Functionalizable Sol-Gel Silica Coatings for Corrosion Mitigation
Abstract
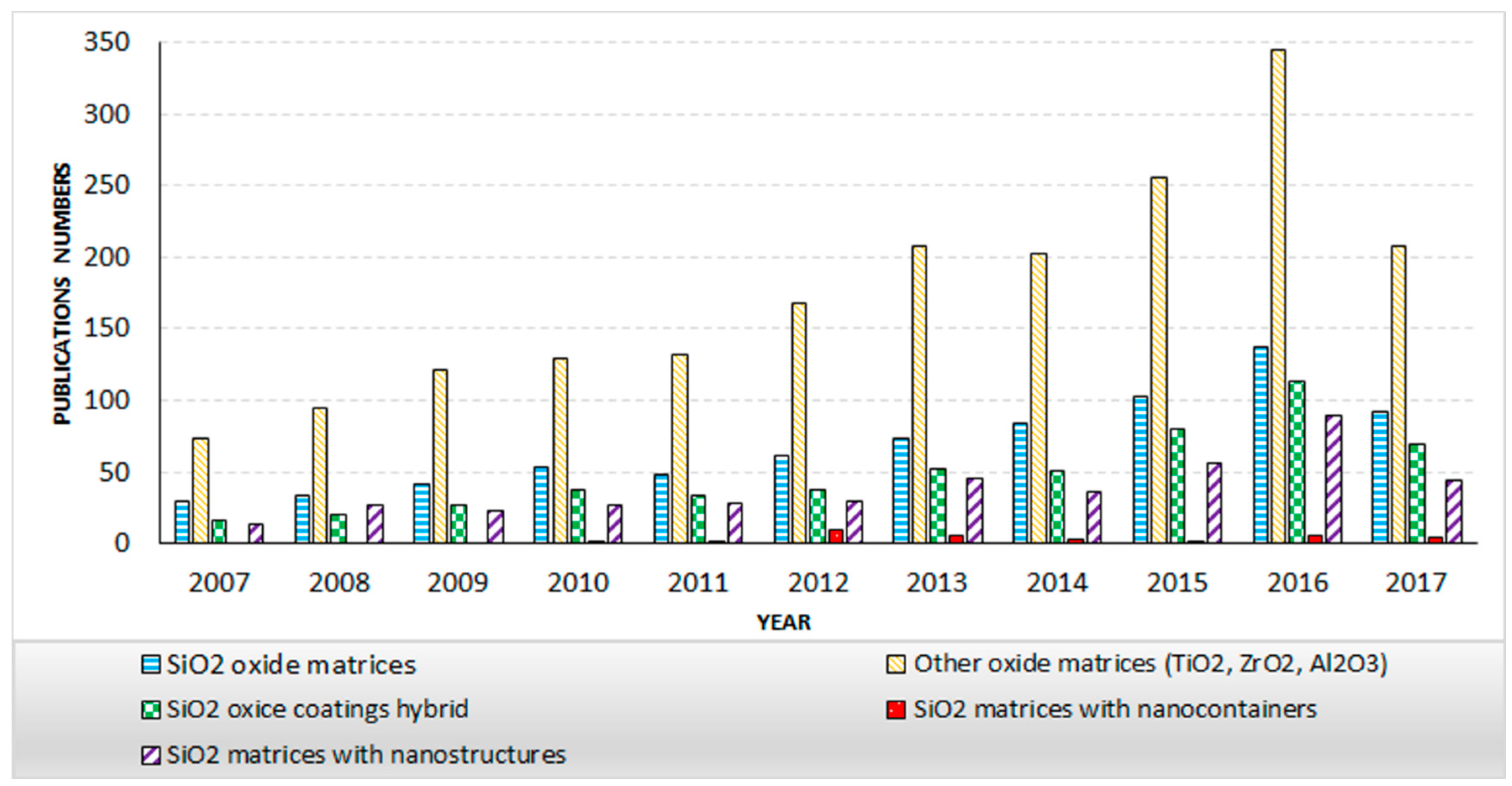
1. Introduction
2. Sol-Gel Method and the Coatings
2.1. Sol-Gel Method
2.2. Coatings
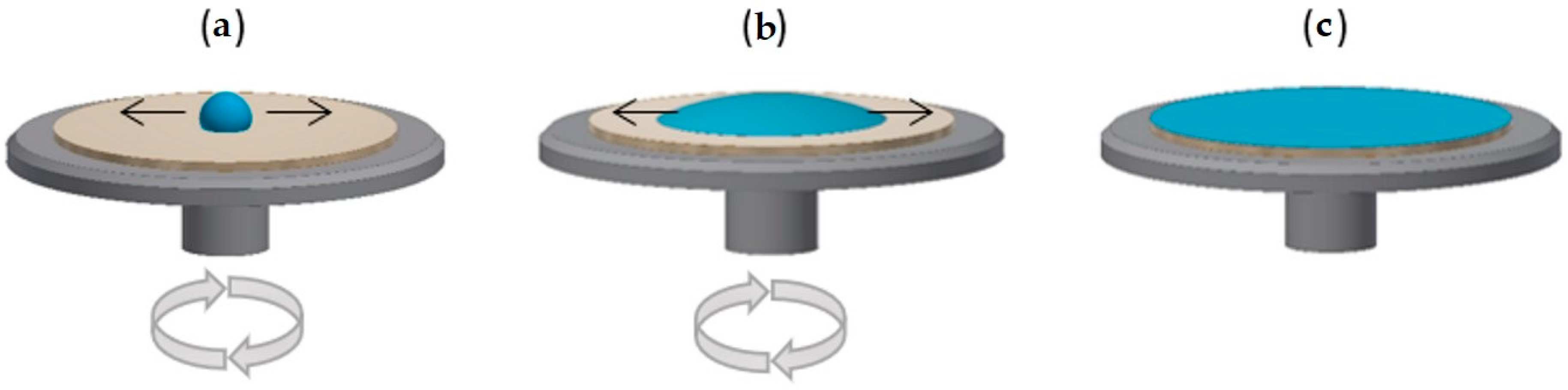
2.2.1. Methods of Obtaining Layered Surfaces
- (1)
- When the liquid viscosity, η and withdrawal speed, U0, are high, such that the curvature of the gravitational meniscus is low, the thickness of the coating layer, h0 can be defined according to the following equation:where ρ is liquid density, g is acceleration of gravity, c1 is constant.h0 = c1(ηU0/ρg)1/2
- (2)
