Predicting Viscosity and Surface Tension at High Temperature of Porcelain Stoneware Bodies: A Methodological Approach
Abstract
1. Introduction
- The vitrification path, which entails melting of fluxing minerals (primarily feldspars and sericite) and leads to a large amount of vitreous phase, typically ranging from 60 to 75% by weight [6];
- The first aimed at estimating the viscosity of the liquid phase, depending on both its composition and temperature;
- The second at evaluating the contribution of the solid fraction to the viscosity of the tile as a whole.
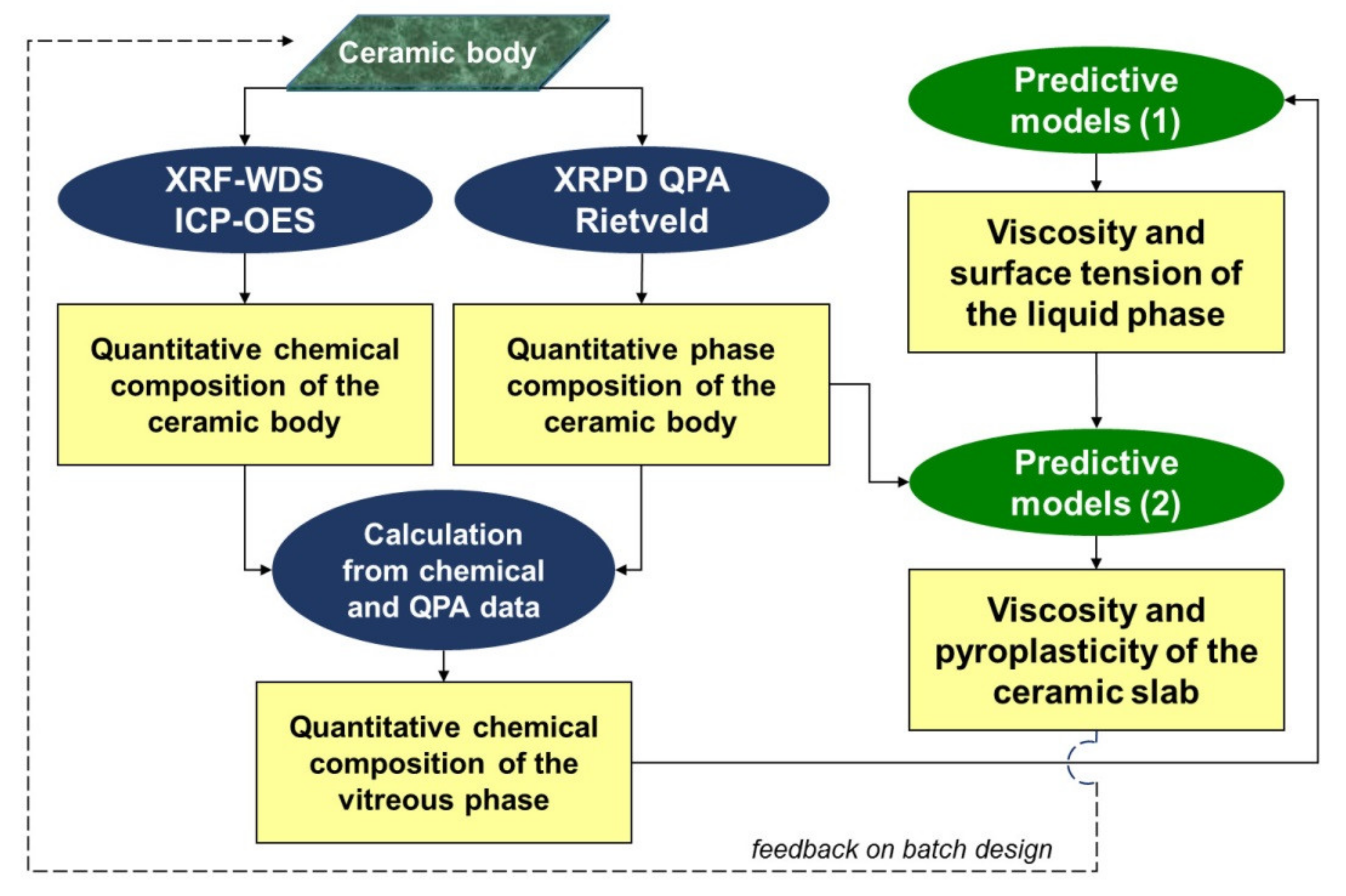
2. Materials and Methods
- (i)
- The maximum content of alumina considered by the Fluegel’s model (maximum Al2O3 19 wt.%) is too low for the systems here in exam (Al2O3 content from 14.5 to 26.0 wt.%, see Table 1);
- (ii)
- Al is considered to act as a glass network modifier (as in alkali glasses) instead of a glass network former, as it occurs in feldspathic melts [37].
3. Results and Discussion
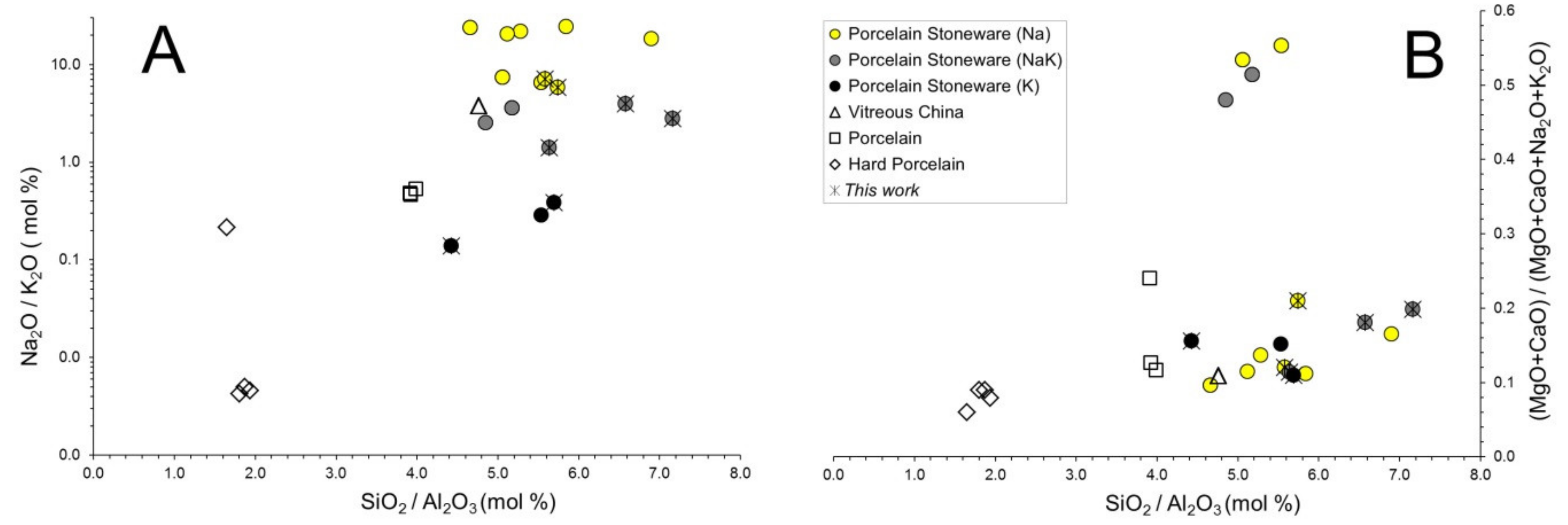
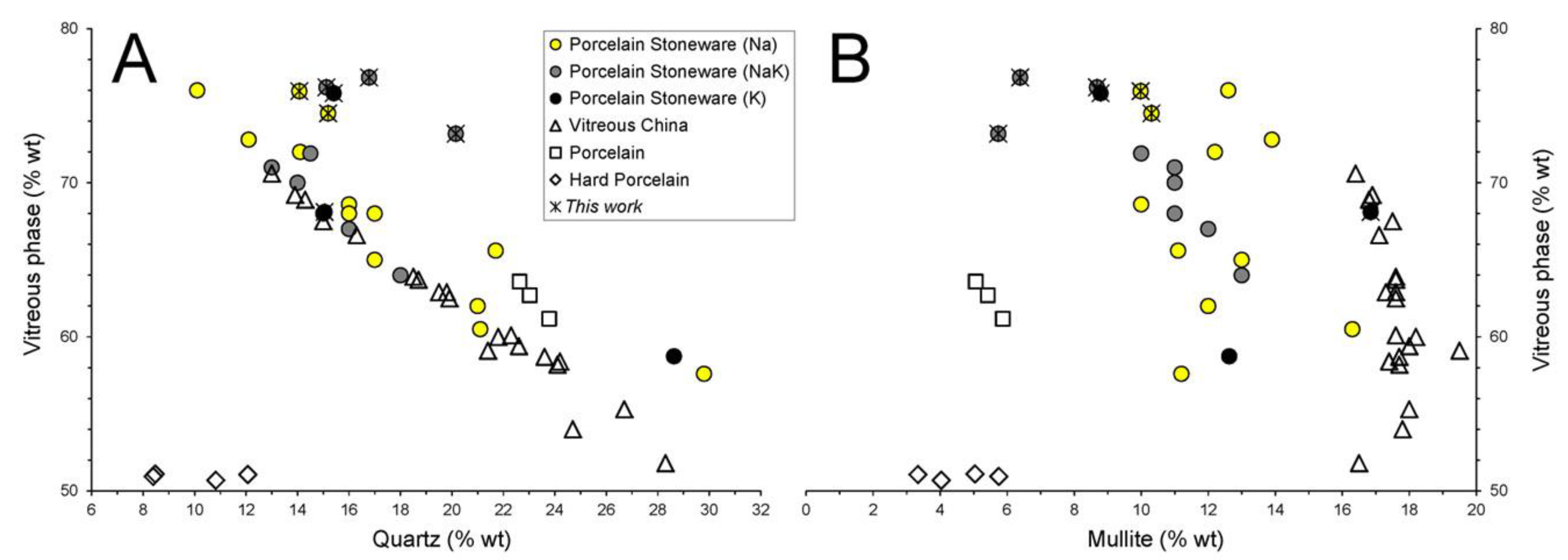
3.1. Chemical and Phase Composition of Ceramic Bodies
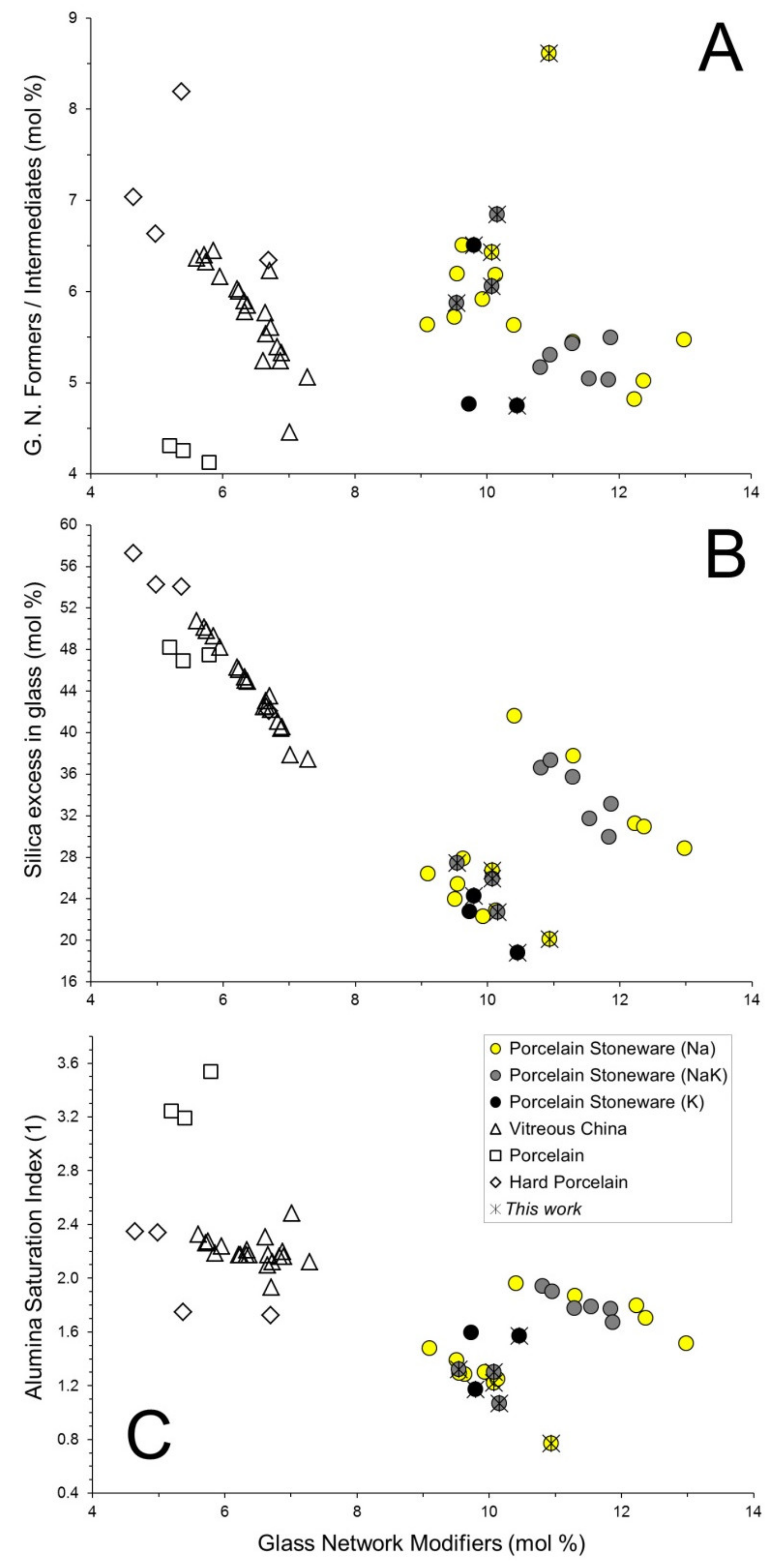
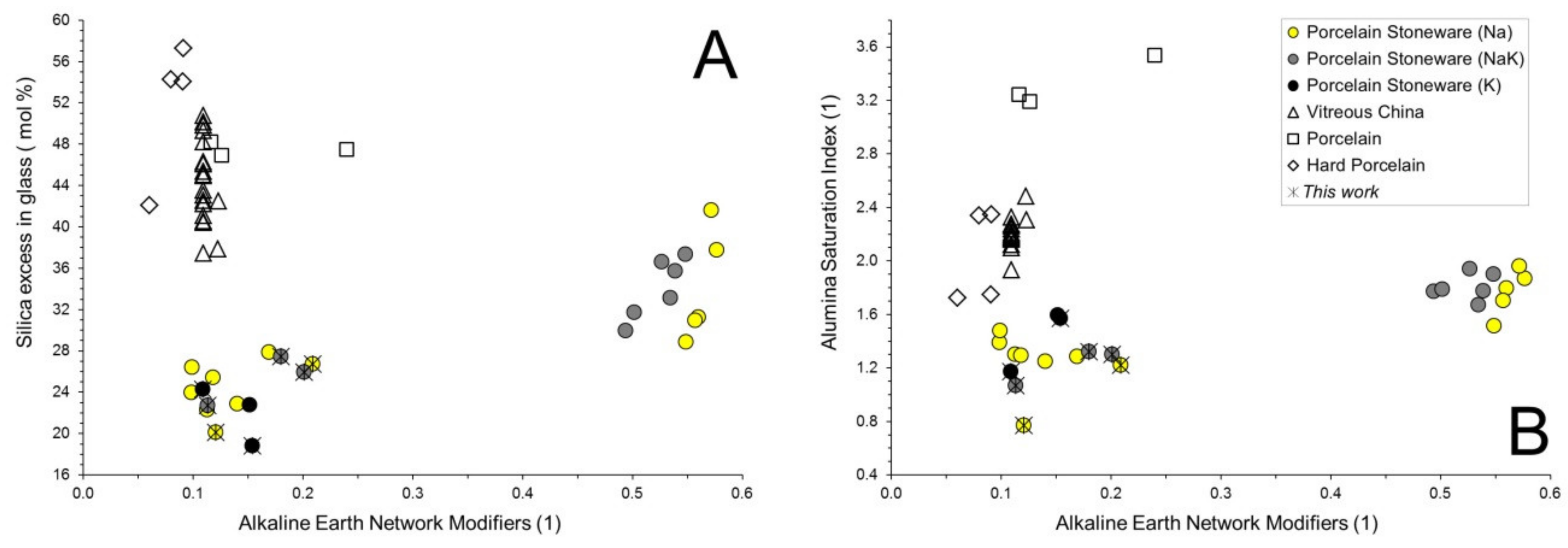
3.2. Chemical Composition of the Vitreous Phases
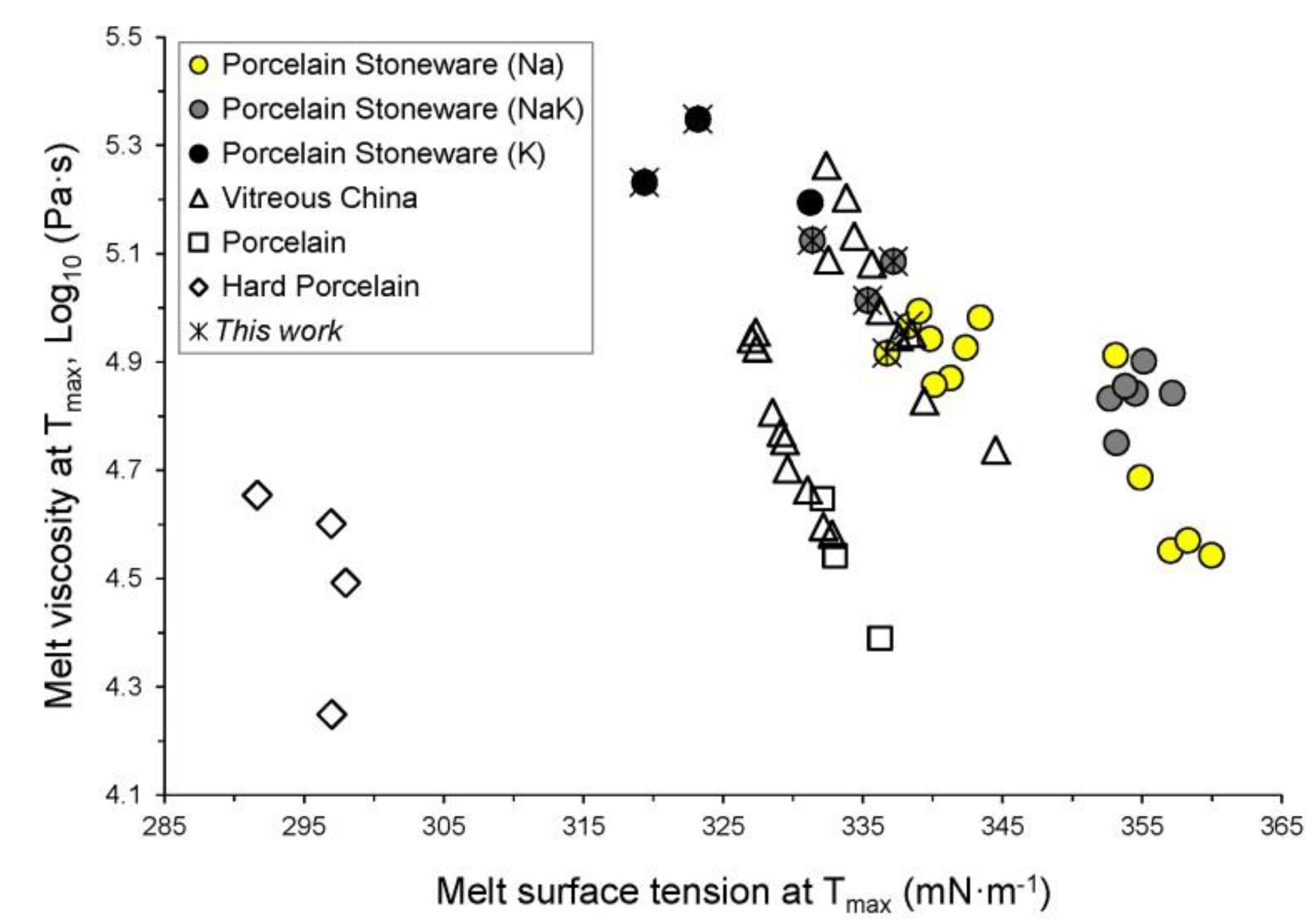
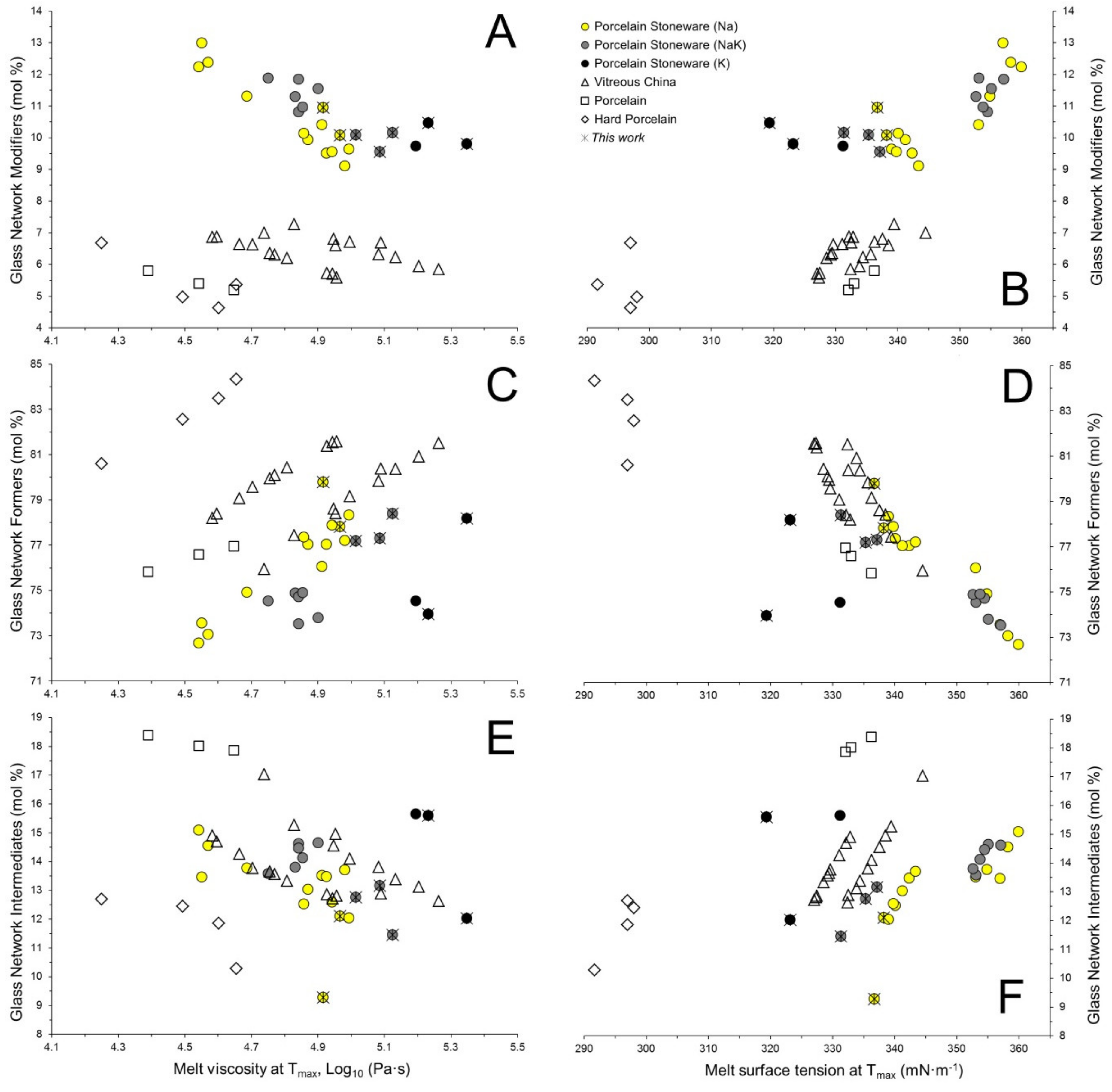
3.3. Physical Properties at High Temperature of the Vitreous Phases
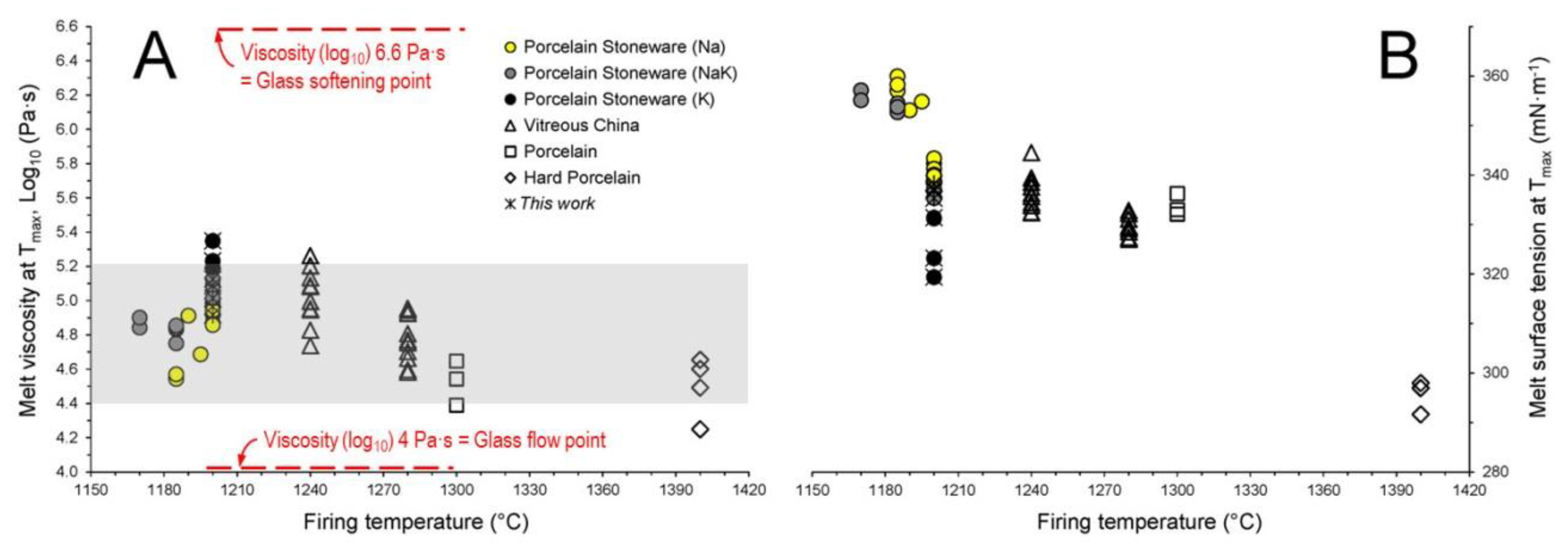
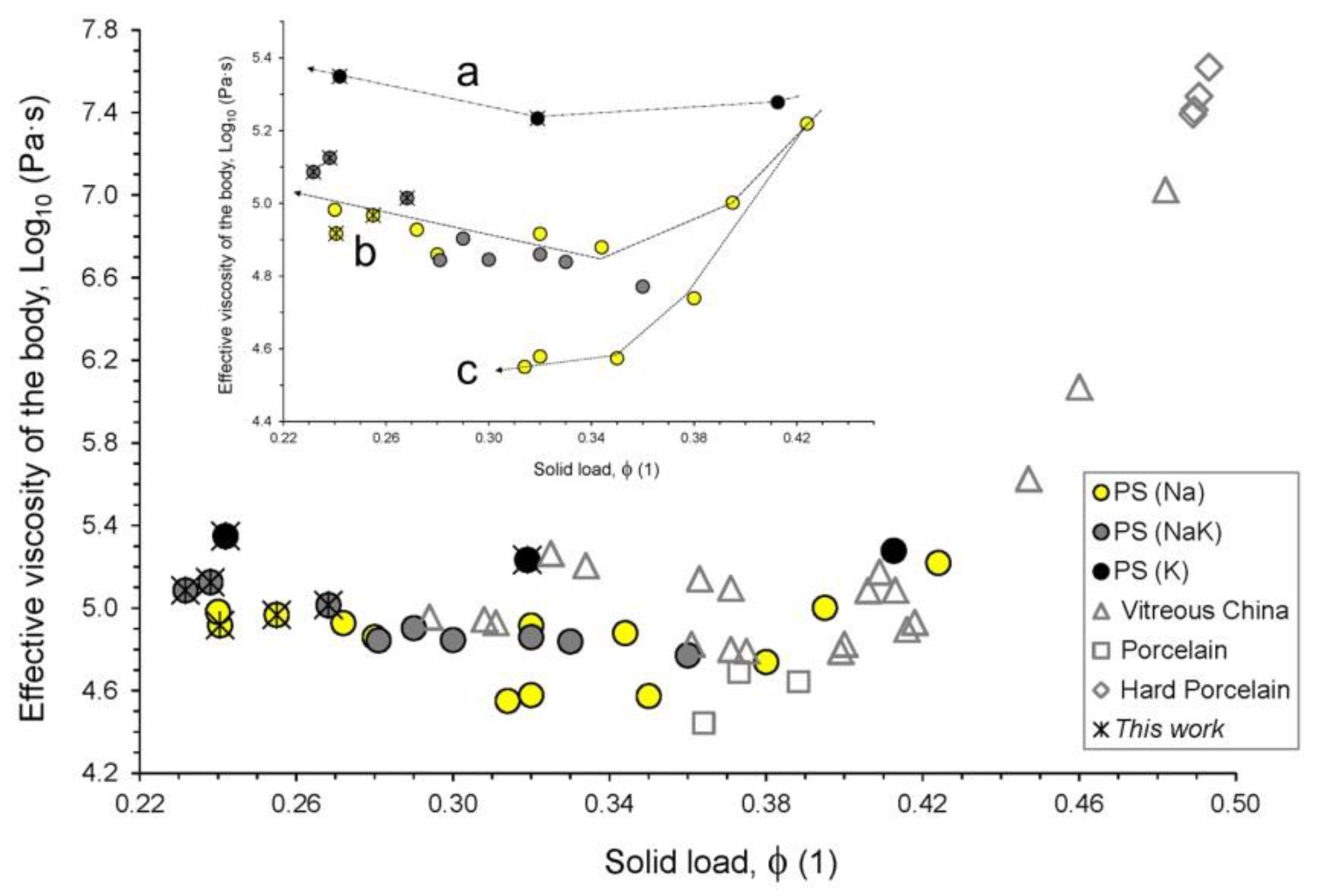
3.4. Repercussions on Firing Behavior
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fiori, C.; Fabbri, C.; Ravaglioli, A. Chemical-mineralogical classification of raw materials and of tile bodies. Int. Ceram. J. 1989, 44, 16–24. [Google Scholar]
- Dondi, M.; Ercolani, G.; Melandri, C.; Mingazzini, C.; Marsigli, M. The chemical composition of porcelain stoneware tiles and its influence on microstructural and mechanical properties. Interceram 1999, 48, 75–83. [Google Scholar]
- Dondi, M.; Raimondo, M.; Zanelli, C. Clays and bodies for ceramic tiles: Reappraisal and technological classification. Appl. Clay Sci. 2014, 96, 91–109. [Google Scholar] [CrossRef]
- Raimondo, M.; Dondi, M.; Zanelli, C.; Guarini, G.; Gozzi, A.; Marani, F.; Fossa, L. Processing and properties of large-sized ceramic slabs. Bol. Soc. Esp. Ceram. Vidr. 2010, 49, 289–296. [Google Scholar]
