Tuning the Surface Plasmon Resonance of Lanthanum Hexaboride to Absorb Solar Heat: A Review
Abstract
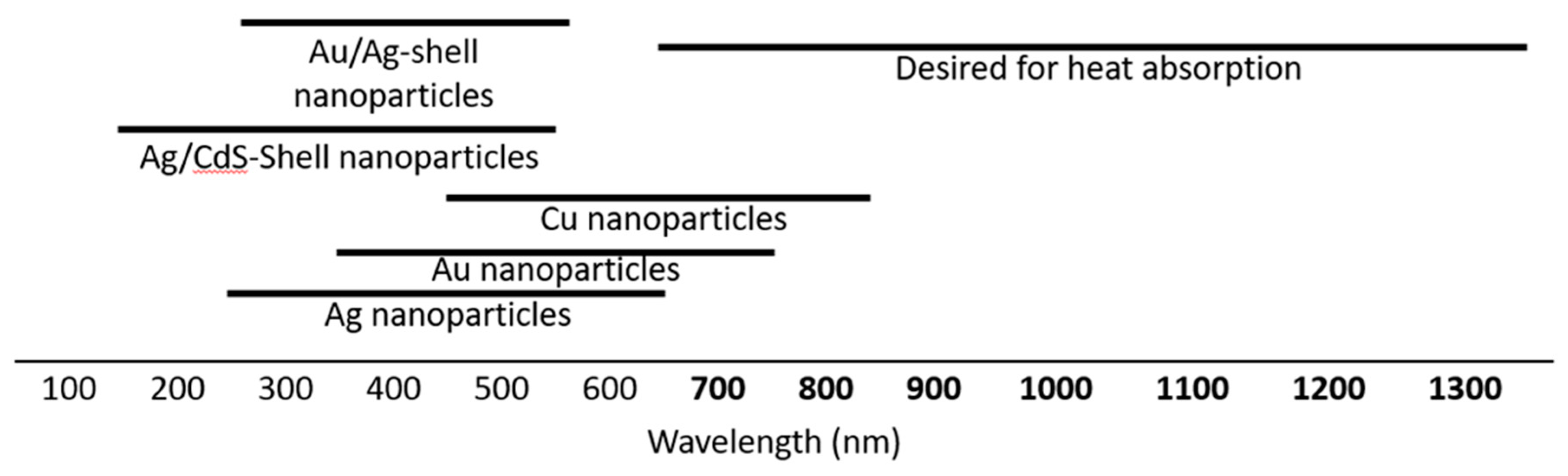
1. Introduction
2. Relating LaB6 Fundamentals to Plasmonics
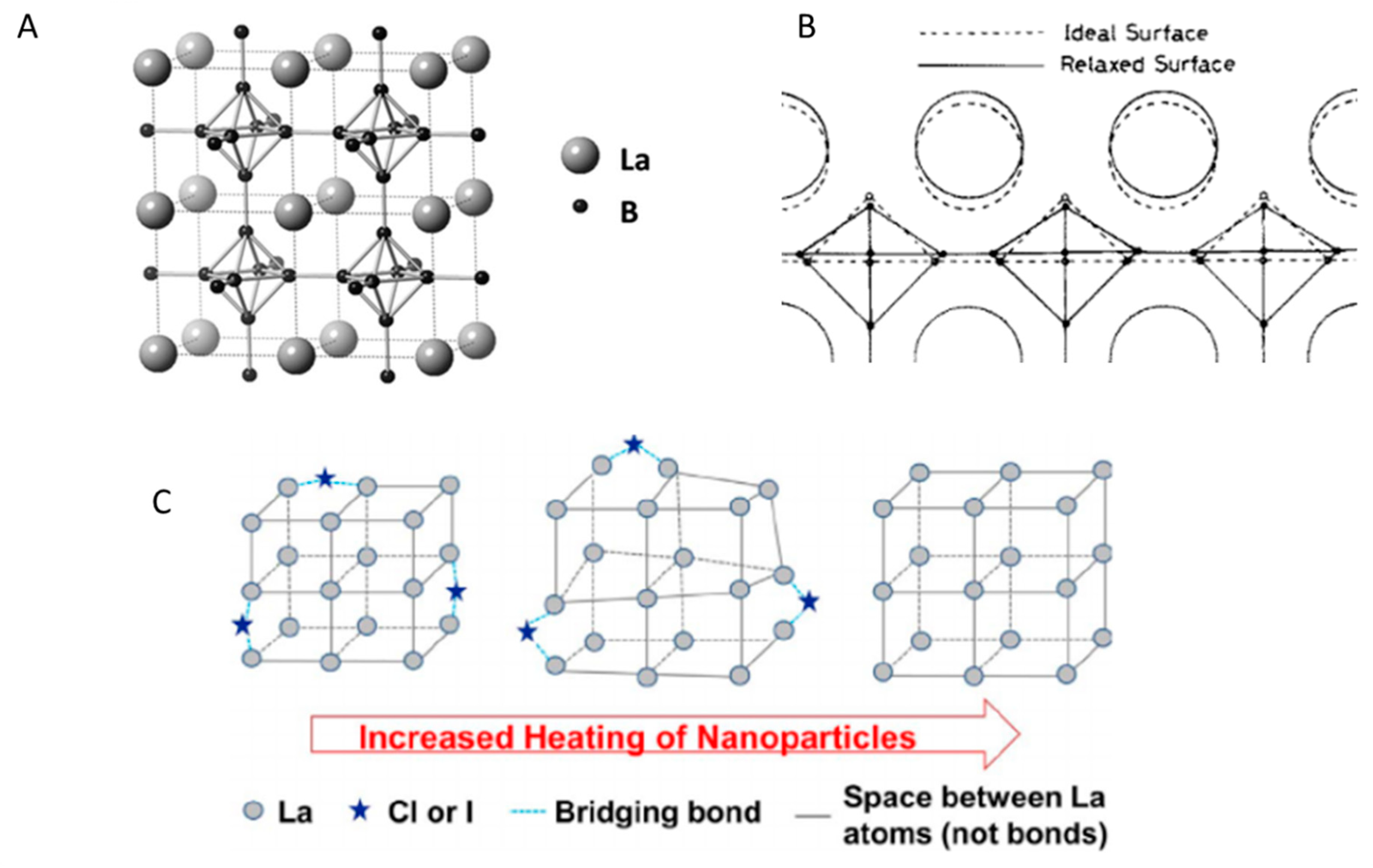
2.1. Crystal Structure of LaB6
2.1.1. Vibrational Structure of LaB6
2.1.2. Structural Defects
3. Controlling the Plasmon of LaB6
3.1. Doping
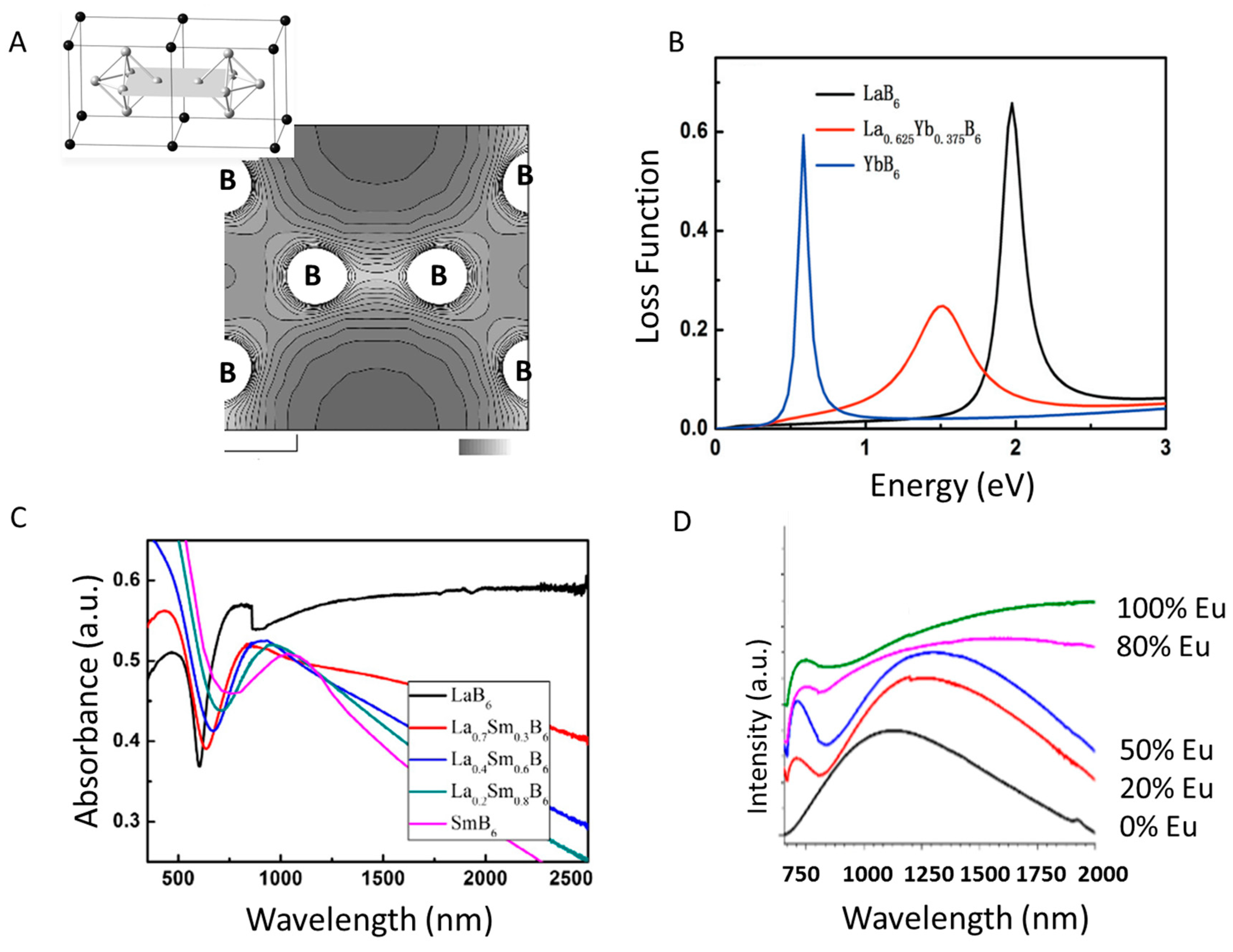
3.1.1. Yb-Doped LaB6 Nanoparticles: Theory
3.1.2. Sm-Doped LaB6 Nanoparticles
3.1.3. Eu-Doped LaB6 Nanoparticles
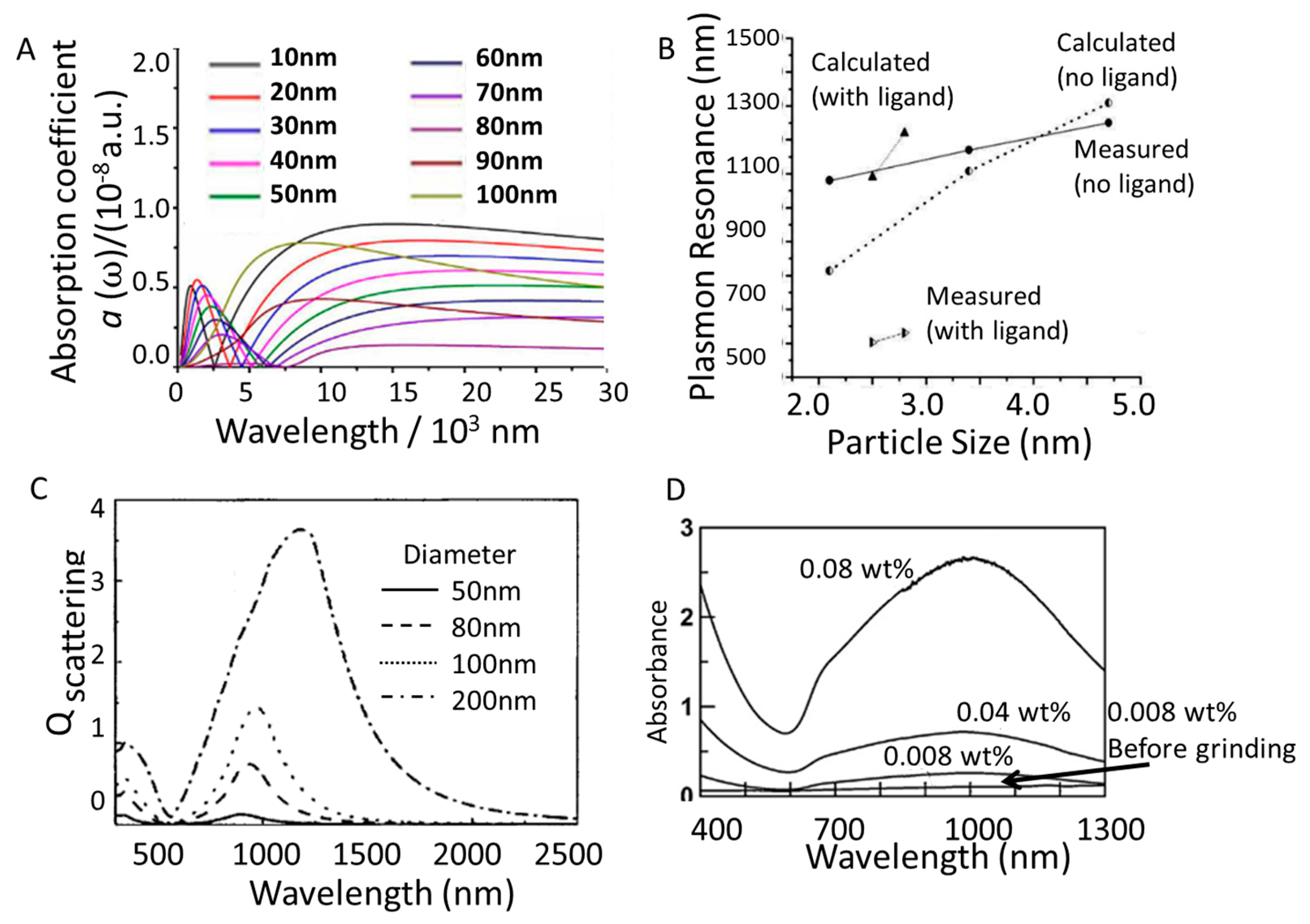
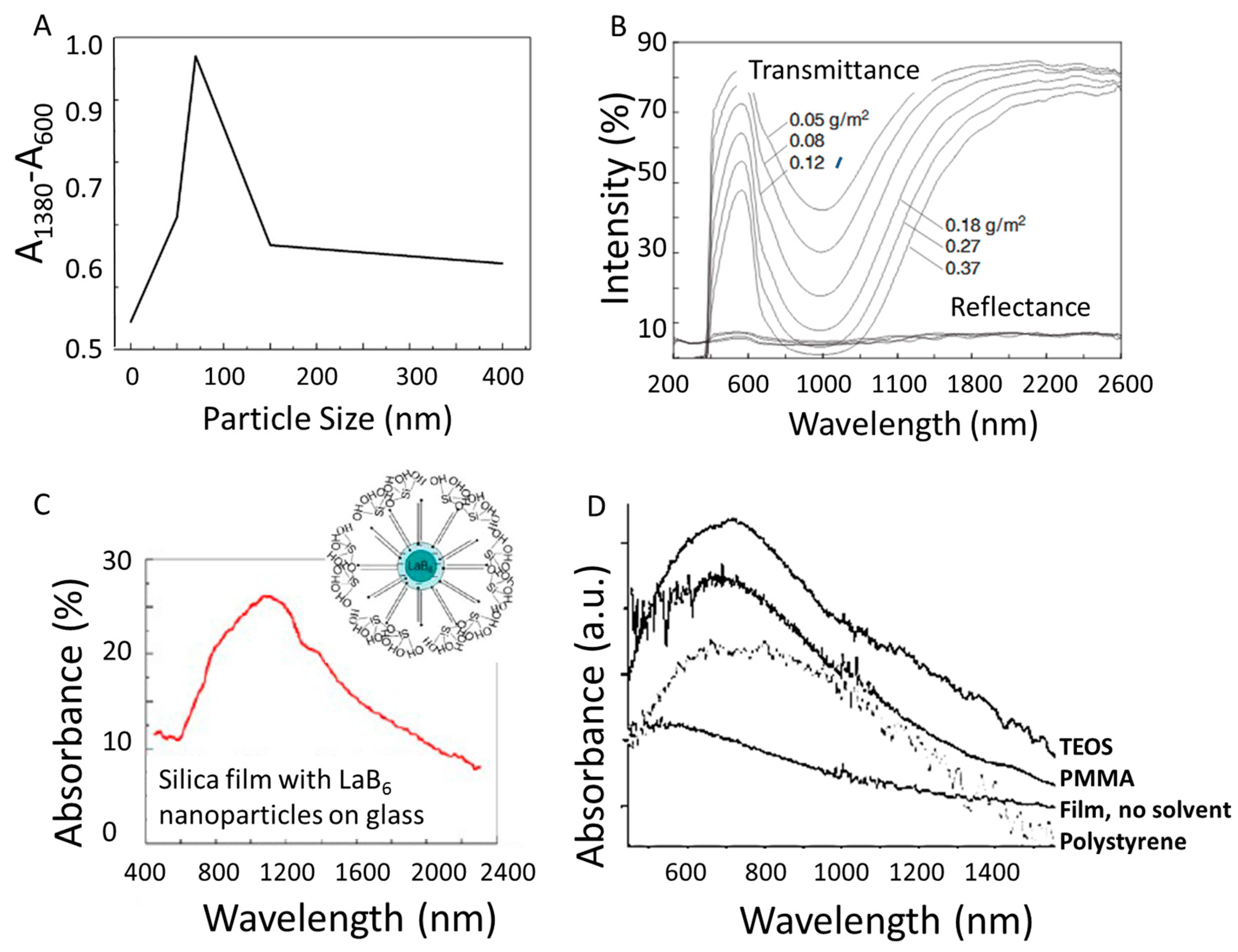
3.2. Nanoparticle Size
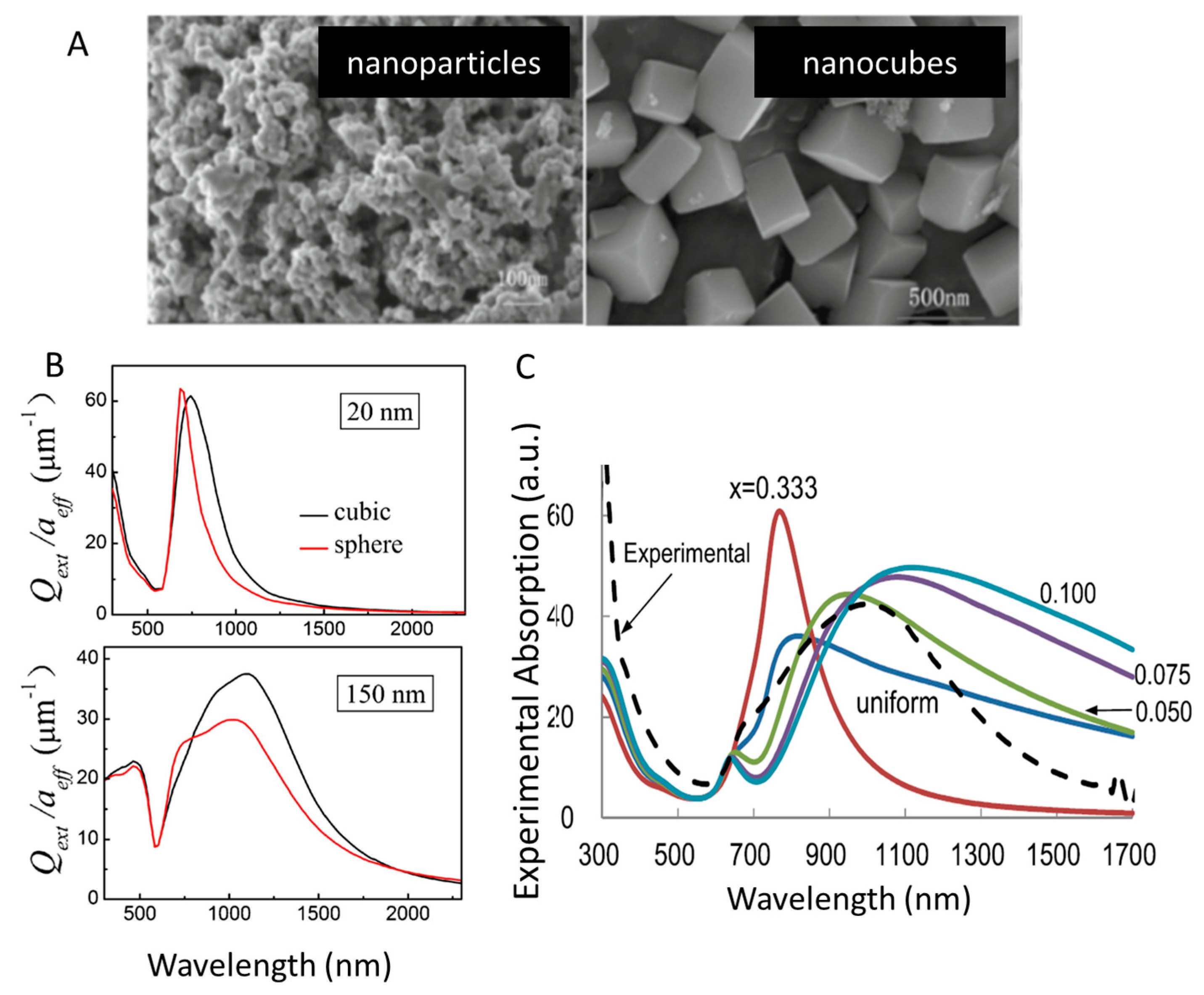
3.3. Morphology
4. Improving LaB6 Plasmonic Applications
Future Challenges of Plasmonic LaB6
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Aslam, U.; Rao, V.G.; Chavez, S.; Linic, S. Catalytic conversion of solar to chemical energy on plasmonic metal nanostructures. Nat. Catal. 2018, 1, 656–665. [Google Scholar] [CrossRef]
- Byers, C.P.; Zhang, H.; Swearer, D.F.; Yorulmaz, M.; Hoener, B.S.; Huang, D.; Hoggard, A.; Chang, W.-S.; Mulvaney, P.; Ringe, E.; et al. From tunable core-shell nanoparticles to plasmonic drawbridges: Active control of nanoparticle optical properties. Sci. Adv. 2015, 1, e1500988. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Wang, W.J.; Li, N.; Li, Z.F.; Lu, W. Plasmonic optical convergence microcavity based on the metal-insulator-metal microstructure. Appl. Phys. Lett. 2017, 110, 231105. