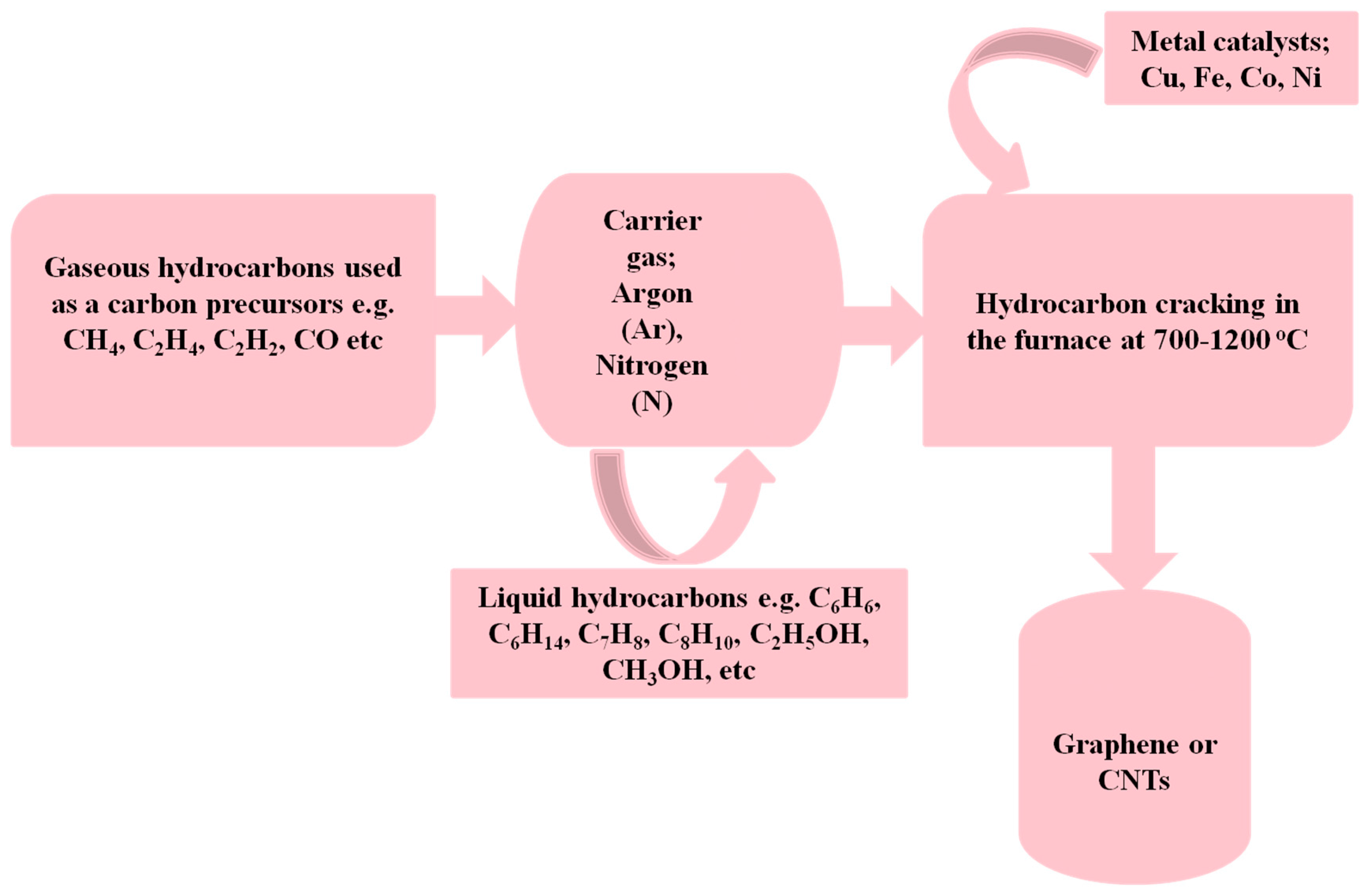
| Source/Precursors | Materials | Reaction Condition: Catalyst and Additives | Reactors | Product/Results | Reference |
|---|---|---|---|---|---|
| Biomass wastes | Leaf, chicken bone, baggase, wood, industrial soot, newspaper | Chemically derived method; H2SO4 | Not specified | rGO sheets | [82] |
| Biomass wastes | Crustacean skin wastes | Catalyst free | Unspecified | Monolayer N-doped-graphene: large size, 99% transmittance | [125] |
| Biomass wastes | Coconut shell | FeCl3 and ZnCl2 | Chemical vapor deposition., the tube was not specified | PGNs: highly interconnected porous structure, good energy density, large surface area, capacitance | [126] |
| Biomass wastes | Grass blades, dog feces, cockroach legs, waste cookies and chocolate | Cu foil | Quartz tube | Monolayer graphene: high quality, low defects, 97% transmittance | [84] |
| Biomass wastes | Dead neam leaves | Pyrolysis in a tube furnace, post-treated with chemical solutions | GQDs: incredible florescence, biocompatibility, size effect on band gap | [127] | |
| Waste plastics | PTFE (SiC) | Catalysts free. The synthetic pathway used to produce graphene does not require an external energy source. It took place in a self-sustained synergistic way. | High-pressure stainless steel reactor | Graphene sheets coated on porous carbon particles with large accessible surface area; with a 28% carbon yield | [128] |
| Waste plastics | PPMA; sapphire (11–20) substrates as a carbon source | Pyrolysis; Cu thin layer | CVD, tube not specified | Thin films of graphene | [129] |
| Solid waste plastics | PE (86%)–PS (14%) | Cu foil; Ambient pressure (AP) CVD process | AP-CVD system with a quartz tube | Lower rate of pyrolysis and injection; higher rate of injection: Large hexagonal shaped single graphene crystal; bilayer or multilayer graphene, respectively. | [130] |
| S/N | Precursors/Raw Materials | Carbonization Atmosphere | Activation Conditions | Chemical Agents | Supplementary Explanation | References |
|---|---|---|---|---|---|---|
| 1 | Almond tree pruning and Almond shell | N2, 600 °C/1 h | 850 °C/30 min | Steam | The diluted steam was physically in touch with the biochars accordingly | [134] |
| 2 | Bagasse | N2, 500 °C/1 h | N/A | ZnCl2 | Single step carbonization-activation, impregnation | [135] |
| 3 | Bamboo | N2, 400–500 °C/2 h | 800 °C/2 h | HCl | Impregnated with 0.1 M HCl | [136] |
| 4 | Coconut shell | N2, 250–750 °C/1 h | 500–900 °C/15 min | K2CO3 | Chemically mediated activation, impregnation ratio 1:1 | [137] |
