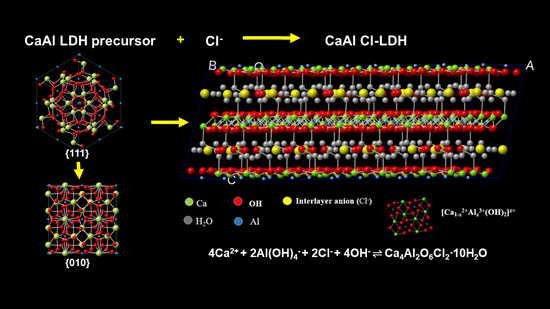
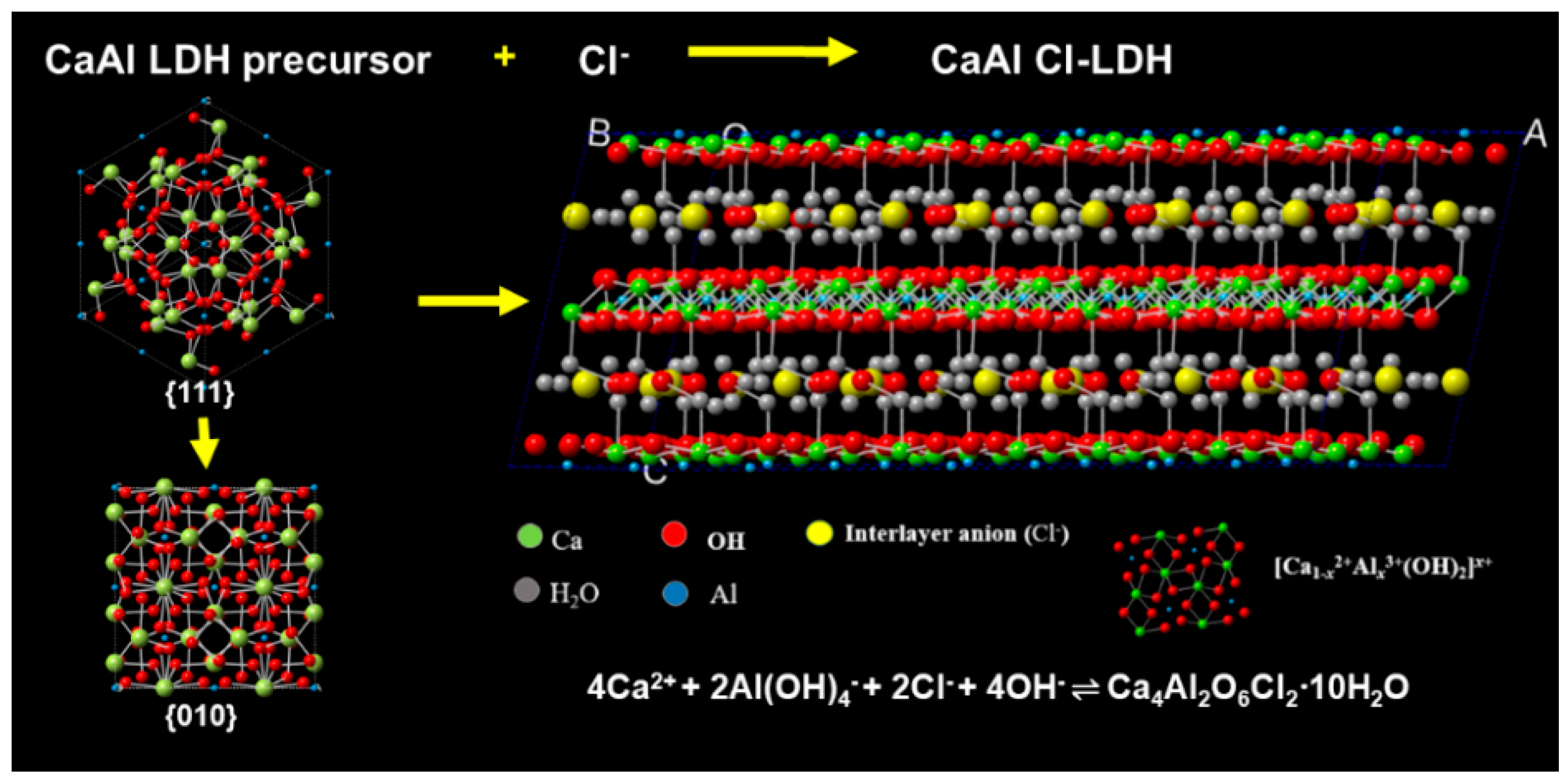
Layered Double Hydroxides Precursor as Chloride Inhibitor: Synthesis, Characterization, Assessment of Chloride