Effect of Air Abrasion on the Number of Particles Embedded in Zironia
Abstract
:1. Introduction
2. Materials and Methods
- size of grain: 60, 110, 250 μm,
- working pressure: 0.2, 0.35 MPa.
Statistical Analyses
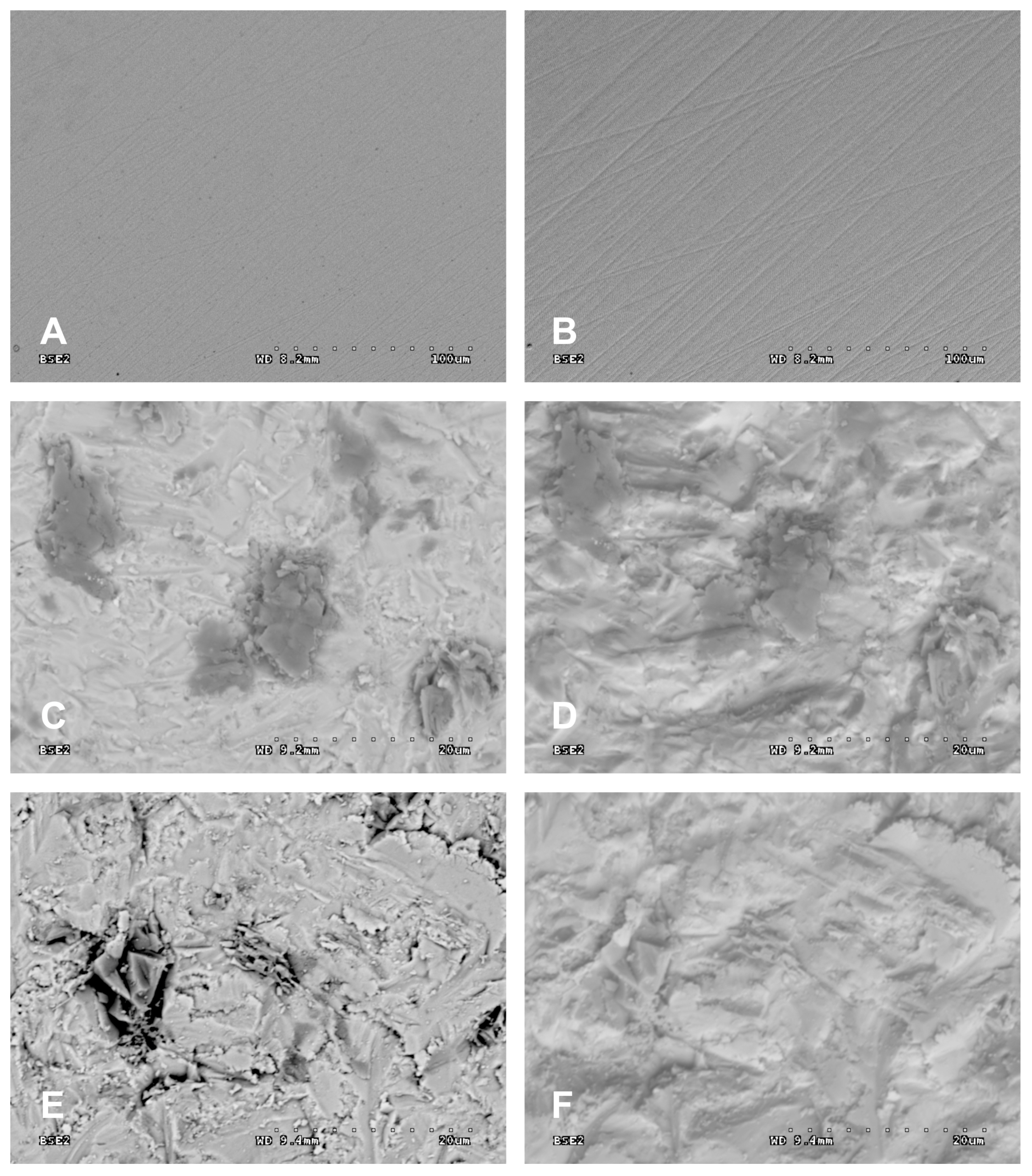
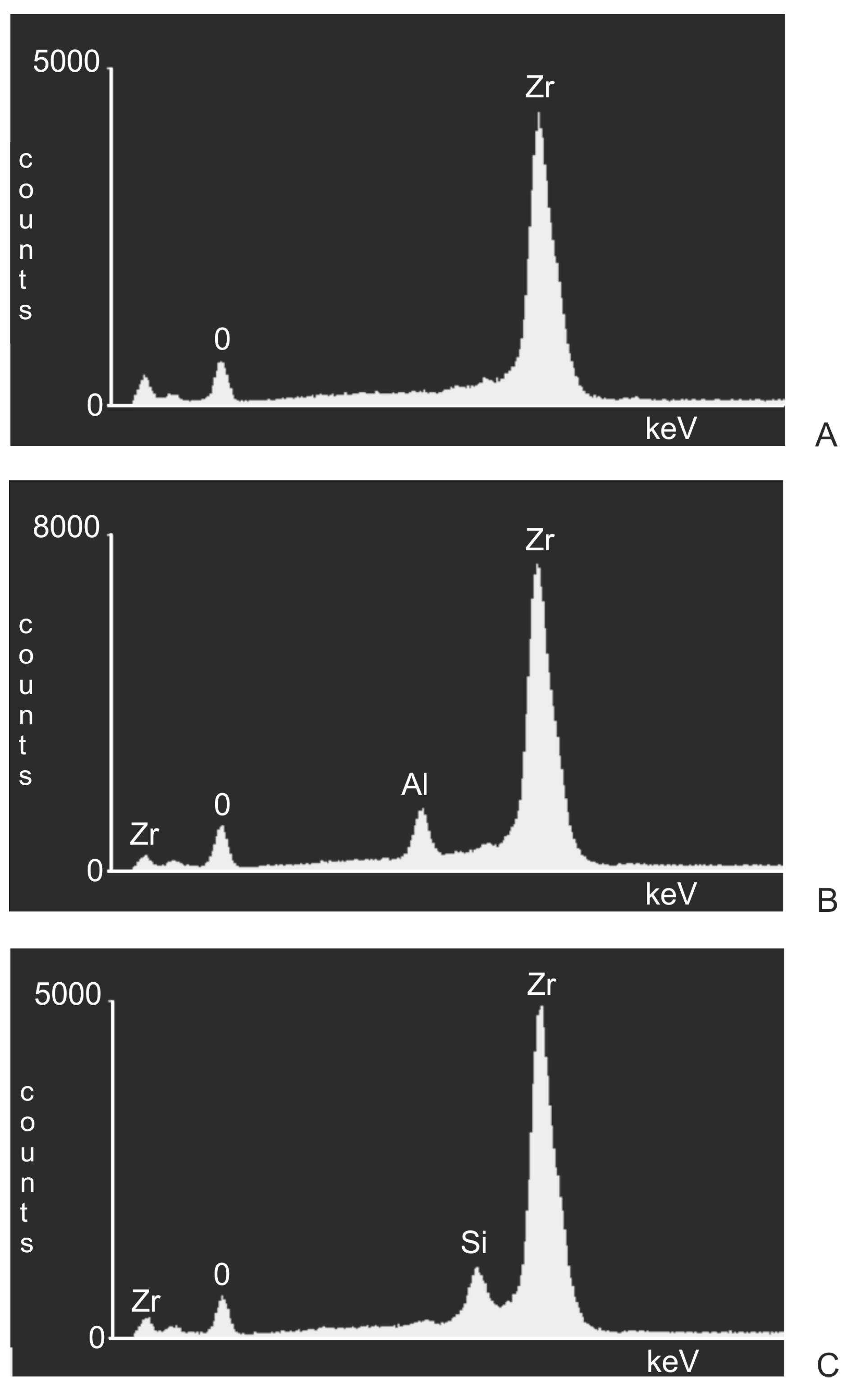
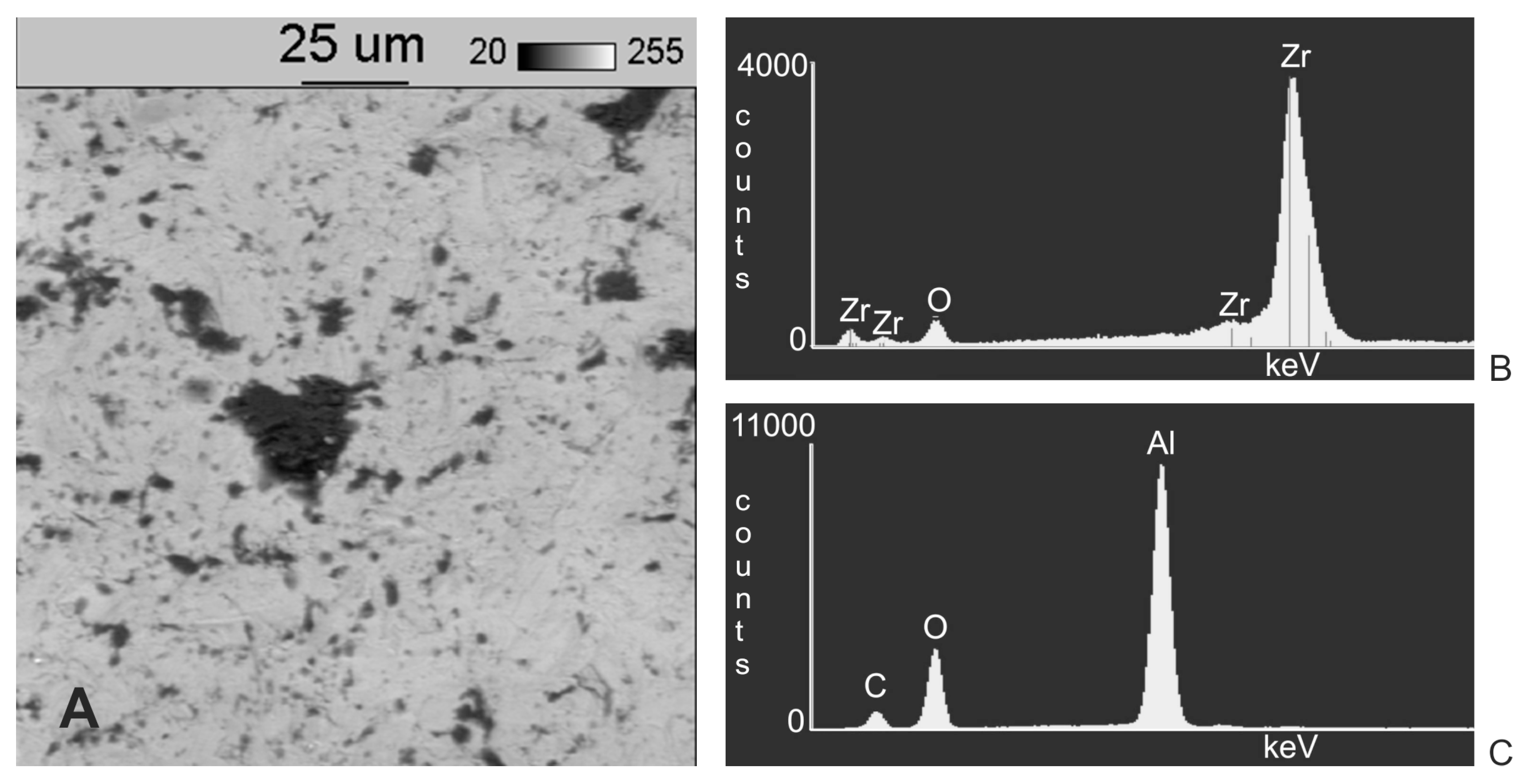