- Da Silva, A.L.; Feltrin, J.; Dal Bó, M.; Bernardin, A.M.; Hotza, D. Effect of reduction of thickness on microstructure and properties of porcelain stoneware tiles. Ceram. Int. 2014, 40, 14693–14699. [Google Scholar] [CrossRef]
- Zanelli, C.; Raimondo, M.; Guarini, G.; Dondi, M. The vitreous phase of porcelain stoneware: Composition, evolution during sintering and physical properties. J. Non-Cryst. Solids 2011, 357, 3251–3260. [Google Scholar] [CrossRef]
- Cambier, F.; Leriche, A. Vitrification. In Processing of Ceramics Part II; Cahn, R.W., Hanson, P., Kramer, E.J., Eds.; VCH: Basel, Switzerland, 1996; Volume 17B, pp. 124–144. [Google Scholar]
- Prado, M.O.; Zanotto, E.D.; Müller, R. Model for sintering polydispersed glass particles. J. Non-Cryst. Solids 2001, 279, 169–178. [Google Scholar] [CrossRef]
- Buchtel, A.M.; Carty, W.M.; Noirot, M.D. Pyroplastic deformation revisited. White Wares Mater. Ceram. Eng. Sci. Proc. 2009, 25, 25–42. [Google Scholar]
- Dos Santos Conserva, L.R.; Melchiades, F.G.; Nastri, S.; Boschi, A.O.; Dondi, M.; Guarini, G.; Raimondo, M.; Zanelli, C. Pyroplastic deformation of porcelain stoneware tiles: Wet vs. dry processing. J. Eur. Ceram. Soc. 2017, 37, 333–342. [Google Scholar] [CrossRef]
- Melchiades, F.G.; dos Santos, L.R.; Nastri, S.; Boschi, A.O. Gres porcelánico esmaltado producido por vía seca: Materias primas fundentes. Bol. Soc. Esp. Ceram. Vidr. 2012, 51, 133–138. [Google Scholar]
- Zanelli, C.; Ardit, M.; Conte, S.; Soldati, R.; Cruciani, G.; Dondi, M. Viscous flow sintering of porcelain stoneware revisited. In Proceedings of the 15th World Congress on QUALICER 2018 Ceramic Tile Quality, Castellón, Spain, 12 February 2018. [Google Scholar]
- Iqbal, Y.; Lee, W.E. Microstructural evolution in triaxial porcelain. J. Am. Ceram. Soc. 2000, 83, 3121–3127. [Google Scholar] [CrossRef]
- Porte, F.; Brydson, R.; Rand, B.; Riley, F.L. Creep viscosity of vitreous china. J. Am. Ceram. Soc. 2004, 87, 923–928. [Google Scholar] [CrossRef]
- Costa, A. Viscosity of high crystal content melts: Dependence on solid fraction. Geophys. Res. Lett. 2005, 32. [Google Scholar] [CrossRef]
- Pascual, M.J.; Durán, A.; Prado, M.O.; Zanotto, E.D. Model for sintering devitrifying glass particles with embedded rigid fibers. J. Am. Ceram. Soc. 2005, 88, 1427–1434. [Google Scholar] [CrossRef]