Adsorption Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of CaAl-Cl LDH
2.2. Chloride Adsorption Kinetics and Isotherms
2.3. Fixation Stability of Cement Mortar Blended with NaCl Solution
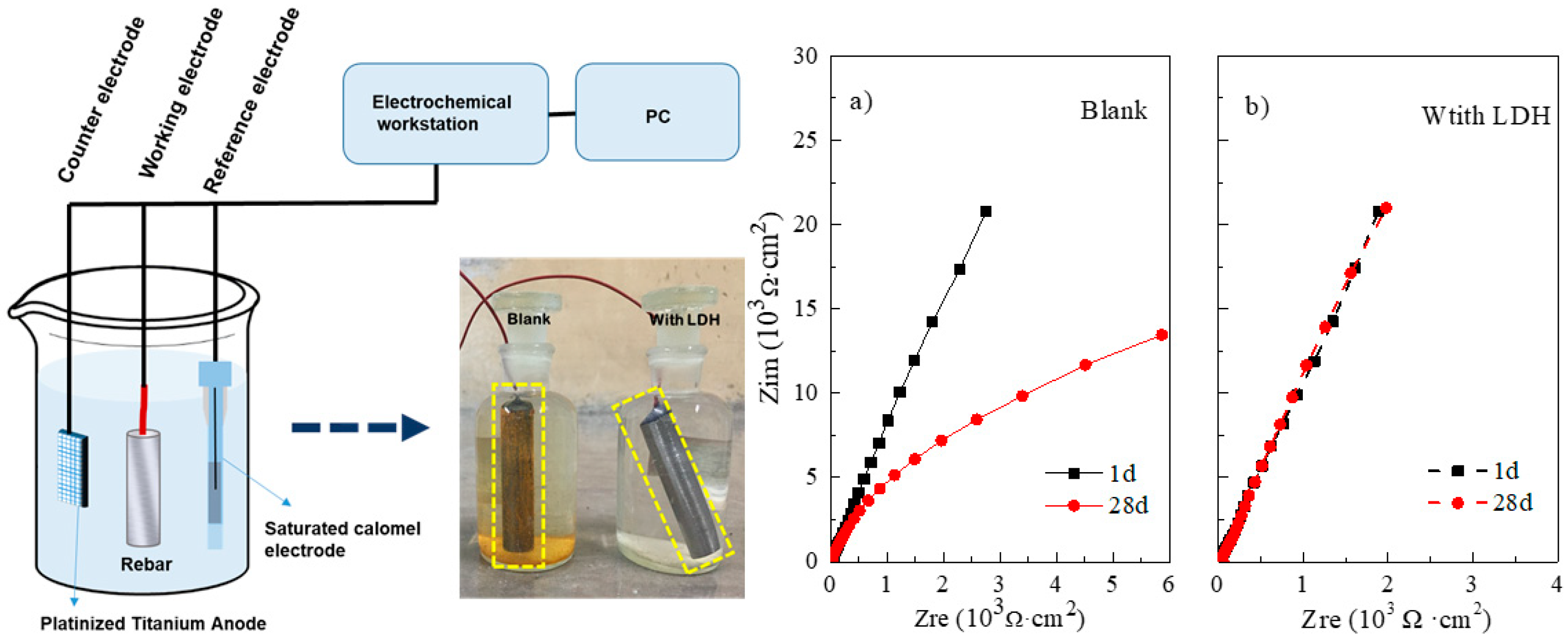
2.4. Influence of Inhibitors on the Corrosion Behavior of Steel
3. Results and Analysis
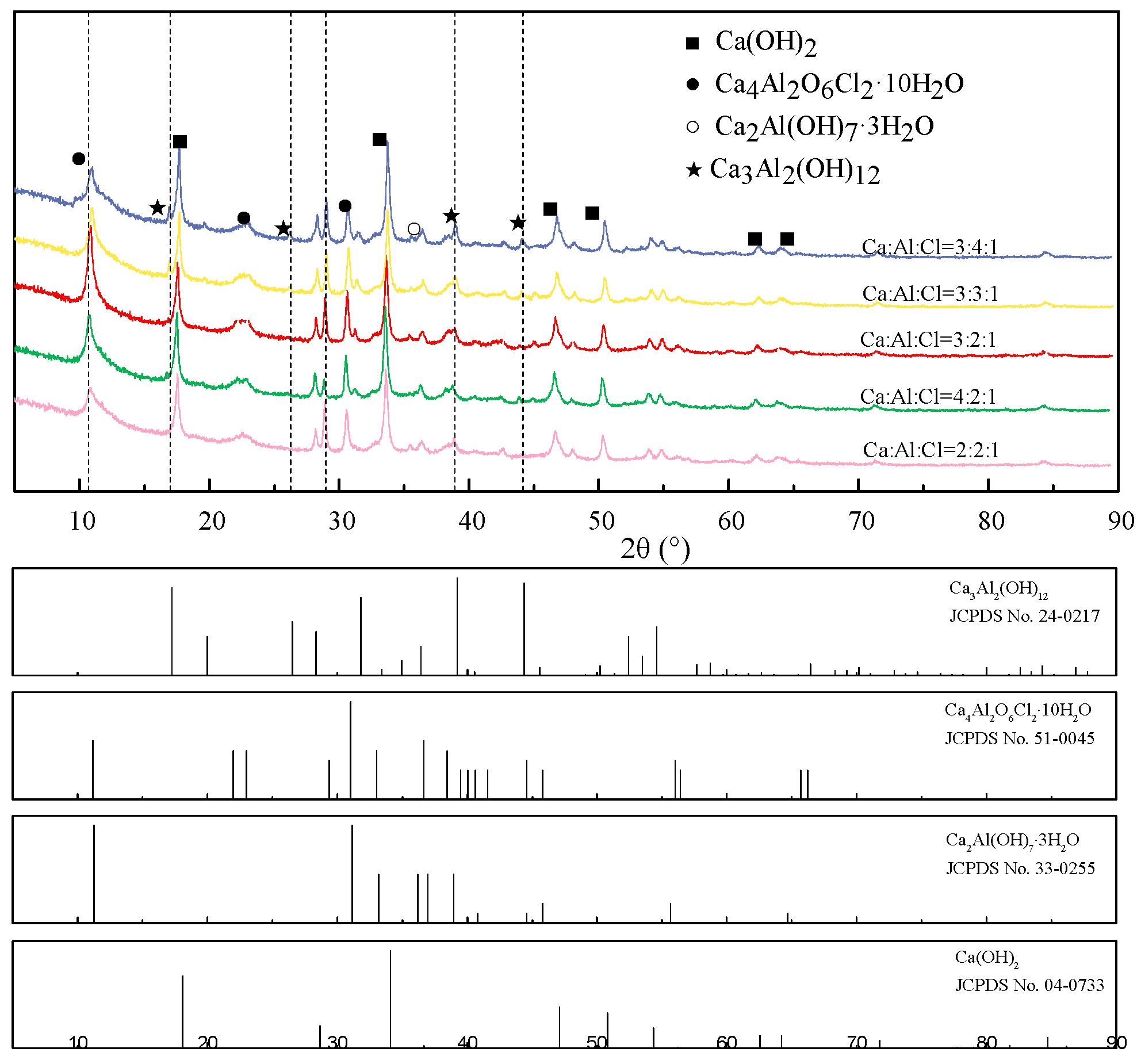
3.1. Synthesis of CaAl-Cl LDH with Various n(Ca: Al: Cl) Ratio