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Noguez, C. Plasmonic Optical Properties and Applications of Metal Nanostructures. Plasmonics 2008, 3, 127–150. [Google Scholar] [CrossRef]
- Takahata, R.; Yamazoe, S.; Koyasu, K.; Tsukuda, T. Surface Plasmon Resonance in Gold Ultrathin Nanorods and Nanowires. J. Am. Chem. Soc. 2014, 136, 8489–8491. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-S.; El-Sayed, M.A. Gold and Silver Nanoparticles in Sensing and Imaging: Sensitivity of Plasmon Response to Size, Shape, and Metal Composition. J. Phys. Chem. B 2006, 110, 19220–19225. [Google Scholar] [CrossRef] [PubMed]
- Xiaoa, L.; Sub, Y.; Qiud, W.; Liua, Y.; Rand, J.; Wua, J.; Lua, F.; Shaod, F.; Tangc, D.; Peng, P. Solar radiation shielding properties of transparent LaB6 filters through experimental and first-principles calculation methods. Ceram. Int. 2016, 42, 14278–14281. [Google Scholar] [CrossRef]
- Bao, L.; Chao, L.; Wei, W.; Tegus, O. Tunable transmission light in nanocrystalline La1−xEuxB6. Mater. Lett. 2015, 139, 187–190. [Google Scholar] [CrossRef]
- Xiao, L.; Su, Y.; Zhou, X.; Chen, H.; Tan, J.; Hu, T.; Yan, J.; Peng, P. Origins of high visible light transparency and solar heat-shielding performance in LaB6. Appl. Phys. Lett. 2012, 101, 041913. [Google Scholar] [CrossRef]