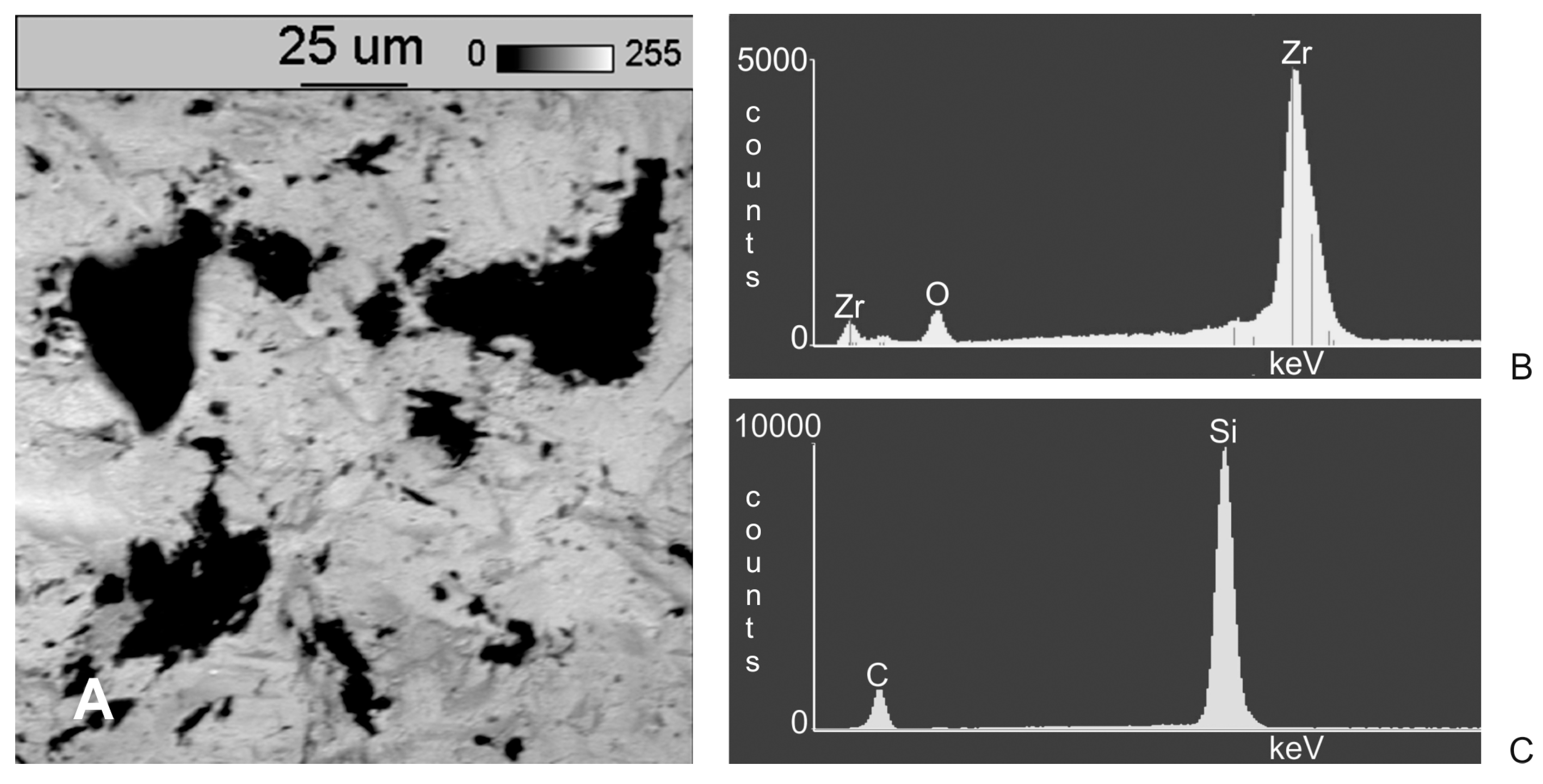
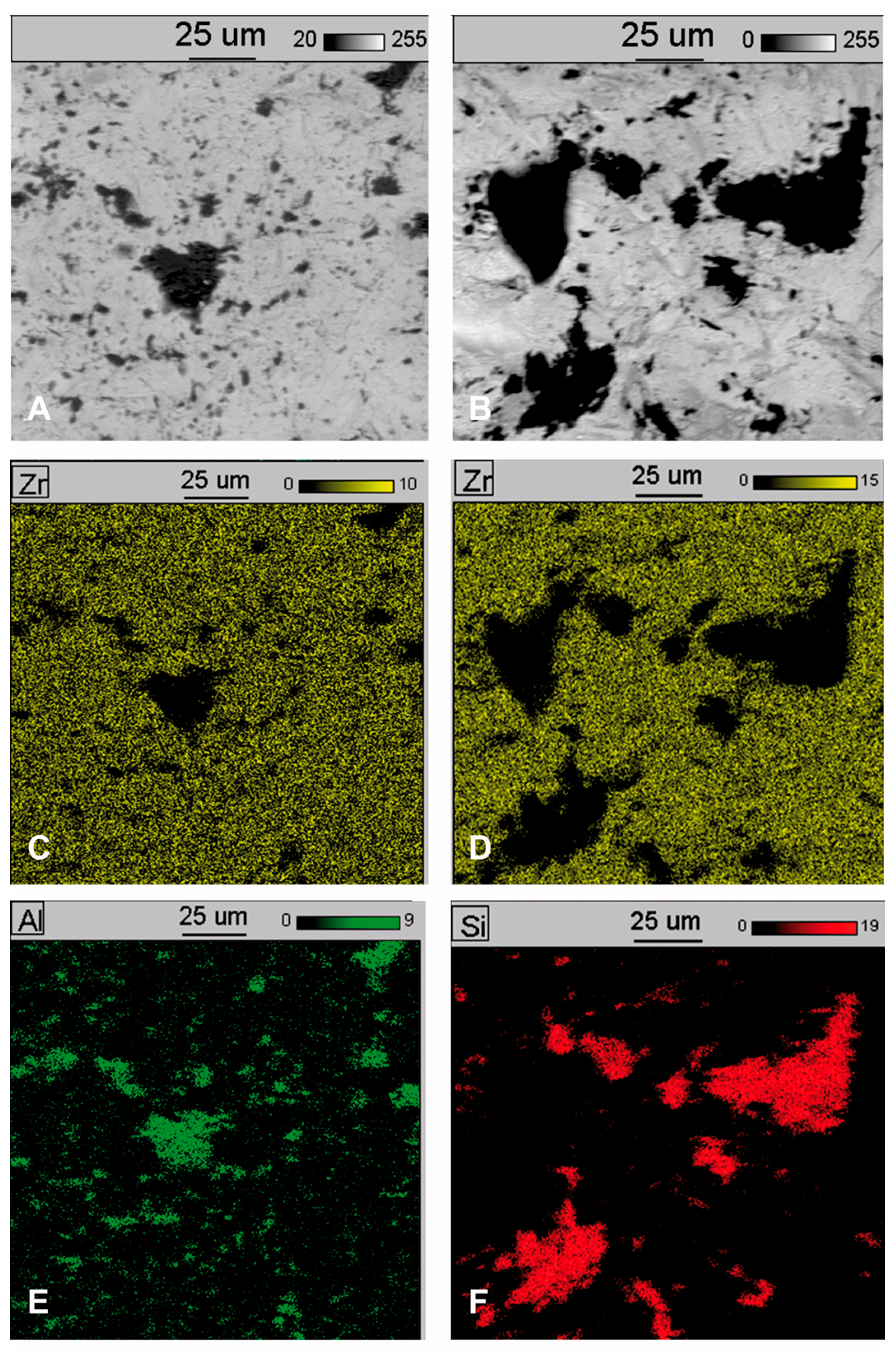
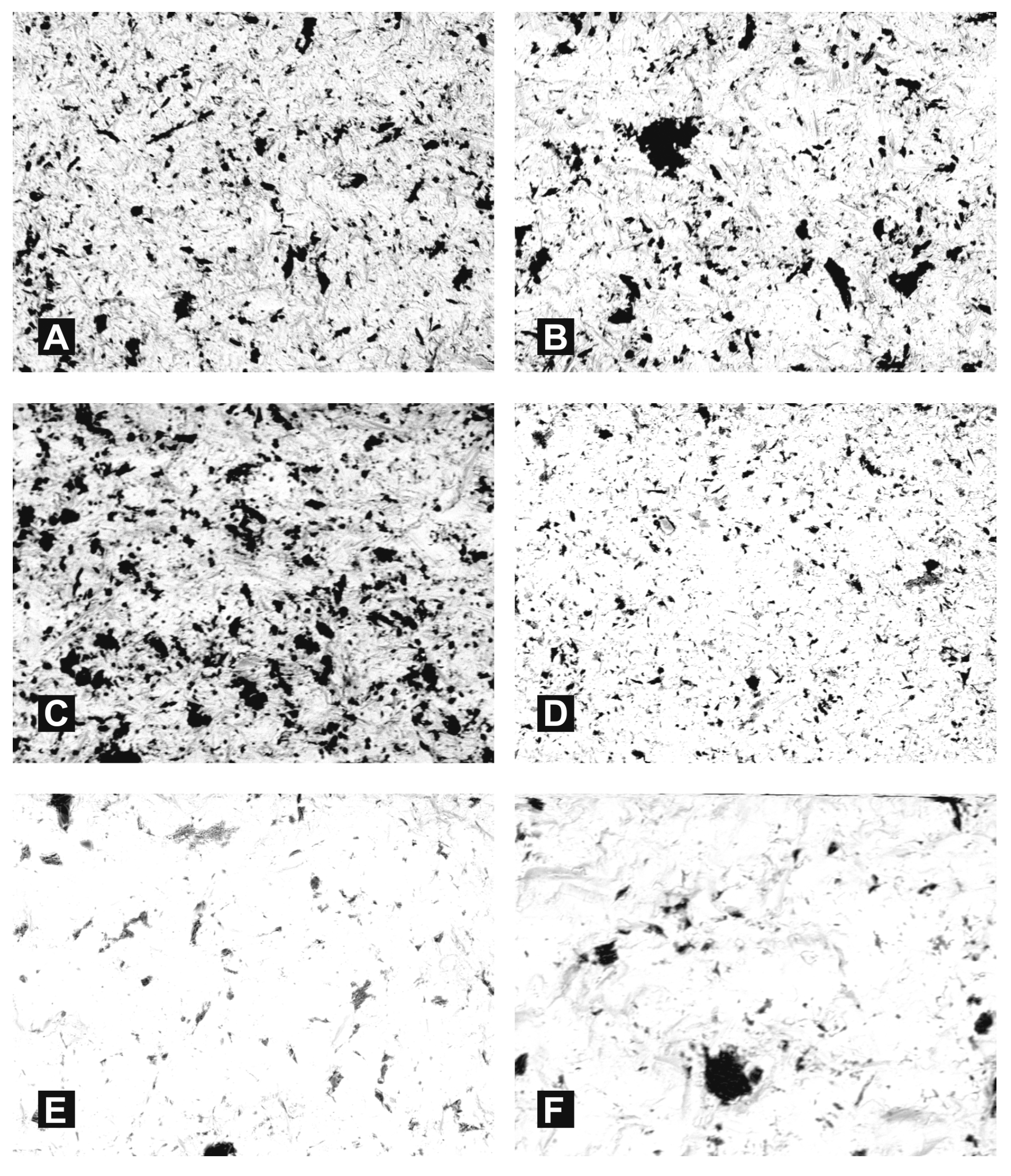
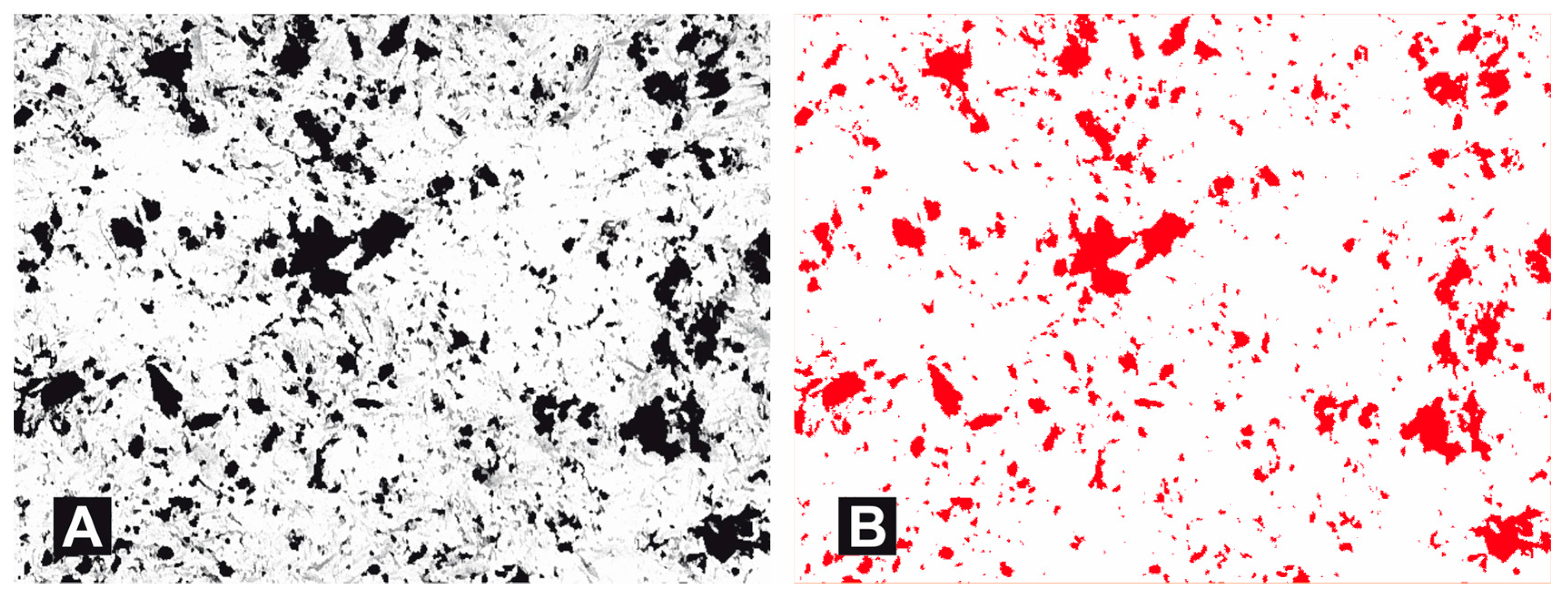