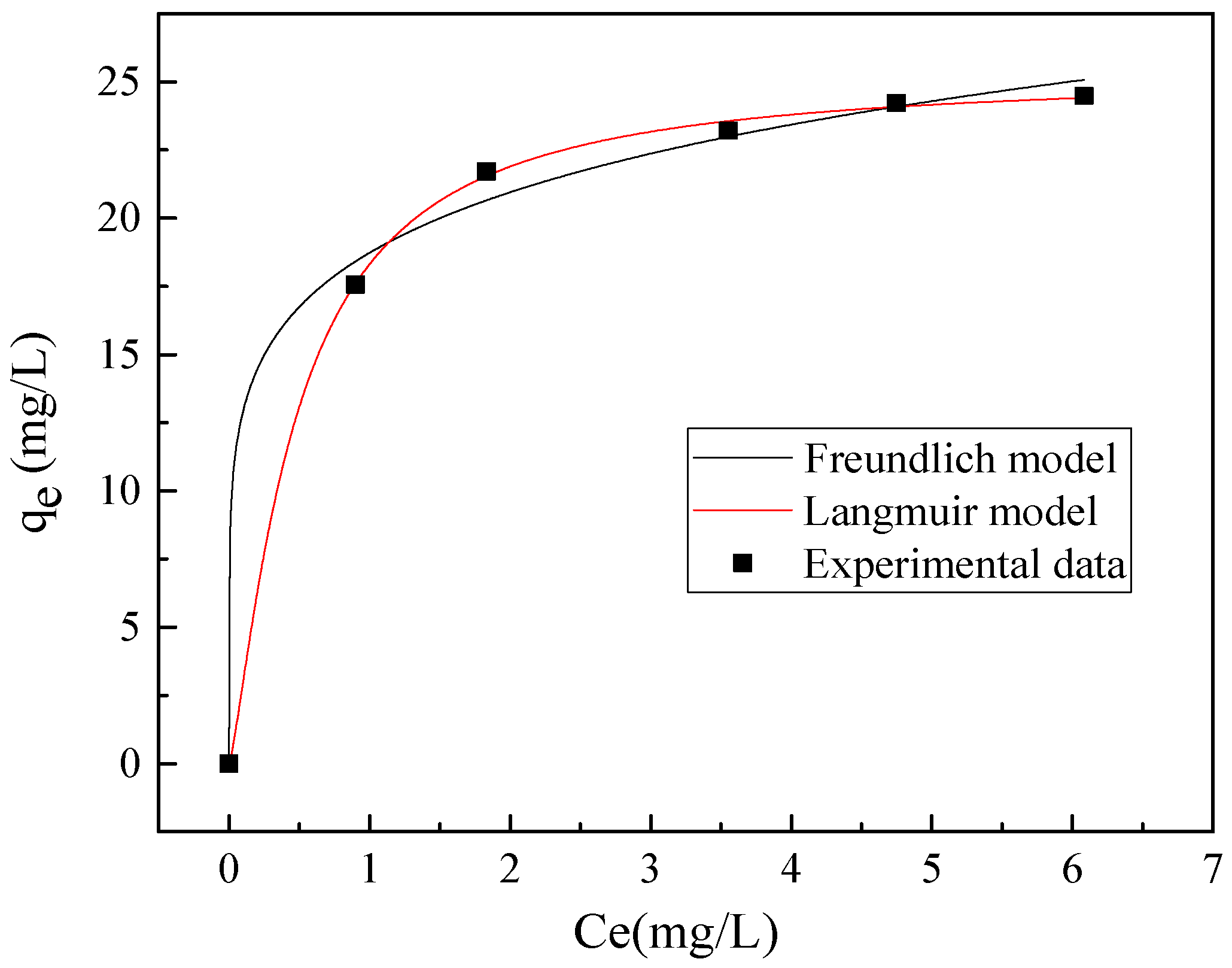
3.2. Kinetic Study and Adsorption Isotherm
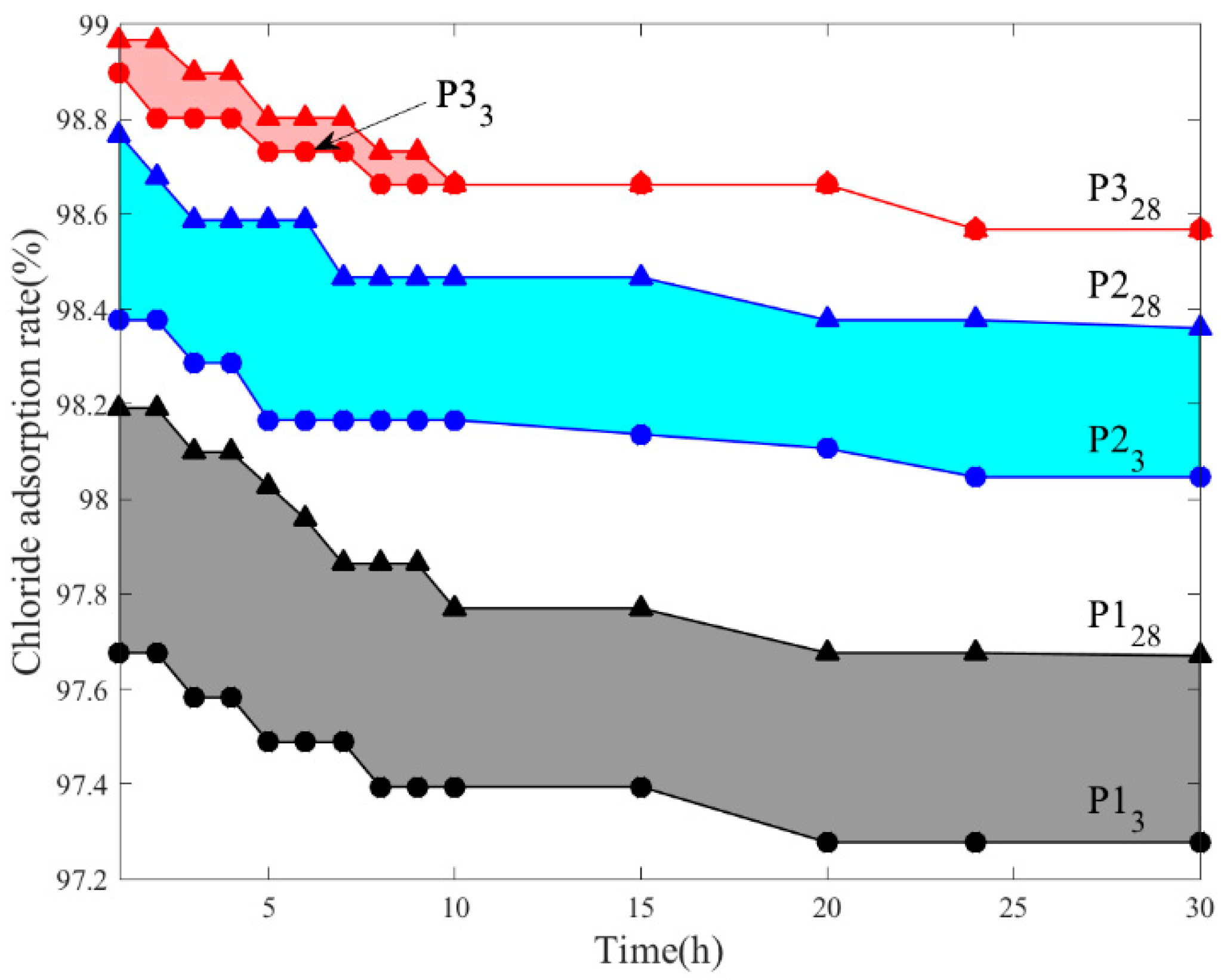
3.3. Chloride Leaching Test
3.4. Electrochemical Evaluation
4. Conclusions
- Chloride ions could be effectively adsorbed by CaAl LDH precursor. The optimal chloride ion removal rate was 87.06% due to the formation of hydrocalumite with Ca: Al: Cl = 3: 2: 1.