- Mattox, T.M.; Ye, X.; Manthiram, K.; Schuck, P.J.; Alivisatos, A.P.; Urban, J.J. Chemical Control of Plasmons in Metal Chalcogenide and Metal Oxide Nanostructures. Adv. Mater. 2015, 27, 5830–5837. [Google Scholar] [CrossRef]
- Lounis, S.D.; Runnerstrom, E.L.; Llordés, A.; Milliron, D.J. Defect Chemistry and Plasmon Physics of Colloidal Metal Oxide Nanocrystals. J. Phys. Chem. Lett. 2014, 5, 1564–1574. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.; Kodanek, T.; Dorfs, D. Tuning the LSPR in copper chalcogenide nanoparticles by cation intercalation, cation exchange and metal growth. Nanoscale 2015, 7, 19519–19527. [Google Scholar] [CrossRef] [PubMed]
- Mattox, T.M.; Bergerud, A.; Agrawal, A.; Milliron, D.J. Influence of Shape on the Surface Plasmon Resonance of Tungsten Bronze Nanocrystals. Chem. Mater. 2014, 26, 1779–1784. [Google Scholar] [CrossRef]
- Zhao, Y.; Pan, H.; Lou, Y.; Qiu, X.; Zhu, J.; Burda, C. Plasmonic Cu2−xS Nanocrystals: Optical and Structural Properties of Copper-Deficient Copper(I) Sulfides. J. Am. Chem. Soc. 2009, 131, 4253–4261. [Google Scholar] [CrossRef] [PubMed]
- González, A.L.; Noguez, C.; Beránek, J.; Barnard, A.S. Size, Shape, Stability, and Color of Plasmonic Silver Nanoparticles. J. Phys. Chem. C 2014, 118, 9128–9136. [Google Scholar] [CrossRef]
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef]
- Liu, P.; Wang, H.; Li, X.; Rui, M.; Zeng, H. Localized surface plasmon resonance of Cu nanoparticles by laser ablation in liquid media. RSC Adv. 2015, 5, 79738–79745. [Google Scholar] [CrossRef]