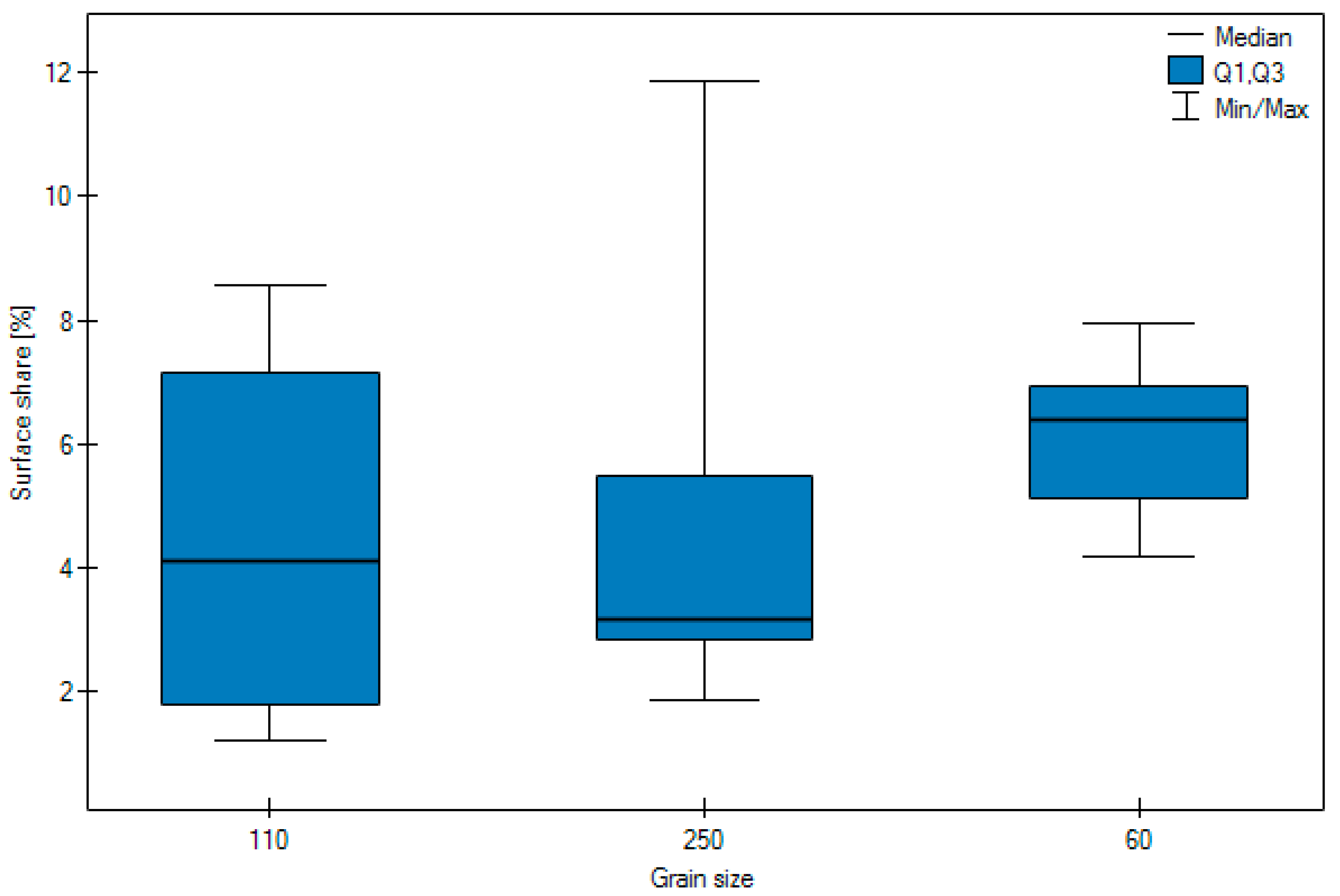
3. Results
4. Discussion
5. Conclusions
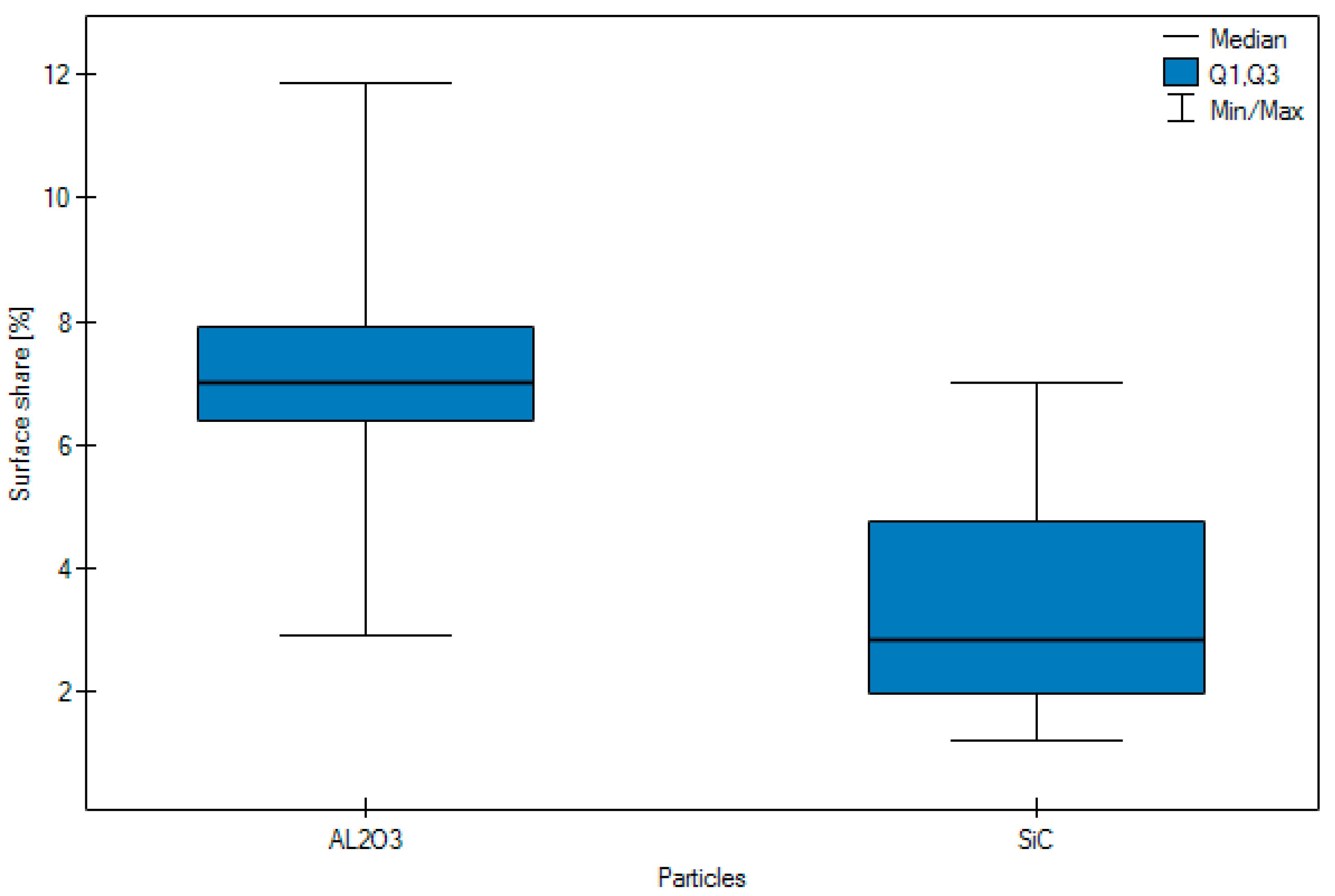
- Following abrasive blasting treatment aluminium oxide and silicon particles were observed embedded in the treated surface of the zirconia.
- A larger quantity of embedded abrasive was observed after blasting with Al2O3 than after blasting with SiC.
- Most particles of abrasive material became embedded when blasting with size 110 grain.