- The adsorption process could be well described by the pseudo-second-order model and Langmuir model. 98.3% chloride ions can be rapidly captured in cement mortar blended with CaAl-Cl LDH precursor and cannot be easily released again.
- The inhibition performance of steel in the electrolytes with/without CaAl LDH precursor was investigated by using electrochemical measurements. The results indicates that CaAl LDH precursor protect the passive film on steel surface by chloride adsorption.
- This research indicates that CaAl-Cl LDH precursor is a potential and rapid adsorbent for immobilize chloride from sodium chloride water that prevents chloride-induced deterioration in reinforced concrete or mortar.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Soeylev, T.A.; Richardson, M.G. Corrosion inhibitors for steel in concrete: State-of-the-art report. Constr. Build. Mater. 2008, 22, 609–622. [Google Scholar] [CrossRef]
- Auerbach, S.M.; Carrado, K.A.; Dutta, P.K. Handbook of Layered Materials, 4th ed.; CRC Press: New York, NY, USA, 2004. [Google Scholar]
- Yao, J.; Kong, Q.; Zhu, H.; Long, Y.; Shen, D. Adsorption characteristics of nitrite on Friedel’s salt under the landfill circumstance. Chem. Eng. J. 2014, 254, 479–485. [Google Scholar] [CrossRef]
- Glasser, F.P.; Kindness, A.; Stronach, S.A. Stability and solubility relationships in AFm phases—Part 1. Chloride, sulfate and hydroxide. Cem. Concr. Res. 1999, 29, 861–866. [Google Scholar] [CrossRef]
- Chen, Y.; Shui, Z.; Chen, W.; Chen, G. Chloride binding of synthetic Ca-Al-NO3 LDHs in hardened cement paste. Constr. Build. Mater. 2015, 93, 1051–1058. [Google Scholar] [CrossRef]
- Xu, J.; Song, Y.; Zhao, Y.; Jiang, L.; Mei, Y.; Chen, P. Chloride removal and corrosion inhibitions of nitrate, nitrite-intercalated Mg-Al layered double hydroxides on steel in saturated calcium hydroxide solution. Appl. Clay Sci. 2018, 163, 129–136. [Google Scholar] [CrossRef]
- Tatematsu, H.; Sasaki, T. Repair materials system for chloride-induced corrosion of reinforcing bars. Cem. Concr. Comp. 2003, 25, 123–129. [Google Scholar] [CrossRef]
- Yoon, S.; Moon, J.; Bae, S.; Duan, X.; Giannelis, E.P.; Monteiro, P.M. Chloride adsorption by calcined layered double hydroxides in hardened Portland cement paste. Mater. Chem. Phys. 2014, 145, 376–386. [Google Scholar] [CrossRef]
- Birnin-Yauri, U.A.; Glasser, F.P. Friedel’s salt, Ca2Al(OH)6(Cl,OH)·2H2O: Its solid solutions and their role in chloride binding. Cem. Concr. Res. 1998, 28, 1713–1723. [Google Scholar] [CrossRef]
- Abdel-Wahab, A.; Batchelor, B. Chloride removal from recycled cooling water using ultra-high lime with aluminum process. Water Environ. Res. 2002, 74, 256–263. [Google Scholar] [CrossRef]
- Abate, C.; Ssheetz, B.E. Aqueous phase equilibria in the system CaO-Al2O3-CaCl2-H2O: The significance and stability of Friedel’s salt. J. Am. Ceram. Soc. 1995, 78, 939–944. [Google Scholar] [CrossRef]
- Chinese National Standard. Common Portland Cement; GB175-2007; Chinese National Standard: Beijing, China, 2007. [Google Scholar]
- Chinese National Standard. Test Code for Hydraulic Concrete; SL 352-2006; Ministry of water resources of the People’s Republic of China: Beijing, China, 2006.