- Duan, H.; Xuan, Y. Synthesis and optical absorption of Ag/CdS core/shell plasmonic nanostructure. Sol. Energy Mater. Sol. Cells 2014, 121, 8–13. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, H.; Li, Z.; Wang, P.; Yang, L.; Fang, Y. The Study of Surface Plasmon in Au/Ag Core/Shell Compound Nanoparticles. Plasmonics 2012, 7, 509–513. [Google Scholar] [CrossRef]
- Schmidt, P.H.; Joy, D.C.; Longinotti, L.D.; Leamy, H.J.; Ferris, S.D.; Fisk, Z. Anisotropy of thermionic electron emission values for LaB6 single-crystal emitter cathodes. Appl. Phys. Lett. 1976, 29, 400–401. [Google Scholar] [CrossRef]
- Ahmed, H.; Broers, A.N. Lanthanum Hexaboride Electron Emitter. J. Appl. Phys. 1972, 43, 2185–2192. [Google Scholar] [CrossRef]
- Goebel, D.M.; Hirooka, Y.; Sketchley, T.A. Large-area lanthanum hexaboride electron emitter. Rev. Sci. Instrum. 1985, 56, 1717–1722. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, J.; Zhang, Q.; Zhao, G.; Yang, G.; Zhang, J.; Zhou, O.; Qin, L.-C. Field emission of electrons from single LaB6 nanowires. Adv. Mater. 2006, 18, 87–91. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, J.; Liu, D.; Lin, Z.; Huang, Q.; Bao, L.; Ma, R.; Wei, Y. Synthesis and properties of nanostructured dense LaB6 cathodes by arc plasma and reactive spark plasma sintering. Acta Mater. 2010, 58, 4978–4985. [Google Scholar] [CrossRef]