- Fluegel, A. Glass viscosity calculation based on a global statistical modelling approach. Glass Technol.-Part A 2007, 48, 13–30. [Google Scholar]
- Urbain, G.; Bottinga, Y.; Richet, P. Viscosity of liquid silica, silicates and alumino-silicates. Geochim. Cosmochim. Acta 1982, 46, 1061–1072. [Google Scholar] [CrossRef]
- Giordano, D.; Russell, J.K.; Dingwell, D.B. Viscosity of magmatic liquids: A model. Earth Planet. Sci. Lett. 2008, 271, 123–134. [Google Scholar] [CrossRef]
- Giordano, D.; Dingwell, D.B. Non-Arrhenian multicomponent melt viscosity: A model. Earth Planet. Sci. Lett. 2003, 208, 337–349. [Google Scholar] [CrossRef]
- Zanelli, C.; Baldi, G.; Dondi, M.; Ercolani, G.; Guarini, G.; Raimondo, M. Glass–ceramic frits for porcelain stoneware bodies: Effects on sintering, phase composition and technological properties. Ceram. Int. 2008, 34, 455–465. [Google Scholar] [CrossRef]
- Carty, W.M.; Senapati, U. Porcelain–Raw materials, processing, phase evolution, and mechanical behavior. J. Am. Ceram. Soc. 1998, 81, 3–20. [Google Scholar] [CrossRef]
- Carty, W.M. Observations on the glass phase composition in porcelains. Ceram. Eng. Sci. Proc. 2002, 23, 79–94. [Google Scholar]
- Raimondo, M.; Zanelli, C.; Guarini, G.; Dondi, M.; Fabbroni, R.; Cortesi, T. Process of pyroplastic shaping for special-purpose porcelain stoneware tiles. Ceram. Int. 2009, 35, 1975–1984. [Google Scholar] [CrossRef]
- Jiang, Z.H.; Zhang, Q.-Y. The structure of glass: A phase equilibrium diagram approach. Prog. Mater. Sci. 2014, 61, 144–215. [Google Scholar] [CrossRef]
- Martín-Márquez, J.; Rincón, J.M.; Romero, M. Effect of microstructure on mechanical properties of porcelain stoneware. J. Eur. Ceram. Soc. 2010, 30, 3063–3069. [Google Scholar] [CrossRef]
- Lassinantti Gualtieri, M.; Romagnoli, M.; Gualtieri, A.F. Influence of body composition on the technological properties and mineralogy of stoneware: A DOE and mineralogical–microstructural study. J. Eur. Ceram. Soc. 2011, 31, 673–685. [Google Scholar] [CrossRef]
- Bernasconi, A.; Diella, V.; Pagani, A.; Pavese, A.; Francescon, F.; Young, K.; Stuart, J.; Tunnicliffe, L. The role of firing temperature, firing time and quartz grain size on phase-formation, thermal dilatation and water absorption in sanitary-ware vitreous bodies. J. Eur. Ceram. Soc. 2011, 31, 1353–1360. [Google Scholar] [CrossRef]