- When the liquid viscosity, η and withdrawal speed, U0, are low, the equation is balanced by considering the liquid-vapour surface tension, γLV and the thickness of the coating layer, h0, can then be defined according to the following equation:
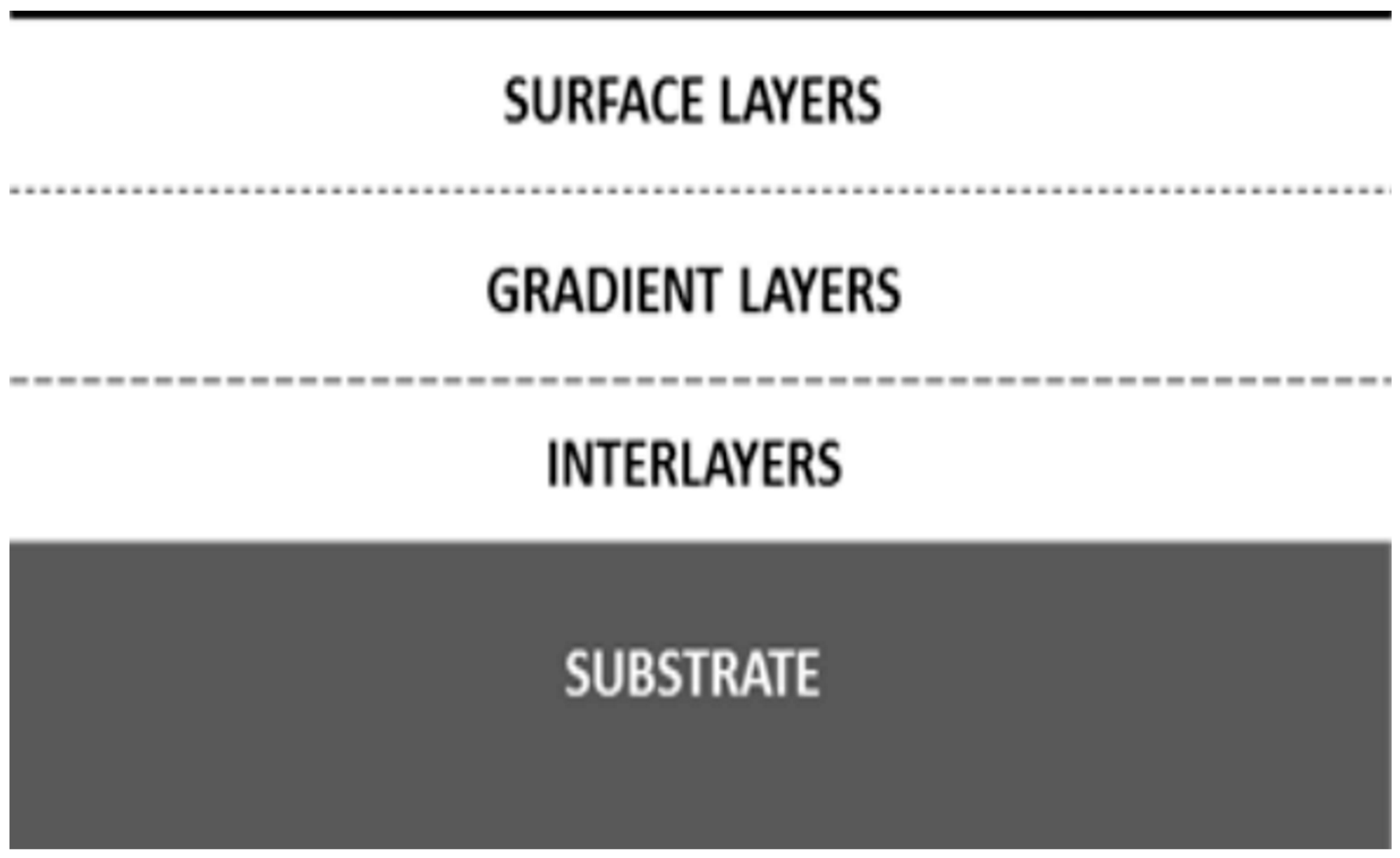
2.2.2. Interlayers
2.2.3. Gradient Layers-Organic-Inorganic Hybrid Coating Materials
2.2.4. Surface Layers
3. Investigative Techniques
3.1. Microscopy
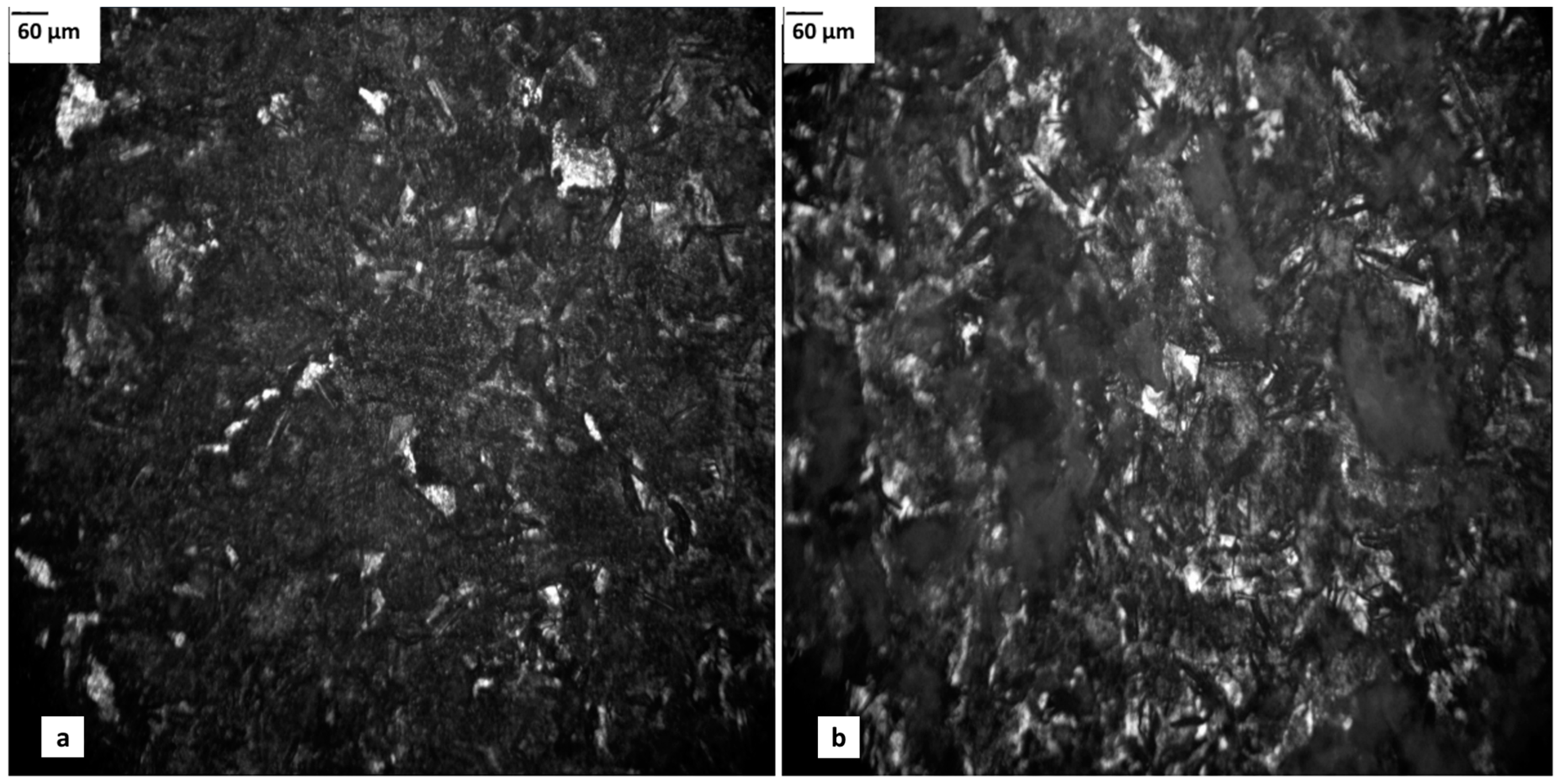
3.1.1. Light Microscopy (LM)-Metallographic Microscopy (MM)
3.1.2. Atomic Force Microscopy (AFM)
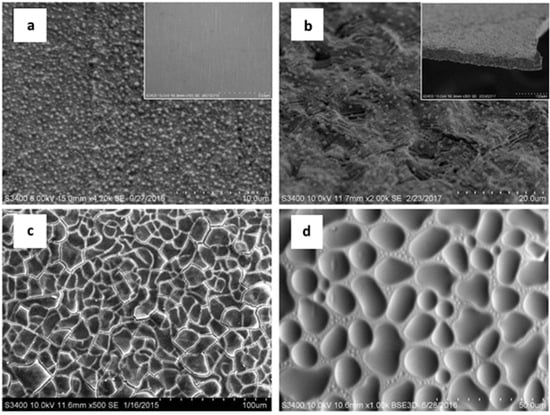
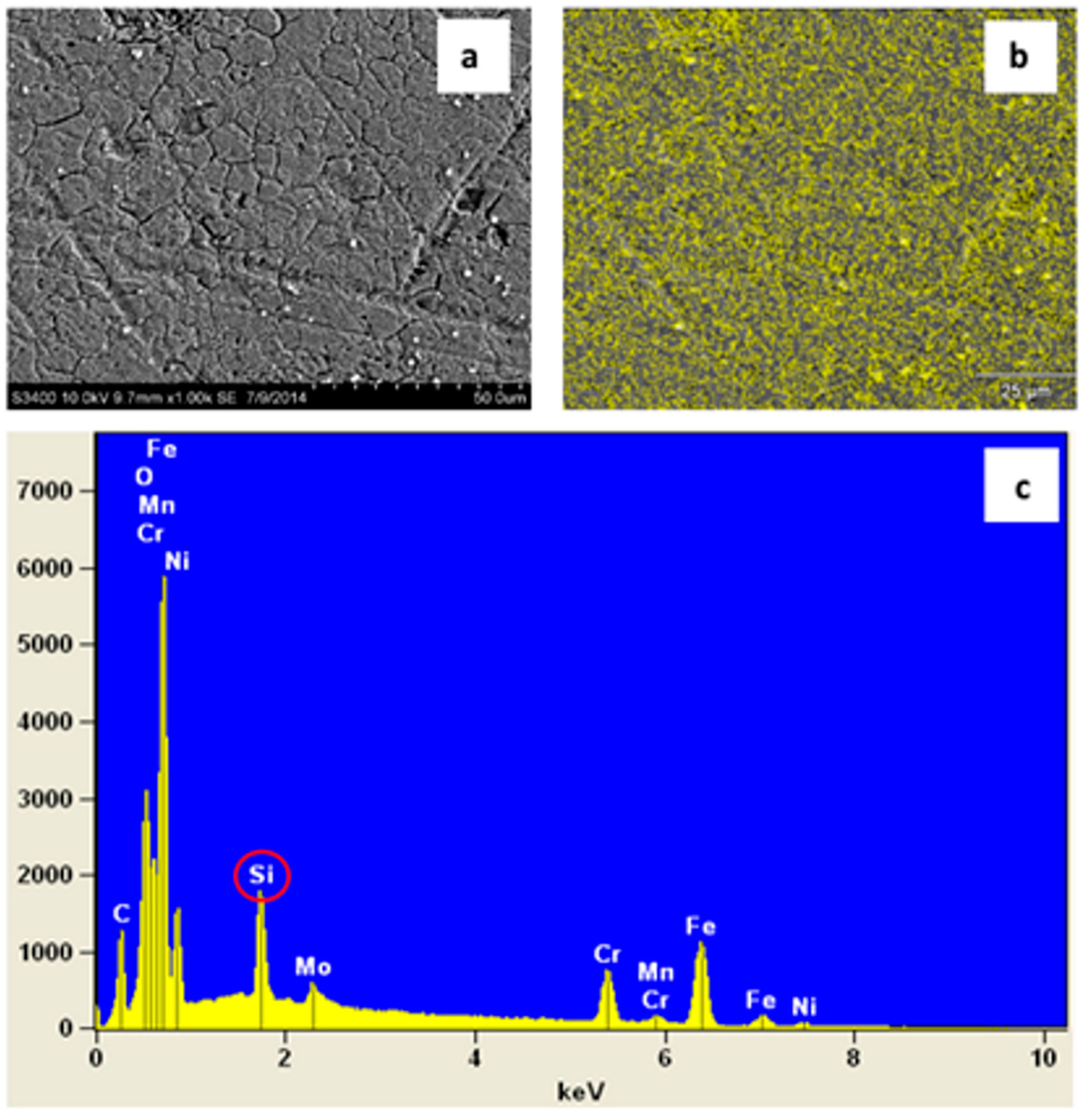
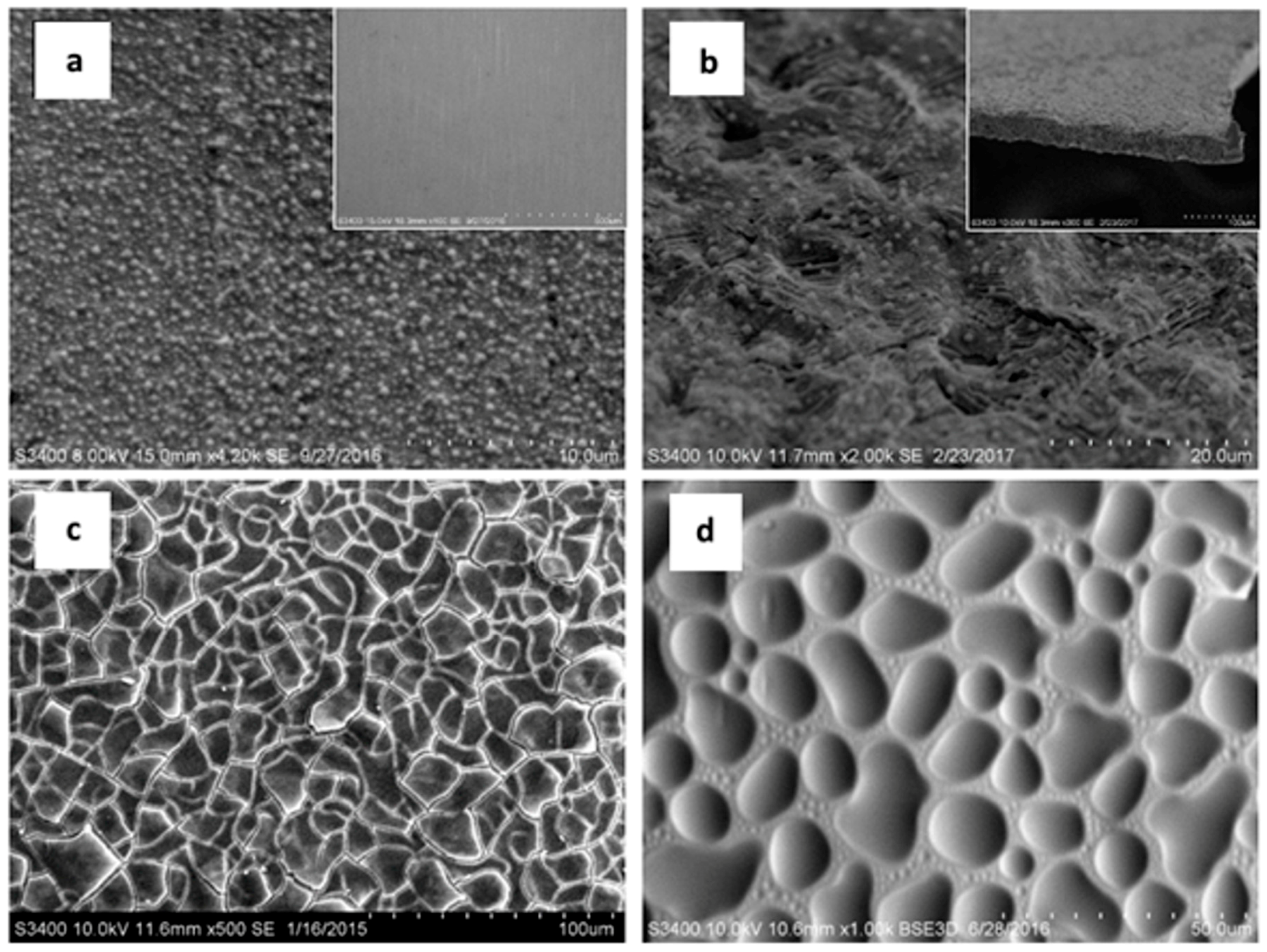