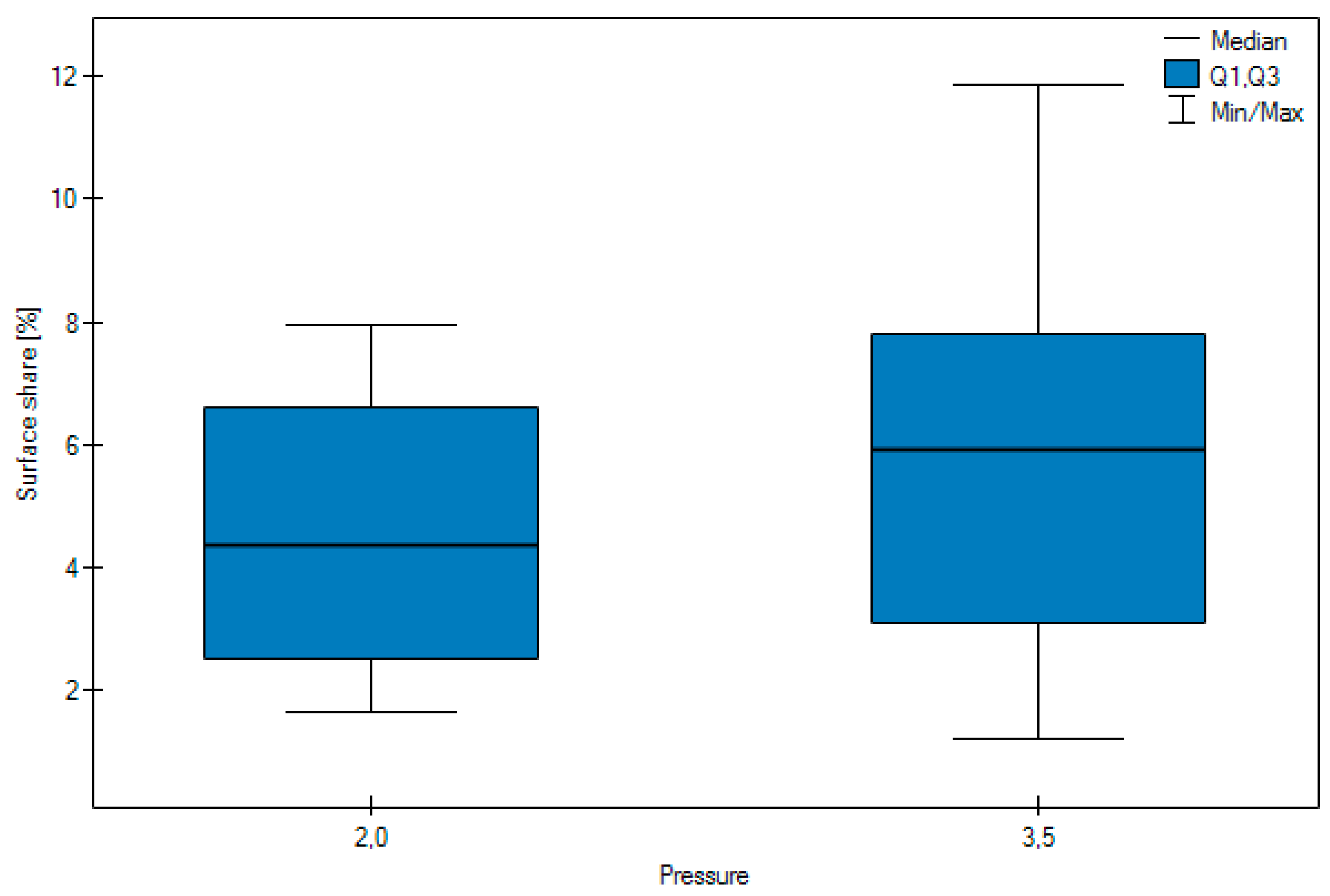
- No significant difference was observed in the surface share of the abrasive depending on the pressure.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gołębiowski, M.; Sobczyk-Guzenda, A.; Szymański, W.; Klimek, L. Influence of parameters of stream abrasive treatment of titanium surfaces on contact angle and surface free energy. Mater. Eng. 2010, 31, 978–980. [Google Scholar]
- Hautaniemi, J.A.; Hero, H.; Juhanoja, J.T. On the bonding of porcelain on titanium. J. Mater. Sci. 1992, 3, 186–191. [Google Scholar] [CrossRef]
- Hofstede, T.M.; Ercoli, C.; Graser, G.N.; Tallents, R.H.; Moss, M.E.; Zero, D.T. Influence of metal surface finishing on porcelain porosity and beam fail loads at the metal-ceramic interface. J. Prosthet. Dent. 2000, 84, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Luthardt, R.G.; Holzhhuter, M.; Sandkuhl, M.O.; Herold, V.; Schnapp, J.D.; Kuhlisch, E.; Walter, M. Reliability and properties of ground Y-TZP zircon ceramics. J. Dent. Res. 2002, 81, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Blatz, M.B.; Sadan, A.; Kern, M. Resin–ceramic bonding: A review of the literature. J. Prosthet. Dent. 2003, 89, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, M.; Vallittu, P.K. Effect of surface conditioning methods on the bond strength of luting cement to ceramics. Dent. Mater. 2003, 19, 725–731. [Google Scholar] [CrossRef]
- Vanni, L.; Valter, S. Low temperature degradation-aging of zirconia: Critical review of relevant aspects in dentistry. Dent. Mater. 2010, 26, 807–820. [Google Scholar]
- Tada, K.; Sato, T.; Yoshinari, M.C. Influence of surface treatment on bond strength of veneering ceramics fused to zirconia. Dent. Mater. 2012, 31, 287–296. [Google Scholar] [CrossRef]
- Stawarczyk, B.; Ozcan, M.; Roos, M.; Trottmann, A.; Hämmerle, C.H. Fracture load and failure analysis of zirconia single crowns veneered with pressed and layered ceramics after chewing simulation. Dent. Mater. J. 2011, 30, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Kosmac, T.; Oblak, C.; Jevnikar, P.; Funduk, P.; Marion, L. Strength and reliability of surface treated Y-TZP dental ceramics. J. Biomed. Mater. Res. 2000, 53, 304–313. [Google Scholar] [CrossRef]
- Zhang, Y.; Pajares, A.; Lawn, B.R. Fatigue and damage tolerance of Y-TZP ceramics in layered biomechanical systems. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 71, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Aboushelib, M.N.; Kleverlaan, C.J.; Feilzer, A.J. Microtensile bond strength of different components of core veneered all-ceramic restorations. Part II. Zirconia veneering ceramics. Dent. Mater. 2006, 22, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Aboushelib, M.N. Microtensile bond strength and impact energy of fracture of CAD-vennered zirconia restorations. J. Prosthodont. 2009, 21, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Denry, I.; Kelly, J.R. State of the art of zirconia for dental applications. Dent. Mater. 2008, 24, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Kosmac, T.; Oblak, C.; Jevnikar, P.; Funduk, N.; Marion, L. The effect of surface grinding and sandblasting on flexural strength and reliability of Y-TPZ zirconia ceramic. Dent. Mater. 1999, 15, 426–433. [Google Scholar] [CrossRef]
- Huang, H. Machining characteristics and surface integrity of yttria stabilized tetragonal zirconia in high speed deep grinding. Mater. Sci. Eng. A 2003, 345, 155–163. [Google Scholar] [CrossRef]
- Guzzato, M.; Albakry, M.; Ringer, S.P.; Swain, M.V. Strenght, fracture, toughness and microstructure of a selection of all-ceramic materials. Part II. Zirconia-based dental ceramics. Dent. Mater. 2004, 20, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Amaral, M.; Valandro, L.F.; Bottino, M.A.; Souza, R.O. Low-temperature degradation of a Y-TZP ceramic after surface treatments. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.L.; Covey, D.L.; Lautenschlager, E.P. Bond characteristic of porcelain fused to milled titanium. Dent. Mater. 1994, 10, 134–140. [Google Scholar] [CrossRef]