- Amigò, J.M.; Clausell, J.V.; Esteve, V.J.; Delgado, M.; Reventòs, M.M.; Ochando, L.E.; Debaerdemaeker, T.; Martı, F. X-ray powder diffraction phase analysis and thermomechanical properties of silica and alumina porcelains. J. Eur. Ceram. Soc. 2004, 24, 75–81. [Google Scholar] [CrossRef]
- Gualtieri, A.F. Thermal Behavior of the Raw Materials Forming Porcelain Stoneware Mixtures by Combined Optical and In Situ X-Ray Dilatometry. J. Am. Ceram. Soc. 2007, 90, 1222–1231. [Google Scholar] [CrossRef]
- Ban, T.; Okada, K. Structure refinement of mullite by the Rietveld method and a new method for estimation of chemical composition. J. Am. Ceram. Soc. 1992, 75, 227–230. [Google Scholar] [CrossRef]
- Giordano, D.; Dingwell, D.B.; Romano, C. Viscosity of a Teide phonolite in the welding interval. J. Volcanol. Geotherm. Res. 2000, 103, 239–245. [Google Scholar] [CrossRef]
- Giordano, D.; Romano, C.; Papale, P.; Dingwell, D.B. The viscosity of trachytes, and comparison with basalts, phonolites, and rhyolites. Chem. Geol. 2004, 213, 49–61. [Google Scholar] [CrossRef]
- Giordano, D.; Mangiacapra, A.; Potuzak, M.; Russell, J.K.; Romano, C.; Dingwell, D.B.; Di Muro, A. An expanded non-Arrhenian model for silicate melt viscosity: A treatment for metaluminous, peraluminous and peralkaline liquids. Chem. Geol. 2006, 229, 42–56. [Google Scholar] [CrossRef]
- Romano, C.; Giordano, D.; Papale, P.; Mincione, V.; Dingwell, D.B.; Rosi, M. The dry and hydrous viscosities of alkaline melts from Vesuvius and Phlegrean Fields. Chem. Geol. 2003, 202, 23–38. [Google Scholar] [CrossRef]
- Whittington, A.; Richet, P.; Linard, Y.; Holtz, F. The viscosity of hydrous phonolites and trachytes. Chem. Geol. 2001, 174, 209–223. [Google Scholar] [CrossRef]
- Le Losq, C.; Neuville, D.R.; Florian, P.; Henderson, G.S.; Massiot, D. The role of Al3+ on rheology and structural changes in sodium silicate and aluminosilicate glasses and melts. Geochim. Cosmochim. Acta 2014, 126, 495–517. [Google Scholar] [CrossRef]
- Dietzel, A. Relation between the surface tension and the structure of molten glass. Kolloid-Z. 1942, 100, 368–380. [Google Scholar] [CrossRef]
- Appen, A.A. Chemistry of Glass (Russ.); Khimiya: Leningrad, Russia, 1974; Volume 351. [Google Scholar]