- Back, T.C.; Schmid, A.K.; Fairchild, S.B.; Boeckl, J.J.; Cahay, M.; Derkink, F.; Chen, G.; Sayir, A. Work function characterization of directionally solidified LaB6–VB2 eutectic. Ultramicroscopy 2017, 183, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, X.; Ning, S.; Xiao, Y.; Zhang, J. The electronic structure and work functions of single crystal LaB6 typical crystal surfaces. Vacuum 2017, 143, 245–250. [Google Scholar] [CrossRef]
- Ning, S.-Y.; Iitaka, T.; Yang, X.-Y.; Wang, Y.; Zhao, J.-J.; Li, Z.; Zhang, J.-X. Enhanced thermionic emission performance of LaB6 by Ce doping. J. Alloys Compd. 2018, 760, 1–5. [Google Scholar] [CrossRef]
- Torgasin, K.; Morita, K.; Zen, H.; Masuda, K.; Katsurayama, T.; Murata, T.; Suphakul, S.; Yamashita, H.; Nogi, T.; Kii, T.; et al. Thermally assisted photoemission effect on CeB6 and LaB6 for application as photocathodes. Phys. Rev. Accel. Beams 2017, 20, 073401. [Google Scholar] [CrossRef]
- Voss, J.; Vojvodic, A.; Chou, S.H.; Howe, R.T.; Abild-Pedersen, F. Inherent Enhancement of Electronic Emission from Hexaboride Heterostructure. Phys. Rev. Appl. 2014, 2, 024004. [Google Scholar] [CrossRef]
- Schelm, S.; Smith, G.B.; Garrett, P.D.; Fisher, W.K. Tuning the surface-plasmon resonance in nanoparticles for glazing applications. J. Appl. Phys. 2005, 97, 124314. [Google Scholar] [CrossRef]
- Schelm, S.; Smith, G.B. Dilute LaB6 nanoparticles in polymer as optimized clear solar control glazing. Appl. Phys. Lett. 2003, 82, 4346–4348. [Google Scholar] [CrossRef]