3.1.3. Scanning Electron Microscopy (SEM)
3.1.4. Transmission Electron Microscopy (TEM)
3.2. Spectroscopy
3.2.1. Electrochemical Impedance Spectroscopy (EIS)
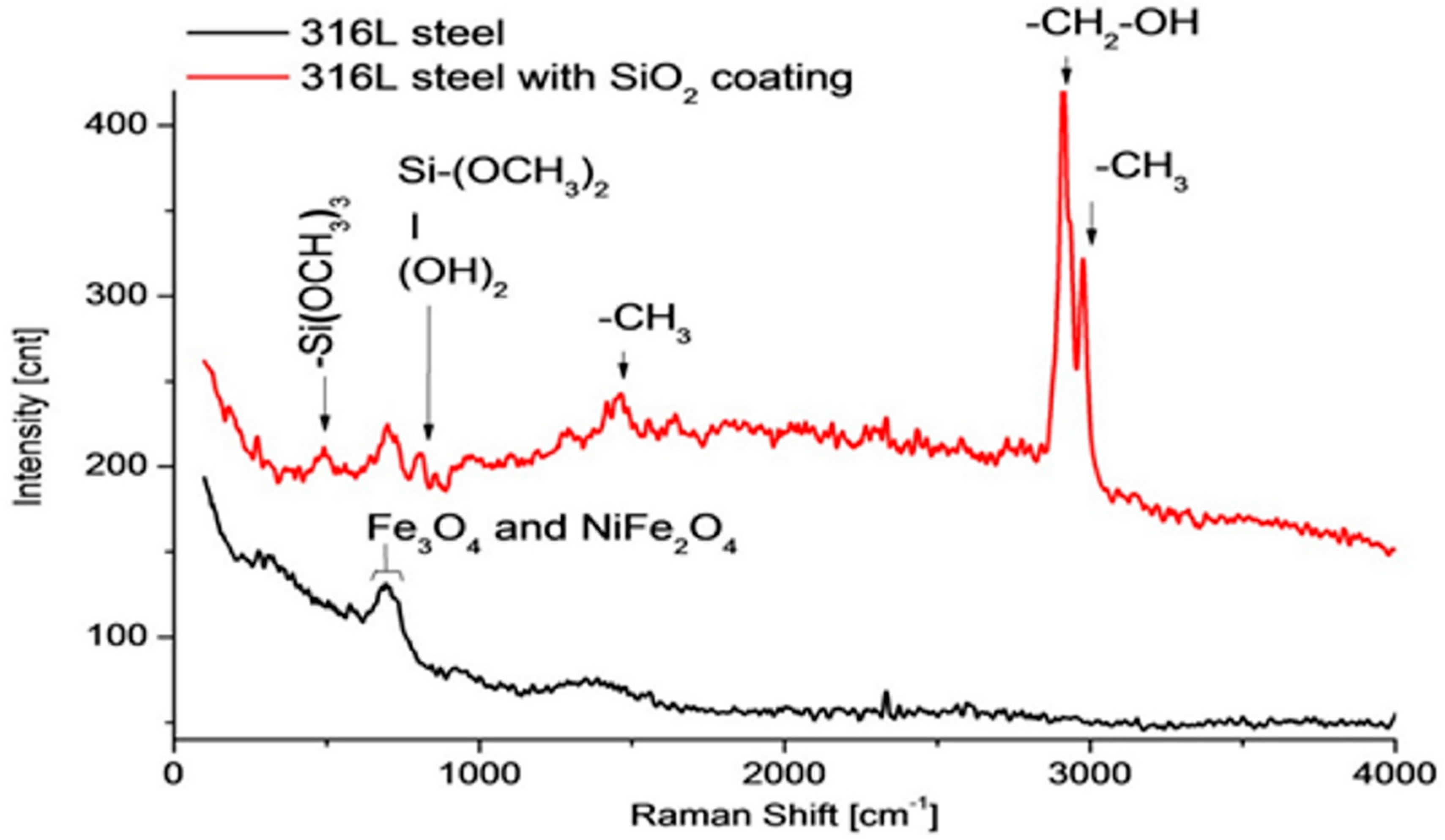
3.2.2. Raman Spectroscopy and Fourier Transform Infrared Spectroscopy (FTIR)
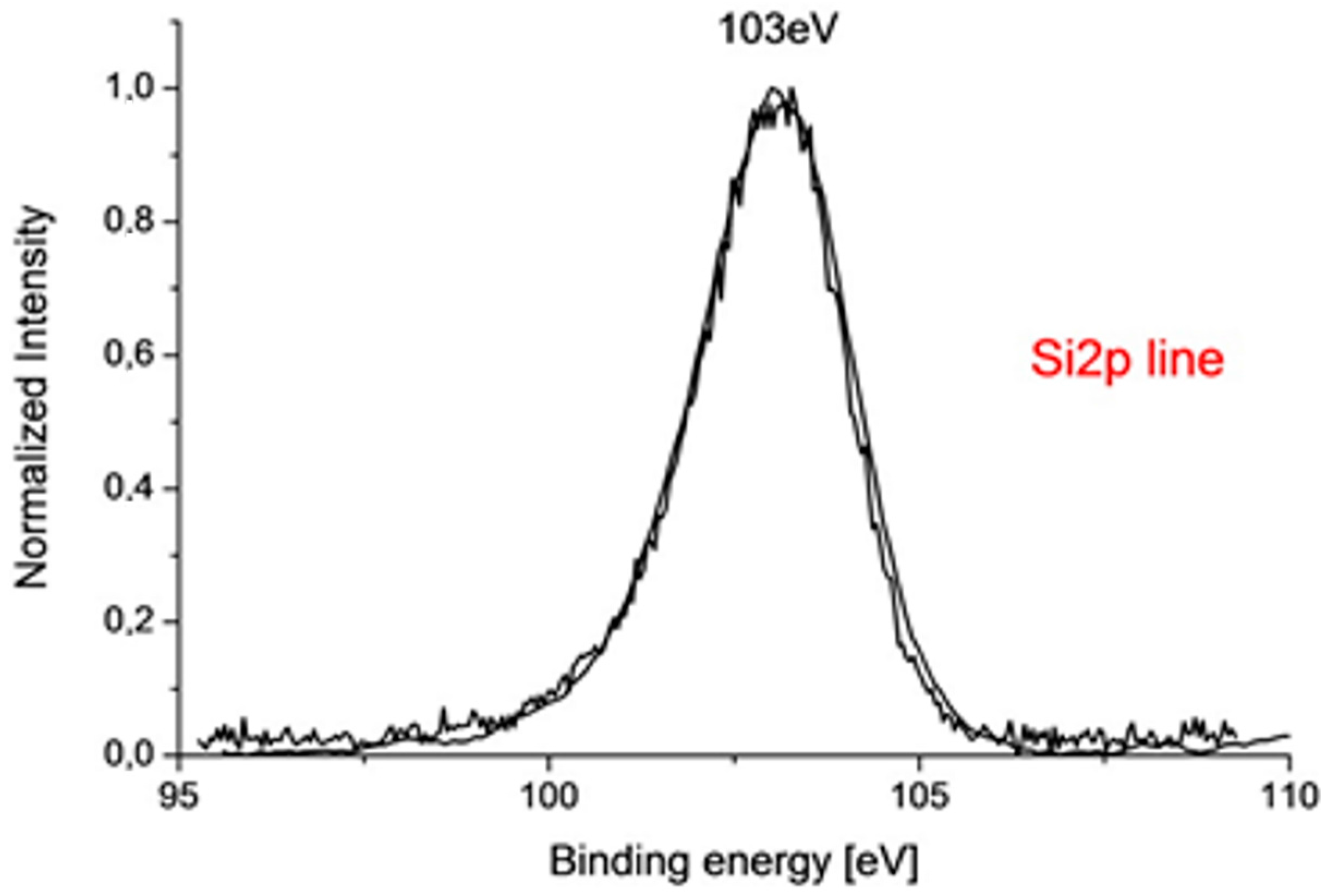
3.2.3. X-ray Photoelectron Spectroscopy (XPS)
4. Conclusions
4.1. In General
4.2. In Detail
- Obtaining anticorrosive coatings using organic-inorganic hybrid materials.This area is related to the still poorly-recognized processes that occur during gel formation, namely, the chemical and physical processes that take place between organic and inorganic components that define the structural characteristics of the layer, (such as the spacing or interpenetration between polymer chains and inorganic matrices, or the occurrence of phase-separation processes involving the formation of systems which can be partially mixed with water during gelling [55]), which can have a significant influence on the final properties of coatings.
- Decreasing the porosity of protective coatings obtained with the sol-gel method.Generally, most sol-gel materials are described as mesoporous. Porosity can be modified by the use of nanoparticle/nanofiller doping, such as montmorillonite [6], ZrO2 [12], as well as active organic or inorganic compounds, such as corrosion inhibitors (cerium(III) compounds [62]) and, also sometimes, precursors with nonhydrolysable organic groups, e.g., methyl or ethyl groups. Moreover, by appropriate control of the aging-time of hydrolysates (e.g., 24 h), it is possible to thicken the structure using progressing sol-gel reactions (discussed in chapter 2.1). In the case of sol-gel coating materials, the right selection of substrates (which determine resultant components) and the arrangement of layers, frequently allows the separation of the substrate from the working environment at the required level in actual real-life scenarios.