- Martín-Márquez, J.; Rincón, J.M.; Romero, M. Mullite development on firing in porcelain stoneware bodies. J. Eur. Ceram. Soc. 2010, 30, 1599–1607. [Google Scholar]
- Watson, E.B.; Harrison, T.M. Zircon saturation revisited: Temperature and composition effects in a variety of crustal magma types. Earth Planet. Sci. Lett. 1983, 64, 295–304. [Google Scholar] [CrossRef]
- Dickinson, J.E., Jr.; Hess, P.C. Rutile solubility and titanium coordination in silicate melts. Geochim. Cosmochim. Acta 1985, 49, 2289–2296. [Google Scholar] [CrossRef]
- Hess, K.U.; Dingwell, D.B.; Gennaro, C.; Mincione, V. Viscosity–temperature behaviour of dry melts in the Qz–Ab–Or system. Chem. Geol. 2001, 174, 133–142. [Google Scholar] [CrossRef]
- Park, J.H.; Min, D.J.; Song, H.S. Amphoteric behavior of alumina in viscous flow and structure of CaO-SiO2(-MgO)-Al2O3 slags. Metall. Mater. Trans. B 2004, 35, 269–275. [Google Scholar] [CrossRef]
- Neuville, D.R.; Cormier, L.; Massiot, D. Al environment in tectosilicate and peraluminous glasses: A 27Al MQ-MAS NMR, Raman, and XANES investigation. Geochim. Cosmochim. Acta 2004, 68, 5071–5079. [Google Scholar] [CrossRef]
- Neuville, D.R.; Mysen, B.O. Role of aluminium in the silicate network: In situ, high-temperature study of glasses and melts on the join SiO2-NaAlO2. Geochim. Cosmochim. Acta 1996, 60, 1727–1737. [Google Scholar] [CrossRef]
- Costa, A.; Caricchi, L.; Bagdassarov, N. A model for the rheology of particle-bearing suspensions and partially molten rocks. Geochem. Geophys. 2009, 10, 1–13. [Google Scholar] [CrossRef]
- Krieger, I.M.; Dougherty, T.J. A mechanism for non-Newtonian flow in suspensions of rigid spheres. J. Rheol. 1959, 3, 137–152. [Google Scholar] [CrossRef]
| wt.% | Sample | Amount | SiO2 | TiO2 | ZrO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | Calculated From |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Porcelain stoneware | 1200 | 58.7 | 64.9 | 0.7 | — | 21.2 | 1.5 | 0.4 | 0.6 | 1.7 | 8.8 | [26] |
| STD.6 | 65.0 | 67.7 | 0.5 | — | 19.6 | 1.3 | 2.9 | 2.1 | 4.9 | 1.1 | [10] | |
| QF | 62.0 | 68.6 | 0.5 | 0.5 | 19.6 | 1.3 | 2.8 | 1.7 | 3.8 | 1.0 | ||
| QM | 68.0 | 69.6 | 0.5 | 1.0 | 19.1 | 1.2 | 2.5 | 1.5 | 3.6 | 0.9 | ||
| KM | 70.0 | 66.7 | 0.4 | — | 21.2 | 1.2 | 2.5 | 1.5 | 3.9 | 2.6 | ||