- Burnat, B.; Walkowiak-Przybył, M.; Błaszczyk, T.; Klimek, L. Corrosion behaviour of polished and sandblasted titanium alloys in PBS solution. Acta Bioeng. Biomech. 2013, 15, 87–95. [Google Scholar] [PubMed]
- Van Niekerk, A.J.; Caputa, T. Modern Prosthetic Restorations in Art and Craft. Selected Texts, 3rd ed.; Company Elamed: Katowice, Poland, 2007; pp. 228–234. [Google Scholar]
- Szala, J. Application of computer picture analysis methods to quantitative assessment of structure in materials. Sci. J. Silesian Univ. Technol. Ser. Metall. 2008, 82, 34–42. [Google Scholar]
- Rzychoń, T.; Kiełbus, A.; Lityńska-Dobrzyńska, L. Microstructure, microstructural stability and mechanical properties of sand-cast Mg-4Al-4RE alloy. Mater. Charact. 2013, 83, 21–34. [Google Scholar] [CrossRef]
- Pytel, M.; Góral, M.; Nowotnik, A. The porosity assessment of ceramic topcoat in thermal barrier coatings deposited by aps method. Adv. Manuf. Sci. Technol. 2016, 40, 53–65. [Google Scholar]
- Bednarczyk, I.; Kuc, D.; Tomaszewska, A.; Mrugała, A. The Influence of Extrusion Process on the Microstructure and Mechanical Properties of Magnesium Alloys. Arch. Metall. Mater. 2017, 62, 545–550. [Google Scholar] [CrossRef]
- Kościelniak, B.; Roskosz, S.; Cwajna, J. Evaluation of carbides in turbine blade made of IN713C superalloy after hot isostatic pressing. Arch. Metall. Mater. 2017, 62, 257–262. [Google Scholar] [CrossRef]
- Kuc, D.; Szala, J.; Bednarczyk, I. Quantitative evaluation of the microstructure and mechanical properties of hot rolled 23MnB4 steel grade for cold upsetting. Arch. Metall. Mater. 2017, 62, 551–556. [Google Scholar] [CrossRef]
- Pietnicki, K.; Wołowiec, E.; Klimek, L. Modeling of the number of stubble stuck elements after abrasive jet machining-processing. Arch. Foundry Eng. 2011, 11, 51–54. [Google Scholar]

| Descriptive Statistics | Particles | Pressure (Mpa) | Grain Size (μm) | ||||
|---|---|---|---|---|---|---|---|
| Al2O3 | SiC | 0.2 | 0.35 | 250 | 110 | 60 | |
| Arithmetic mean | 7.10 | 3.29 | 4.56 | 5.83 | 4.99 | 4.51 | 6.085 |
| Median | 7.02 | 2.84 | 4.35 | 5.91 | 3.17 | 4.10 | 6.385 |
| Standard deviation | 2.34 | 1.59 | 2.06 | 3.21 | 3.62 | 2.78 | 1.0344 |
| Minimum | 2.93 | 1.21 | 1.63 | 1.21 | 1.86 | 1.21 | 4.19 |
| Maximum | 11.86 | 7.01 | 7.94 | 11.86 | 11.86 | 8.57 | 7.94 |
| Lower Quartile < 0 | 6.38 | 1.96 | 2.52 | 3.08 | 2.84 | 1.80 | 5.1175 |
| Upper quartile | 7.91 | 4.75 | 6.61 | 7.79 | 5.49 | 7.14 | 6.945 |
| Test statistics | Z = 7.9545 | Z = 1.9525 | H = 9.8445 | ||||
| p | p < 0.0001 | p = 0.0509 | p = 0.0073 | ||||
| Dunn’s test (post-hoc) | Grain size (μm) | 250 | 0.7237 | 0.0116 | |||
| 110 | 0.7237 | 0.0040 | |||||
| 60 | 0.0116 | 0.0040 | |||||
| Post-Hoc | Grain Size (μm) | Grain Size (μm) | ||
|---|---|---|---|---|
| 250 | 110 | 60 | ||
| Dunn-Bonferroni correction (post-hoc) | 250 | 10,000 | 0.0349 | |
| 110 | 10,000 | 0.0121 | ||
| 60 | 0.0349 | 0.0121 | ||
| Dunn-Sidak correction (post-hoc) | 250 | 0.9789 | 0.0345 | |
| 110 | 0.9789 | 0.0120 | ||
| 60 | 0.0345 | 0.0120 | ||
| Conover-Iman test (post-hoc) | 250 | 0.7150 | 0.0102 | |
| 110 | 0.7150 | 0.0035 | ||
| 60 | 0.0102 | 0.0035 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Śmielak, B.; Klimek, L. Effect of Air Abrasion on the Number of Particles Embedded in Zironia. Materials 2018, 11, 259. https://doi.org/10.3390/ma11020259
Śmielak B, Klimek L. Effect of Air Abrasion on the Number of Particles Embedded in Zironia. Materials. 2018; 11(2):259. https://doi.org/10.3390/ma11020259
Chicago/Turabian StyleŚmielak, Beata, and Leszek Klimek. 2018. "Effect of Air Abrasion on the Number of Particles Embedded in Zironia" Materials 11, no. 2: 259. https://doi.org/10.3390/ma11020259
APA StyleŚmielak, B., & Klimek, L. (2018). Effect of Air Abrasion on the Number of Particles Embedded in Zironia. Materials, 11(2), 259. https://doi.org/10.3390/ma11020259