- -
- ability to dedicate the composition, both to the selected substrate and to the chosen environment by applying multilayer coatings
- -
- chemical inertion of inorganic oxide matrices and very good adhesion to the metallic substrates
- -
- easy doping or modification of sol-gel matrices
- -
- possibility of modifying the parameters of synthesis and surface or volume functionalization, results in sealing the network and thus increasing barrier properties
- -
- application of sol-gel silica layers to elements working in an aqueous or alkaline environment is limited and forces the progress of development of stable coating materials in various environments
- -
- work on the synthesis of highly corrosion-resistant sol-gel layers with high hardness and abrasion resistance are still in progress
- -
- one of the sol-gel synthesis routes is based on acid catalysis, which translates into low pH of sols, which in turn limit their application to the acids sensitive substrates, e.g., magnesium
- -
- there is also a problem of using some sol-gel organic-inorganic hybrids directly on the surface of metallic materials, which results from the separation of the organic phase in the inorganic matrix.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zheludkevich, M.L.; Salvado, I.M.; Ferreira, M.G.S. Sol-gel coatings for corrosion protection of metals. J. Mater. Chem. 2005, 15, 5099–5111. [Google Scholar] [CrossRef]
- Vasconcelos, D.C.; Carvalho, J.A.; Mantel, M.; Vasconcelos, W. Corrosion resistance of stainless steel coated with sol-gel silica. J. Non-Cryst. Solids 2000, 273, 135–139. [Google Scholar] [CrossRef]
- Figueira, R.B.; Silva, C.J.R.; Pereira, E.V. Organic–inorganic hybrid sol-gel coatings for metal corrosion protection: A review of recent progress. J. Coat. Technol. Res. 2015, 12, 1–35. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Giovanardi, R.; Veronesi, P. Modification of Ti6Al4V implant surfaces by biocompatible TiO2/PCL hybrid layers prepared via sol-gel dip coating: Structural characterization, mechanical and corrosion behavior. Mater. Sci. Eng. C 2017, 74, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liang, K.; Mei, L.; Gu, S.; Wang, S. Corrosion protection of mild steel by zirconia sol-gel coatings. J. Mater. Sci. Lett. 2001, 20, 1081–1083. [Google Scholar] [CrossRef]
- Hang, T.T.X.; Truc, T.A.; Olivier, M.G.; Vandermiers, C.; Guérit, N.; Pébère, N. Corrosion protection mechanisms of carbon steel by an epoxy resin containing indole-3 butyric acid modified clay. Prog. Org. Coat. 2010, 69, 410–416. [Google Scholar] [CrossRef]
- Kasten, L.S.; Grant, J.T.; Grebasch, N.; Voevodin, N.; Arnold, F.E.; Donley, M.S. An XPS study of cerium dopants in sol-gel coatings for aluminum 2024-T3. Surf. Coat. Technol. 2001, 140, 11–15. [Google Scholar] [CrossRef]
- Khramov, A.N.; Voevodin, N.N.; Balbyshev, V.N.; Donley, M.S. Hybrid organo-ceramic corrosion protection coatings with encapsulated organic corrosion inhibitors. Thin Solid Films 2004, 447–448, 549–557. [Google Scholar] [CrossRef]
- Lakshmi, R.V.; Yoganandan, G.; Kavya, K.T.; Basu, B.J. Effective corrosion inhibition performance of Ce3+ doped sol-gel nanocomposite coating on aluminum alloy. Prog. Org. Coat. 2013, 76, 367–374. [Google Scholar] [CrossRef]
- Yu, F.; Akid, R. Corrosion protection of AA2024-T3 alloy by modified hybrid titania-containing sol-gel coatings. Prog. Org. Coat. 2017, 102, 120–129. [Google Scholar] [CrossRef]
- Małecka, J.; Krzak-Roś, J. Preparation of SiO2 coating by sol-gel method, to improve high-temperature corrosion resistance of a γ-TiAl phase based alloy. Adv. Mater. Sci. 2013, 12, 5–12. [Google Scholar] [CrossRef]
- Kiruthika, P.; Subasri, R.; Jyothirmayi, A.; Sarvani, K.; Hebalkar, N.Y. Effect of plasma surface treatment on mechanical and corrosion protection properties of UV-curable sol-gel based GPTS-ZrO2 coatings on mild steel. Surf. Coat. Technol. 2010, 204, 1270–1276. [Google Scholar] [CrossRef]
- Tkaczyk, M.; Krzak-Roś, J.; Kaleta, J. Evaluation of mechanical and physicochemical properties of protection coatings obtained by the sol-gel method. Mater. Sci. 2012, 48, 323–331. [Google Scholar] [CrossRef]
- Osouli-Bostanabad, K.; Tutunchi, A.; Eskandarzade, M. The influence of pre-bond surface treatment over the reliability of steel epoxy/glass composites bonded joints. Int. J. Adhes. Adhes. 2017, 75, 145–154. [Google Scholar] [CrossRef]
- Rugmini Ammal, P.; Prajila, M.; Joseph, A. Physicochemical studies on the inhibitive properties of a 1,2,4-triazole Schiff’s base, HMATD, on the corrosion of mild steel in hydrochloric acid. Egypt. J. Pet. 2017. [Google Scholar] [CrossRef]
- Claire, L.; Marie, G.; Julien, G.; Jean-Michel, S.; Jean, R.; Marie-Joëlle, M.; Stefano, R.; Michele, F. New architectured hybrid sol-gel coatings for wear and corrosion protection of low-carbon steel. Prog. Org. Coat. 2016, 99, 337–345. [Google Scholar] [CrossRef]
- Winnicki, M.; Małachowska, A.; Rutkowska-Gorczyca, M.; Sokołowski, P.; Ambroziak, A.; Pawłowski, L. Characterization of cermet coatings deposited by low-pressure cold spraying. Surf. Coat. Technol. 2015, 268, 108–114. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Erol, M.; Stark, W.J.; Mohn, D.; Hong, Z.; Mano, J.F. Polymer/bioactive glass nanocomposites for biomedical applications: A review. Compos. Sci. Technol. 2010, 70, 1764–1776. [Google Scholar] [CrossRef]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef] [PubMed]
- Asri, R.I.M.; Harun, W.S.W.; Samykano, M.; Lah, N.A.C.; Ghani, S.A.C.; Tarlochan, F.; Raza, M.R. Corrosion and surface modification on biocompatible metals: A review. Mater. Sci. Eng. C 2017, 77, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Karbowniczek, J.; Cordero-Arias, L.; Virtanen, S.; Misra, S.K.; Valsami-Jones, E.; Tuchscherr, L.; Rutkowski, B.; Górecki, K.; Bała, P.; Czyrska-Filemonowicz, A.; et al. Electrophoretic deposition of organic/inorganic composite coatings containing ZnO nanoparticles exhibiting antibacterial properties. Mater. Sci. Eng. C 2017, 77, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Sol-Gel Products (MCP-7803)—Global Industry Analysts, Inc. Available online: http://www.strategyr.com/Sol_Gel_Products_Market_Report.asp#sthash.SKtiq856.dpbs (accessed on 24 July 2017).