| QFKM | 64.0 | 68.1 | 0.5 | 0.5 | 19.3 | 1.3 | 2.7 | 1.7 | 4.0 | 1.9 | ||
| QMKM | 67.0 | 68.2 | 0.5 | 1.0 | 19.4 | 1.2 | 2.6 | 1.6 | 3.7 | 1.8 | ||
| STD.6 | 68.6 | 65.9 | 0.5 | 1.0 | 21.3 | 1.2 | 2.8 | 2.0 | 4.4 | 1.0 | ||
| STD.9 | 68.0 | 66.5 | 0.5 | 1.0 | 20.5 | 1.3 | 2.8 | 2.0 | 4.5 | 1.0 | ||
| KM | 71.0 | 66.6 | 0.4 | 1.0 | 20.6 | 1.2 | 2.4 | 1.5 | 3.7 | 2.5 | ||
| QFKM | 71.9 | 67.7 | 0.4 | 0.9 | 20.4 | 1.2 | 2.4 | 1.5 | 3.7 | 1.7 | ||
| QMKM | 68.0 | 68.1 | 0.5 | 1.0 | 19.9 | 1.2 | 2.5 | 1.6 | 3.5 | 1.8 | ||
| Na | 75.9 | 70.3 | 0.9 | — | 18.2 | 0.6 | 0.4 | 0.5 | 7.5 | 1.6 | This Work | |
| NaB | 74.5 | 71.4 | 0.3 | — | 17.9 | 0.8 | 0.6 | 1.0 | 6.4 | 1.7 | ||
| AT | 76.7 | 70.8 | 0.6 | — | 18.3 | 0.8 | 0.3 | 1.0 | 5.9 | 2.3 | ||
| ATP | 72.9 | 70.8 | 0.5 | — | 17.8 | 0.8 | 0.7 | 0.8 | 5.5 | 3.1 | ||
| NaK | 76.2 | 68.7 | 0.9 | — | 19.0 | 0.6 | 0.4 | 0.4 | 4.8 | 5.2 | ||
| K | 75.8 | 68.2 | 0.8 | — | 18.6 | 0.6 | 0.4 | 0.4 | 2.2 | 8.9 | ||
| KB | 68.1 | 67.3 | 0.3 | — | 18.3 | 0.8 | 0.7 | 0.4 | 1.0 | 11.2 | ||
| 2 | 72.8 | 70.2 | 0.4 | — | 19.9 | 0.6 | 0.1 | 0.6 | 7.8 | 0.4 | [27] | |
| 7 | 76.0 | 70.2 | 0.4 | — | 20.3 | 0.5 | 0.1 | 0.6 | 7.5 | 0.4 | ||
| 21 | 65.6 | 70.4 | 0.4 | — | 19.3 | 0.6 | 0.2 | 0.7 | 8.1 | 0.4 | ||
| 22 | 72.0 | 71.0 | 0.5 | — | 18.5 | 0.6 | 0.4 | 0.7 | 7.9 | 0.5 | ||
| 24 | 57.6 | 72.2 | 0.5 | — | 17.8 | 0.6 | 0.5 | 0.7 | 7.3 | 0.5 | ||
| 25 | 60.5 | 71.4 | 0.5 | — | 18.5 | 0.7 | 0.2 | 0.7 | 7.7 | 0.4 | ||
| Vitreous china | 0-1240-50 | 51.8 | 67.2 | 0.5 | — | 24.1 | 1.3 | 0.3 | 0.3 | 4.3 | 2.0 | [28] |
| 20-1240-50 | 55.3 | 69.3 | 0.5 | — | 21.8 | 1.2 | 0.3 | 0.2 | 4.7 | 1.9 | ||
| 40-1240-50 | 58.7 | 70.7 | 0.5 | — | 20.9 | 1.1 | 0.3 | 0.2 | 4.5 | 1.8 | ||
| 60-1240-50 | 59.4 | 71.4 | 0.5 | — | 20.3 | 1.1 | 0.3 | 0.2 | 4.4 | 1.8 | ||
| 80-1240-50 | 59.1 | 73.1 | 0.5 | — | 18.6 | 1.1 | 0.3 | 0.2 | 4.4 | 1.8 | ||
| 0-1280-50 | 58.4 | 70.2 | 0.5 | — | 21.4 | 1.1 | 0.3 | 0.2 | 4.5 | 1.8 | ||
| 20-1280-50 | 58.2 | 70.5 | 0.5 | — | 21.1 | 1.1 | 0.3 | 0.2 | 4.5 | 1.8 | ||
| 40-1280-50 | 60.1 | 71.3 | 0.4 | — | 20.6 | 1.1 | 0.3 | 0.2 | 4.4 | 1.7 | ||
| 60-1280-50 | 62.5 | 72.4 | 0.4 | — | 19.8 | 1.0 | 0.3 | 0.2 | 4.2 | 1.7 | ||
| 80-1280-50 | 62.9 | 72.6 | 0.4 | — | 19.7 | 1.0 | 0.3 | 0.2 | 4.2 | 1.7 | ||
| 0-1240-18 | 54.0 | 70.3 | 0.5 | — | 21.4 | 1.2 | 0.3 | 0.2 | 4.1 | 1.9 | ||