| 5 | Coconut shell | N2, 400–800 °C/1 h | 800 °C/60–270 min | Steam | Chars get in touch with N2 and H2O afterward | [138] |
| 6 | Coconut shell | N2, 850 °C/1 h | 850 °C/5–80 min | CO2 | One step Pyrolysis/activation | [139] |
| 7 | Coffee waste | N2, 700 °C | 700 °C/2–3 h | CO2/ZnCl2 and KOH | Heating rate of 10 °C/min; Impregnation ratio 2:1 to 3:1 | [140] |
| 8 | Date tree frond | N2, 400 °C/3 h | N/A | H3PO4 | Single step carbonization-activation | [141] |
| 9 | Ground nut shell | N2, 800 °C/5 min | N/A | ZnCl2 | One step and two step activation, respectively | [142] |
| 10 | Ground nut shell | N2, 800 °C/5 min | N/A | H3PO4 | One step and two step activation, respectively | [142] |
| 11 | Ground nut shell | N2, 800 °C/5 min | N/A | KOH | Both one step and two step activation | [142] |
| 12 | Hazelnut Baggase | N2, 500–700 °C/2h | N/A | ZnCl2 | One step carbonization/activation | [143] |
| 13 | Hazelnut Baggase | N2, 500–700 °C/2 h | N/A | KOH | One step carbonization/activation | [143] |
| 14 | Kenaf Fibre | N2, 400 °C/2 h | 700 °C/1 h | CO2/KOH | Impregnation of the char was done via KOH at 1:4 ratio | [144] |
| 15 | Mango seed shell | N2, 500 °C/1 h | N/A | ZnCl2 | One step carbonization-activation, impregnation | [145] |
| 16 | Neem Husk | N2, 200–500 °C/10 min | N/A | KOH | One step carbonization-activation, most favorable at 350 | [146] |
| 17 | Olive waste cake | N2, 350–650 °C/2 h | N/A | H3PO4 | single step carbonization-activation | [147] |
| 18 | Oil palm shell | N2, 500 °C/3 h; CO2/1 h | 500 °C/1 h | ZnCl2/CO2 | Chemical activation coupled by physical activation; N2, gas was later replaced by flowing CO2 gas for one hour. | [148] |
| 19 | Palm kernel shell | N2, 400 °C/1 h | 800–1000 °C; 15–40 min | KOH | Carbonization followed by impregnation for 2 h | [149] |
| 20 | Palm shell | N2, 400–800 °C/3 h | 400–800 °C/90 min | CO2/ZnCl2 | Physical activation, 65% ZnCl2 | [150] |
| 21 | Palm oil trunk | N2, 500 °C/3 h; CO2/1 h | 500 °C/1 h | H3PO4/CO2 | The ratio of the acid to the precursor of 0.9 was used, followed by carbonization and activation using CO2 | [97] |
| 22 | Rice husk | N2, 500 °C/1 h | N/A | ZnCl2 | One step carbonization-activation, impregnation | [151] |
| 23 | Walnut shell | N2, 600 °C/1 h | 850 °C/30 min | Steam | Chars were subsequently in contact with diluted steam | [152] |
| Carbon Nanomaterials | Dimensions | Hybridization | Experimental Specific Surface Area (m2 g−1) | Thermal Conductivity (W m−1 K−1) | Electrical Conductivity (S cm−1) | Tenacity | Hardness |