- Babiarczuk, B.; Szczurek, A.; Donesz-Sikorska, A.; Rutkowska, I.; Krzak, J. The influence of an acid catalyst on the morphology, wettabillity, adhesion and chemical structure properties of TiO2 and ZrO2 sol-gel thin films. Surf. Coat. Technol. 2016, 285, 134–145. [Google Scholar] [CrossRef]
- Philipp, G.; Schmidt, H. New materials for contact lenses prepared from Si- and Ti-alkoxides by the sol-gel process. J. Non-Cryst. Solids 1984, 63, 283–292. [Google Scholar] [CrossRef]
- Chiola, V.; Ritsko, J.E.; Vanderpool, C.D. Process for Producing Low-Bulk Density Silica. U.S. Patent 3556725, 26 February 1969. [Google Scholar]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: San Diego, CA, USA, 1990. [Google Scholar]
- Schmidt, H. New type of non-crystalline solids between inorganic and organic materials. J. Non-Cryst. Solids 1985, 73, 681–691. [Google Scholar] [CrossRef]
- Ono, S.; Tsuge, H.; Nishi, Y.; Hirano, S. Improvement of Corrosion Resistance of Metals by an Environmentally Friendly Silica Coating Method. J. Sol-Gel Sci. Technol. 2004, 29, 147–153. [Google Scholar] [CrossRef]
- Haas, K.-H. Hybrid Inorganic-Organic Polymers Based on Organically Modified Si-Alkoxides. Adv. Eng. Mater. 2000, 2, 571–582. [Google Scholar] [CrossRef]
- Hay, J.N.; Raval, H.M. Preparation of Inorganic Oxides via a Non-Hydrolytic Sol-Gel Route. J. Sol-Gel Sci. Technol. 1998, 13, 109–112. [Google Scholar] [CrossRef]
- Andrianainarivelo, M.; Corriu, R.J.P.; Leclercq, D.; Mutin, P.H.; Vioux, A. Nonhydrolytic Sol-Gel process: Aluminium and zirconium titanate gels. J. Sol-Gel Sci. Technol. 1997, 8, 89–93. [Google Scholar] [CrossRef]
- Metroke, T.L.; Apblett, A. Effect of solvent dilution on corrosion protective properties of Ormosil coatings on 2024-T3 aluminum alloy. Prog. Org. Coat. 2004, 51, 36–46. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Izquierdo-Barba, I.; Gil, F.J. Localized corrosion of 316L stainless steel with SiO2-CaO films obtained by means of sol-gel treatment. J. Biomed. Mater. Res. A 2003, 67, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Atik, M.; Luna, F.P.; Messaddeq, S.H.; Aegerter, M.A. Ormocer (ZrO2-PMMA) films for stainless steel corrosion protection. J. Sol-Gel Sci. Technol. 1997, 8, 517–522. [Google Scholar] [CrossRef]
- Tiwari, A.; Wang, R.; Wei, B. Advanced Surface Engineering Materials; J. Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 33–83. [Google Scholar]
- Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) and Establishing a European Chemicals Agency; Regulation (EC) No 1907/2006-REACH; EU-OSHA: Bilbao, Spain, 2006.
- Tiwari, A.; Hihara, L.H.; Rawlins, J.W. Intelligent Coatings for Corrosion Control; Elsevier Inc.: Philadelphia, PA, USA, 2015; pp. 1–15, 59–333, 363–407, 585–602. [Google Scholar]
- Zhang, F.; Di, C.; Berdunov, N.; Hu, Y.; Hu, Y.; Gao, X.; Meng, Q.; Sirringhaus, H.; Zhu, D. Ultrathin Film Organic Transistors: Precise Control of Semiconductor Thickness via Spin-Coating. Adv. Mater. 2013, 25, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Uzum, A.; Fukatsu, K.; Kanda, H.; Kimura, Y.; Tanimoto, K.; Yoshinaga, S.; Jiang, Y.; Ishikawa, Y.; Uraoka, Y.; Ito, S. Silica-sol-based spin-coating barrier layer against phosphorous diffusion for crystalline silicon solar cells. Nanoscale Res. Lett. 2014, 9, 659. [Google Scholar] [CrossRef] [PubMed]
- Ataie, S.A.; Zakeri, A. Improving tribological properties of (Zn–Ni)/nano Al2O3 composite coatings produced by ultrasonic assisted pulse plating. J. Alloys Compd. 2016, 674, 315–322. [Google Scholar] [CrossRef]
- Kuo, Y.-L.; Chang, K.-H. Atmospheric pressure plasma enhanced chemical vapor deposition of SiOx films for improved corrosion resistant properties of AZ31 magnesium alloys. Surf. Coat. Technol. 2015, 283, 194–200. [Google Scholar] [CrossRef]
- Ma, G.; Yan, S.; Wu, D.; Miao, Q.; Liu, M.; Niu, F. Microstructure evolution and mechanical properties of ultrasonic assisted laser clad yttria stabilized zirconia coating. Ceram. Int. 2017, 43, 9622–9629. [Google Scholar] [CrossRef]
- Song, G.L. Corrosion Prevention of Magnesium Alloys; Woodhead Publishing: Cambridge, UK, 2013; pp. 469–485. [Google Scholar]
- Liu, L.; Wang, D.K.; Martens, D.L.; Smart, S.; Diniz da Costa, J.C. Interlayer-free microporous cobalt oxide silica membranes via silica seeding sol-gel technique. J. Membr. Sci. 2015, 492, 1–8. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Serra, R.; Montemor, M.F.; Yasakau, K.A.; Salvado, I.M.M.; Ferreira, M.G.S. Nanostructured sol-gel coatings doped with cerium nitrate as pre-treatments for AA2024-T3. Electrochim. Acta 2005, 51, 208–217. [Google Scholar] [CrossRef]
- Moutarlier, V.; Neveu, B.; Gigandet, M.P. Evolution of corrosion protection for sol-gel coatings doped with inorganic inhibitors. Surf. Coat. Technol. 2008, 202, 2052–2058. [Google Scholar] [CrossRef]
- Barua, N.K.; Ragini, T.; Subasri, R. Sol-gel derived single-layer zeolite-based coatings on glass for broadband antireflection properties. J. Non-Cryst. Solids 2017, 469, 51–55. [Google Scholar] [CrossRef]
- Fedel, M.; Callone, E.; Fabbian, M.; Deflorian, F.; Dirè, S. Influence of Ce3+ doping on molecular organization of Si-based organic/inorganic sol-gel layers for corrosion protection. Appl. Surf. Sci. 2017, 414, 82–91. [Google Scholar] [CrossRef]
- Zhong, X.; Li, Q.; Hu, J.; Yang, X.; Luo, F.; Dai, Y. Effect of cerium concentration on microstructure, morphology and corrosion resistance of cerium–silica hybrid coatings on magnesium alloy AZ91D. Prog. Org. Coat. 2010, 69, 52–56. [Google Scholar] [CrossRef]