| 20-1240-18 | 62.9 | 72.2 | 0.4 | — | 20.0 | 1.0 | 0.3 | 0.2 | 4.2 | 1.7 | ||
| 40-1240-18 | 63.7 | 72.9 | 0.4 | — | 19.4 | 1.0 | 0.3 | 0.2 | 4.1 | 1.6 | ||
| 60-1240-18 | 66.6 | 73.6 | 0.4 | — | 19.1 | 1.0 | 0.3 | 0.2 | 3.9 | 1.6 | ||
| 80-1240-18 | 67.5 | 74.3 | 0.4 | — | 18.4 | 1.0 | 0.3 | 0.2 | 3.9 | 1.6 | ||
| 0-1280-18 | 60.0 | 72.0 | 0.4 | — | 19.9 | 1.1 | 0.3 | 0.2 | 4.4 | 1.7 | ||
| 20-1280-18 | 63.9 | 73.0 | 0.4 | — | 19.3 | 1.0 | 0.3 | 0.2 | 4.1 | 1.6 | ||
| 40-1280-18 | 68.9 | 74.1 | 0.4 | — | 18.8 | 0.9 | 0.2 | 0.2 | 3.8 | 1.5 | ||
| 60-1280-18 | 69.2 | 74.3 | 0.4 | — | 18.6 | 0.9 | 0.2 | 0.2 | 3.8 | 1.5 | ||
| 80-1280-18 | 70.6 | 74.3 | 0.4 | — | 18.7 | 0.9 | 0.2 | 0.2 | 3.7 | 1.5 | ||
| Porcelain | S5 | 62.7 | 67.4 | 0.9 | — | 25.1 | 0.6 | — | 0.5 | 1.4 | 4.1 | [29] |
| S6 | 61.2 | 66.9 | 0.5 | — | 25.5 | 0.8 | — | 0.6 | 1.3 | 4.4 | ||
| S7 | 63.6 | 66.3 | 0.6 | — | 26.0 | 0.8 | 0.5 | 0.5 | 1.3 | 4.1 | ||
| Hard porcelain | S1 | 51.1 | 74.1 | 0.6 | — | 17.8 | 0.8 | — | 0.3 | — | 6.5 | [29] |
| S2 | 50.7 | 75.4 | 0.7 | — | 16.7 | 0.9 | — | 0.4 | — | 6.0 | ||
| S3 | 51.1 | 76.7 | 0.7 | — | 14.5 | 0.8 | — | 0.4 | — | 7.0 | ||
| S4 | 50.9 | 72.0 | 0.9 | — | 17.5 | 0.9 | — | 0.3 | 1.0 | 7.2 |
| Porcelain Stoneware | Tmax | Viscosity | Surface Tension | Tg, Glass Transition | Sample | Tmax | Viscosity | Surface Tension | Tg, Glass Transition | |
|---|---|---|---|---|---|---|---|---|---|---|
| °C | log10 Pa·s | mN·m−1 | °C | °C | log10 Pa·s | mN·m−1 | °C | |||
| 1200 | 1200 | 5.19 | 331.2 | 761 | Vitreous China | 0-1240-50 | 1240 | 4.74 | 344.5 | 769 |
| STD.6 | 1185 | 4.55 | 357.0 | 727 | 20-1240-50 | 1240 | 4.83 | 339.4 | 762 | |
| QF | 1195 | 4.69 | 354.9 | 744 | 40-1240-50 | 1240 | 4.95 | 337.6 | 767 | |
| QM | 1190 | 4.91 | 353.1 | 752 | 60-1240-50 | 1240 | 5.00 | 336.3 | 768 | |
| KM | 1170 | 4.84 | 357.1 | 739 | 80-1240-50 | 1240 | 5.09 | 332.5 | 767 | |
| QFKM | 1185 | 4.75 | 353.2 | 737 | 0-1280-50 | 1280 | 4.58 | 332.8 | 767 | |
| QMKM | 1185 | 4.83 | 352.7 | 743 | 20-1280-50 | 1280 | 4.60 | 332.2 | 767 | |
| STD.6 | 1185 | 4.54 | 360.0 | 736 | 40-1280-50 | 1280 | 4.66 | 331.0 | 769 | |
| STD.9 | 1185 | 4.57 | 358.3 | 734 | 60-1280-50 | 1280 | 4.75 | 329.4 | 773 | |
| KM | 1170 | 4.9 | 355.1 | 741 | 80-1280-50 | 1280 | 4.77 | 329.2 | 774 | |