- Bogomol, I.; Nishimura, T.; Nesterenko, Y.; Vasylkiv, O.; Sakka, Y.; Loboda, P. The bending strength temperature dependence of the directionally solidified eutectic LaB6–ZrB2 composite. J. Alloys Compd. 2011, 509, 6123–6129. [Google Scholar] [CrossRef]
- Chen, D.; Min, G.; Wu, Y.; Yu, H.; Zhang, L. The preparation and composition design of boron-rich lanthanum hexaboride target for sputtering. J. Alloys Compd. 2015, 638, 380–386. [Google Scholar] [CrossRef]
- Gridneva, I.V.; Lazorenko, V.I.; Lotsko, D.V.; Mil’man, Y.V.; Paderno, Y.B.; Chugunova, S.I. Mechanical properties of single-crystal lanthanum hexaboride with local loading. Sov. Powder Metall. Met. Ceram. 1990, 29, 967–972. [Google Scholar] [CrossRef]
- Loboda, P.I.; Kysla, H.P.; Dub, S.M.; Karasevs’ka, O.P. Mechanical properties of the monocrystals of lanthanum hexaboride. Mater. Sci 2009, 45, 108–113. [Google Scholar] [CrossRef]
- Mitterer, C.; Komenda-Stallmaier, J.; Losbichler, P.; Schmölz, P.; Störi, H. Decorative boride coatings based on LaB6. Surf. Coat. Technol. 1995, 74–75, 1020–1027. [Google Scholar] [CrossRef]
- Bogomol, I.; Nishimura, T.; Vasylkiv, O.; Sakka, Y.; Loboda, P. High-temperature strength of directionally reinforced LaB6–TiB2 composite. J. Alloys Compd. 2010, 505, 130–134. [Google Scholar] [CrossRef]
- Buckingham, J.D. Thermionic emission properties of a lanthanum hexaboride/rhenium cathode. Br. J. Appl. Phys. 1965, 16, 1821. [Google Scholar] [CrossRef]