- Liu, J.; Cai, J.; Shi, L.; Liu, J.; Zhou, X.; Mu, S.; Hong, J. The inhibition behavior of a water-soluble silane for reinforcing steel in 3.5% NaCl saturated Ca(OH)2 solution. Constr. Build. Mater. 2018, 189, 95–101. [Google Scholar] [CrossRef]
- Verbruggen, H.; Terryn, H.; De Graeve, I. Inhibitor evaluation in different simulated concrete pore solution for the protection of steel rebars. Constr. Build. Mater. 2016, 124, 887–896. [Google Scholar] [CrossRef]
- Manera, M.; Vennesland, O.; Bertolini, L. Chloride threshold for rebar corrosion in concrete with addition of silica fume. Corros. Sci. 2008, 50, 554–560. [Google Scholar] [CrossRef]
- Angst, U.; Elsener, B.; Larsen, C.K.; Vennesland, O. Critical chloride content in reinforced concrete—A review. Cem. Concr. Res. 2009, 39, 1122–1138. [Google Scholar] [CrossRef]
- Liu, R.; Jiang, L.; Huang, G.; Zhu, Y.; Liu, X.; Chu, H.; Xiong, C. The effect of carbonate and sulfate ions on chloride threshold level of reinforcement corrosion in mortar with/without fly ash. Constr. Build. Mater. 2016, 113, 90–95. [Google Scholar] [CrossRef]
- Figueira, R.B.; Sadovski, A.; Melo, A.P.; Pereira, E.V. Chloride threshold value to initiate reinforcement corrosion in simulated concrete pore solutions: The influence of surface finishing and pH. Constr. Build. Mater. 2017, 141, 183–200. [Google Scholar] [CrossRef]
- Geng, C.; Xu, Y.; Weng, D.; Wu, X. A time-saving method to determine the chloride threshold level for depassivation of steel in concrete. Constr. Build. Mater. 2010, 24, 903–909. [Google Scholar] [CrossRef]
- Abdel-Wahab, A.; Batchelor, B.; Schwantes, J. An equilibrium model for chloride removal from recycled cooling water using the ultra-high lime with aluminum process. Water Environ. Res. 2005, 77, 3059–3065. [Google Scholar] [CrossRef]
- Kano, F.; Abe, I.; Kamaya, H.; Ueda, I. Fractal model for adsorption on activated carbon surfaces: Langmuir and Freundlich adsorption. Surf. Sci. 2000, 467, 131–138. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1362–1403. [Google Scholar] [CrossRef]
- Baseri, H.; Tizro, S. Treatment of nickel ions from contaminated water by magnetite based nanocomposite adsorbents: Effects of thermodynamic and kinetic parameters and modeling with Langmuir and Freundlich isotherms. Process Saf. Environ. 2017, 109, 465–477. [Google Scholar] [CrossRef]
- Chi, L.; Wang, Z.; Sun, Y.; Lu, S.; Yao, Y. Crystalline/Amorphous Blend Identification from Cobalt Adsorption by Layered Double Hydroxides. Materials 2018, 11, 17069. [Google Scholar] [CrossRef] [PubMed]
- Roeffaers, M.B.; Sels, B.F.; Loos, D.; Kohl, C.; Müllen, K.; Jacobs, P.A. In situ space- and time-resolved sorption kinetics of anionic dyes on individual ldh crystals. Chem. Phys. Chem. 2010, 6, 2295–2299. [Google Scholar] [CrossRef] [PubMed]
- Chi, L.; Wang, Z.; Sun, Y.; Lu, S.; Yao, Y. Removal of cobalt ions from waste water by Friedel’s salt. Mater. Res. Express 2019, 6, 015508. [Google Scholar] [CrossRef]
- Fogg, A.M.; Freij, A.J.; Rohl, A.L.; Ogden, M.I.; Parkinson, G.M. Toward a fundamental understanding of molecular recognition: A synthetic and computational study of morphological control of Ca3Al2(OH)12. J. Phys. Chem. B 2002, 106, 5820–5826. [Google Scholar] [CrossRef]