| QFKM | 1185 | 4.84 | 354.5 | 747 | 0-1240-18 | 1240 | 4.95 | 338.5 | 772 | |
| QMKM | 1185 | 4.86 | 353.8 | 748 | 20-1240-18 | 1240 | 5.08 | 335.6 | 774 | |
| Na | 1200 | 4.92 | 336.7 | 719 | 40-1240-18 | 1240 | 5.13 | 334.4 | 775 | |
| NaB | 1200 | 4.97 | 338.3 | 727 | 60-1240-18 | 1240 | 5.20 | 333.8 | 779 | |
| AT | 1200 | 5.09 | 337.2 | 737 | 80-1240-18 | 1240 | 5.26 | 332.4 | 781 | |
| ATP | 1200 | 5.01 | 335.3 | 730 | 0-1280-18 | 1280 | 4.70 | 329.6 | 769 | |
| NaK | 1200 | 5.13 | 331.4 | 738 | 20-1280-18 | 1280 | 4.81 | 328.5 | 775 | |
| K | 1200 | 5.35 | 323.2 | 752 | 40-1280-18 | 1280 | 4.93 | 327.4 | 782 | |
| KB | 1200 | 5.23 | 319.4 | 742 | 60-1280-18 | 1280 | 4.94 | 327.0 | 783 | |
| 2 | 1200 | 4.93 | 342.4 | 726 | 80-1280-18 | 1280 | 4.96 | 327.3 | 785 | |
| 7 | 1200 | 4.98 | 343.4 | 733 | Porcelain | S5 | 1300 | 4.65 | 332.1 | 808 |
| 21 | 1200 | 4.87 | 341.3 | 719 | S6 | 1300 | 4.54 | 333.0 | 802 | |
| 22 | 1200 | 4.86 | 340.1 | 716 | S7 | 1300 | 4.39 | 336.2 | 798 | |
| 24 | 1200 | 4.99 | 339.0 | 726 | Hard porcelain | S1 | 1400 | 4.49 | 298.0 | 817 |
| 25 | 1200 | 4.94 | 339.8 | 722 | S2 | 1400 | 4.60 | 296.9 | 823 | |
| S3 | 1400 | 4.65 | 291.6 | 814 | ||||||
| S4 | 1400 | 4.25 | 297.0 | 790 | ||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conte, S.; Zanelli, C.; Ardit, M.; Cruciani, G.; Dondi, M. Predicting Viscosity and Surface Tension at High Temperature of Porcelain Stoneware Bodies: A Methodological Approach. Materials 2018, 11, 2475. https://doi.org/10.3390/ma11122475
Conte S, Zanelli C, Ardit M, Cruciani G, Dondi M. Predicting Viscosity and Surface Tension at High Temperature of Porcelain Stoneware Bodies: A Methodological Approach. Materials. 2018; 11(12):2475. https://doi.org/10.3390/ma11122475
Chicago/Turabian StyleConte, Sonia, Chiara Zanelli, Matteo Ardit, Giuseppe Cruciani, and Michele Dondi. 2018. "Predicting Viscosity and Surface Tension at High Temperature of Porcelain Stoneware Bodies: A Methodological Approach" Materials 11, no. 12: 2475. https://doi.org/10.3390/ma11122475
APA StyleConte, S., Zanelli, C., Ardit, M., Cruciani, G., & Dondi, M. (2018). Predicting Viscosity and Surface Tension at High Temperature of Porcelain Stoneware Bodies: A Methodological Approach. Materials, 11(12), 2475. https://doi.org/10.3390/ma11122475