- Makhlouf, A.S.H. Handbook of Smart Coatings for Materials Protection; Woodhead Publishing Series in Materials and Surfaces Engineering: Cambridge, UK, 2014; pp. 423–458. [Google Scholar]
- Staub, F.; Olewicz, E. Mikroskop Metalograficzny; PWN: Warsaw, Poland, 1967. [Google Scholar]
- Nouri, E.; Shahmiri, M.; Rezaie, H.R.; Talayian, F. Investigation of structural evolution and electrochemical behaviour of zirconia thin films on the 316L stainless steel substrate formed via sol-gel process. Surf. Coat. Technol. 2011, 205, 5109–5115. [Google Scholar] [CrossRef]
- Qiao, L.; Xie, F.; Xie, M.; Gong, C.; Wang, W.; Gao, J. Characterization and photoelectrochemical performance of Zn-doped TiO2 films by sol-gel method. Trans. Nonferrous Met. Soc. China 2016, 26, 2109–2116. [Google Scholar] [CrossRef]
- Cheraghi, H.; Shahmiri, M.; Sadeghian, Z. Corrosion behavior of TiO2–NiO nanocomposite thin films on AISI 316L stainless steel prepared by sol-gel method. Thin Solid Films 2012, 522, 289–296. [Google Scholar] [CrossRef]
- Suzana, A.F.; Ferreira, E.A.; Benedetti, A.V.; Carvalho, H.W.P.; Santilli, C.V.; Pulcinelli, S.H. Corrosion protection of chromium-coated steel by hybrid sol-gel coatings. Surf. Coat. Technol. 2016, 299, 71–80. [Google Scholar] [CrossRef]
- Ali, S.M.; Al lehaibi, H.A. Nano-structured sol-gel coatings as protective films against zinc corrosion in 0.5 M HCl solution. J. Saudi Chem. Soc. 2017, 21, 473–480. [Google Scholar] [CrossRef]
- Goldstein, J.; Newbury, D.E.; Echlin, P.; Joy, D.C.; Romig, A.D., Jr.; Lyman, C.E.; Fiori, C.; Lifshin, E. Scanning Electron Microscopy and X-Ray Microanalysis. A Text for Biologists, Materials Scientists and Geologists; Plenum Press: New York, NY, USA, 1992. [Google Scholar]
- Acquafredda, P.; Andriani, T.; Lorenzoni, S.; Zanettin, E. Chemical Characterization of Obsidians from Different Mediterranean Sources by Non-destructive SEM-EDS Analytical Method. J. Archaeol. Sci. 1999, 26, 315–325. [Google Scholar] [CrossRef]
- Reimer, L. Transmission Electron Microscopy. Physics of Image Formation and Microanalysis; Springer: Berlin, Germany, 1998; pp. 135–169, 207–288. [Google Scholar]
- Barbacki, A. Mikroskopia Elektronowa; Wydawnictwo Politechniki Poznańskiej: Poznań, Poland, 2003; pp. 84–114. [Google Scholar]
- Williams, D.B.; Carter, C.B. The Transmission Electron Microscope. In Transmission Electron Microscopy; Springer: Boston, MA, USA, 2009; pp. 3–22. [Google Scholar]
- Pepe, A.; Aparicio, M.; Ceré, S.; Durán, A. Preparation and characterization of cerium doped silica sol-gel coatings on glass and aluminum substrates. J. Non-Cryst. Solids 2004, 348, 162–171. [Google Scholar] [CrossRef]
- Rosero-Navarro, N.C.; Paussa, L.; Andreatta, F.; Castro, Y.; Durán, A.; Aparicio, M.; Fedrizzi, L. Optimization of hybrid sol-gel coatings by combination of layers with complementary properties for corrosion protection of AA2024. Prog. Org. Coat. 2010, 69, 167–174. [Google Scholar] [CrossRef]
- Barranco, V.; Carmona, N.; Galván, J.C.; Grobelny, M.; Kwiatkowski, L.; Villegas, M.A. Electrochemical study of tailored sol-gel thin films as pre-treatment prior to organic coating for AZ91 magnesium alloy. Prog. Org. Coat. 2010, 68, 347–355. [Google Scholar] [CrossRef]
- Dubois, F.; Mendibide, C.; Pagnier, T.; Perrard, F.; Duret, C. Raman mapping of corrosion products formed onto spring steels during salt spray experiments. A correlation between the scale composition and the corrosion resistance. Corros. Sci. 2008, 50, 3401–3409. [Google Scholar] [CrossRef]
- Amer, M.S.; Durgam, L.; El-Ashry, M.M. Raman mapping of local phases and local stress fields in silicon–silicon carbide composites. Mater. Chem. Phys. 2006, 98, 410–414. [Google Scholar] [CrossRef]
- Bera, S.; Rout, T.K.; Udayabhanu, G.; Narayan, R. Comparative Study of Corrosion Protection of Sol-Gel Coatings with Different Organic Functionality on Al-2024 substrate. Prog. Org. Coat. 2015, 88, 293–303. [Google Scholar] [CrossRef]



| Reaction | Formula |
|---|---|
| Hydrolysis reaction | M–OR + H2O M–OH + ROH |
| Condensation reactions | |
| Water condensation | M–OH + HO–M M–O–M + H2O |
| Alcohol condensation | M–OR + HO–M M–O–M + ROH |
| Type of Layer | Key Features | Examples |
|---|---|---|
| Inert Layers | Low porosity | SiO2 [1], ZrO2 [5,43], Al2O3 [43], TiO2 [43] |
| Substrate adhesion | ||
| Reduced roughness | ||
| Gradient Layers | Low porosity | Oxide matrices with GPTMS [6]/VETO [49]/PMMA [37] doped by corrosion inhibitors [7,8,46,49], nanostructures [1,6,12,45] or nanocontainers [37] |
| Crack resistance | ||
| Inhibition of corrosion | ||
| Surface Layers | Low porosity | ZrO2 [1,10], SiO2-GPTMS-VETO doped by cerium nitrate [49] |
| Mechanical resistance |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gąsiorek, J.; Szczurek, A.; Babiarczuk, B.; Kaleta, J.; Jones, W.; Krzak, J. Functionalizable Sol-Gel Silica Coatings for Corrosion Mitigation. Materials 2018, 11, 197. https://doi.org/10.3390/ma11020197
Gąsiorek J, Szczurek A, Babiarczuk B, Kaleta J, Jones W, Krzak J. Functionalizable Sol-Gel Silica Coatings for Corrosion Mitigation. Materials. 2018; 11(2):197. https://doi.org/10.3390/ma11020197
Chicago/Turabian StyleGąsiorek, Jolanta, Anna Szczurek, Bartosz Babiarczuk, Jerzy Kaleta, Walis Jones, and Justyna Krzak. 2018. "Functionalizable Sol-Gel Silica Coatings for Corrosion Mitigation" Materials 11, no. 2: 197. https://doi.org/10.3390/ma11020197
APA StyleGąsiorek, J., Szczurek, A., Babiarczuk, B., Kaleta, J., Jones, W., & Krzak, J. (2018). Functionalizable Sol-Gel Silica Coatings for Corrosion Mitigation. Materials, 11(2), 197. https://doi.org/10.3390/ma11020197