- Glasser, F.P.; Marchand, J.; Samson, E. Durability of concrete—Degradation phenomena involving detrimental chemical reactions. Cem. Concr. Res. 2008, 38, 226–246. [Google Scholar] [CrossRef]
- Beaudoin, J.J.; Ramachandran, V.S.; Feldman, R.F. Interaction of chloride and C-S-H. Cem. Concr. Res. 1990, 20, 875–883. [Google Scholar] [CrossRef]
- Yoon, S.; Ha, J.; Chae, S.R.; Kilcoyne, D.A.; Monteiro, P.J.M. X-ray spectromicroscopic study of interactions between NaCl and calcium silicate hydrates. Mag. Concrete Res. 2014, 66, 141–149. [Google Scholar] [CrossRef]
- Matschei, T.; Lothenbach, B.; Glasser, F.P. The AFm phase in Portland cement. Cem. Concr. Res. 2007, 37, 118–130. [Google Scholar] [CrossRef]
- Marinescu, M.V.A.; Brouwers, H.J.H. Estimation of chloride contents in hardened OPC pastes. In Proceedings of the 3rd International RILEM Conference on Modelling the Durability of Reinforced Concrete, Guimaraes, Portugal, 22–24 October 2009; pp. 22–24. [Google Scholar]
- Suryavanshi, A.K.; Scantlebury, J.D.; Lyon, S.B. Mechanism of Friedel’s salt formation in cements rich in tri-calcium aluminate. Cem. Concr. Res. 1996, 26, 717–727. [Google Scholar] [CrossRef]

| Binder | OPC | Sand | Absorb-Mixture | NaCl Solution | Desalinated Water |
|---|---|---|---|---|---|
| P1 | 450 | 1350 | / | 225 | / |
| P2 | 450 | 1350 | / | / | 225 |
| P3 | 427.5 | 1350 | 22.5 | 225 | / |
| n(Ca:Al:Cl) | pH | n(Cl−) g/L | n(Al3+) g/L | n(Ca2+) g/L | R % |
|---|---|---|---|---|---|
| 3:1:1 | 12.65 | 1.707 | 0.400 | 0.053 | 79.96% |
| 3:2:1 | 12.70 | 1.102 | 2.836 | 0.039 | 87.06% |
| 3:3:1 | 12.67 | 1.511 | 5.839 | 0.049 | 82.27% |
| 3:4:1 | 12.78 | 1.816 | 9.185 | 0.037 | 78.69% |
| 2:2:1 | 12.66 | 1.807 | 3.579 | 0.032 | 78.79% |
| 4:2:1 | 12.72 | 1.141 | 2.060 | 0.025 | 86.61% |
| Exp-Qe (mg/g) | k2 (g/mg·min) | Cal-Qe (mg/g) | R2 | |
|---|---|---|---|---|
| 0.12 mol/L | 22.7 | 0.0030 | 23.1 | 0.9997 |
| 0.24 mol/L | 22.2 | 0.0029 | 23.8 | 0.9979 |
| 0.48 mol/L | 25.5 | 0.0024 | 25.7 | 0.9998 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, L.; Wang, Z.; Zhou, Y.; Lu, S.; Yao, Y. Layered Double Hydroxides Precursor as Chloride Inhibitor: Synthesis, Characterization, Assessment of Chloride Adsorption Performance. Materials 2018, 11, 2537. https://doi.org/10.3390/ma11122537
Chi L, Wang Z, Zhou Y, Lu S, Yao Y. Layered Double Hydroxides Precursor as Chloride Inhibitor: Synthesis, Characterization, Assessment of Chloride Adsorption Performance. Materials. 2018; 11(12):2537. https://doi.org/10.3390/ma11122537
Chicago/Turabian StyleChi, Lin, Zheng Wang, Youfang Zhou, Shuang Lu, and Yan Yao. 2018. "Layered Double Hydroxides Precursor as Chloride Inhibitor: Synthesis, Characterization, Assessment of Chloride Adsorption Performance" Materials 11, no. 12: 2537. https://doi.org/10.3390/ma11122537
APA StyleChi, L., Wang, Z., Zhou, Y., Lu, S., & Yao, Y. (2018). Layered Double Hydroxides Precursor as Chloride Inhibitor: Synthesis, Characterization, Assessment of Chloride Adsorption Performance. Materials, 11(12), 2537. https://doi.org/10.3390/ma11122537