- Xu, G.-L.; Chen, J.-D.; Xia, Y.-Z.; Liu, X.-F.; Liu, Y.-F.; Zhou, X.-Z. First-Principles Calculations of Elastic and Thermal Properties of Lanthanum Hexaboride. Chin. Phys. Lett. 2009, 26, 056201. [Google Scholar]
- Hu, P.; Zhang, X.-H.; Han, J.-C.; Luo, X.-G.; Du, S.-Y. Effect of Various Additives on the Oxidation Behavior of ZrB2-Based Ultra-High-Temperature Ceramics at 1800 °C. J. Am. Ceram. Soc. 2010, 93, 345–349. [Google Scholar] [CrossRef]
- Kelly, J.P.; Kanakala, R.; Graeve, O.A. A Solvothermal Approach for the Preparation of Nanostructured Carbide and Boride Ultra-High-Temperature Ceramics. J. Am. Ceram. Soc. 2010, 93, 3035–3038. [Google Scholar] [CrossRef]
- Marchenko, A.A.; Cherepanov, V.V.; Tarashchenko, D.T.; Kazantseva, Z.I.; Naumovets, A.G. A low work function substrate for STM studies of objects with poor tunneling transparency: Lanthanum hexaboride (100). Surf. Sci. 1998, 416, 460–465. [Google Scholar] [CrossRef]
- Monteverde, F.; Alfano, D.; Savino, R. Effects of LaB6 addition on arc-jet convectively heated SiC-containing ZrB2-based ultra-high temperature ceramics in high enthalpy supersonic airflows. Corros. Sci. 2013, 75, 443–453. [Google Scholar] [CrossRef]
- Cortie, M.B.; McDonagh, A.M. Synthesis and Optical Properties of Hybrid and Alloy Plasmonic Nanoparticles. Chem. Rev. 2011, 111, 3713–3735. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Cronin, S.B. A Review of Surface Plasmon Resonance-Enhanced Photocatalysis. Adv. Funct. Mater. 