|---|---|---|---|---|---|---|---|
| Graphite | 3 | sp2 | ~10–20 | Anisotropic: 1500–2000, 5–10 | Anisotropic: 2–3 × 104 | Flexible, non-elastic | High |
| Graphene | 2 | sp2 | ~1500 | 4840–5300 | ~2000 | Flexible, elastic | Uppermost (for single layer) |
| Carbon nanotube | 1 | mostly sp2 | ~1300 | 3500 | Structure-dependent | Flexible, elastic | High |
| Fullerene | 0 | mostly sp2 | 80–90 | 0.4 | 10−10 | Elastic | High |
| Properties | Graphite | Diamond |
|---|---|---|
| Crystal system and form | Hexagonal; substantial lamellar veins and earthy masses | Isometric; cubes and octahedrons |
| Specific Gravity | 2.2 | 3.5 |
| Density (g/cm3) | 2.25 | 3.52 |
| Color/Appearance | Grey black, Black silver, opaque shiny | Variable-pale yellows, browns, grays, and also white, blue, black, reddish, greenish, colorless and sparkling |
| Hardness (Mohs)/Field indicator | 1–2; Soft, slippery, soapy, greasy luster, density and streak | 10; Very Hard (a hardest substance known) |
| Luster | Metallic to dull | Adamantine to waxy |
| Cleavage | Perfect in 1 direction | Perfect in 4 directions forming octahedrons |
| Transparency | Crystals are opaque | Crystals are transparent to translucent in rough crystals |
| Fracture | Flaky | Conchoidal |
| Electrical and Heat conductivity (E&H) | Good conductor of both E&H | Poor electrical conductor; good thermal conductor |
| Burning in the air | At about 700 °C | Most readily at about 900 °C |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasir, S.; Hussein, M.Z.; Zainal, Z.; Yusof, N.A. Carbon-Based Nanomaterials/Allotropes: A Glimpse of Their Synthesis, Properties and Some Applications. Materials 2018, 11, 295. https://doi.org/10.3390/ma11020295
Nasir S, Hussein MZ, Zainal Z, Yusof NA. Carbon-Based Nanomaterials/Allotropes: A Glimpse of Their Synthesis, Properties and Some Applications. Materials. 2018; 11(2):295. https://doi.org/10.3390/ma11020295
Chicago/Turabian StyleNasir, Salisu, Mohd Zobir Hussein, Zulkarnain Zainal, and Nor Azah Yusof. 2018. "Carbon-Based Nanomaterials/Allotropes: A Glimpse of Their Synthesis, Properties and Some Applications" Materials 11, no. 2: 295. https://doi.org/10.3390/ma11020295
APA StyleNasir, S., Hussein, M. Z., Zainal, Z., & Yusof, N. A. (2018). Carbon-Based Nanomaterials/Allotropes: A Glimpse of Their Synthesis, Properties and Some Applications. Materials, 11(2), 295. https://doi.org/10.3390/ma11020295