2013, 23, 1612–1619. [Google Scholar] [CrossRef]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Rycenga, M.; Skrabalak, S.E.; Wiley, B.; Xia, Y. Chemical Synthesis of Novel Plasmonic Nanoparticles. Annu. Rev. Phys. Chem. 2009, 60, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Mattox, T.M.; Groome, C.; Doran, A.; Beavers, C.M.; Urban, J.J. Chloride influence on the formation of lanthanum hexaboride: An in-situ diffraction study. J. Cryst. Growth 2018, 486, 60–65. [Google Scholar] [CrossRef]
- Nagao, T.; Kitamura, K.; Iizuka, Y.; Oshima, C. Surface phonons of LaB6(100): Deformation of boron octahedra at the surface. Surf. Sci. 1993, 290, 436–444. [Google Scholar] [CrossRef]
- Nagao, T.; Kitamura, T.; Iizuka, T.; Umeuchi, M.; Oshima, C. Deformation of octahedra at LaB6(100) surface studied by HREELS. Surf. Sci. 1993, 287–288, 391–395. [Google Scholar] [CrossRef]
- Rokuta, E.; Yamamoto, N.; Hasegawa, Y.; Trenary, M.; Nagao, T.; Oshima, C.; Otani, S. Deformation of boron networks at the LaB6(111) surface. Surf. Sci. 1998, 416, 363–370. [Google Scholar] [CrossRef]
- Li, Y.; Cao, Y.; Jia, D. A general strategy for synthesis of metal nanoparticles by a solid-state redox route under ambient conditions. J. Mater. Chem. A 2014, 2, 3761–3765. [Google Scholar] [CrossRef]
- Polte, J. Fundamental growth principles of colloidal metal nanoparticles—A new perspective. CrystEngComm 2015, 17, 6809–6830. [Google Scholar] [CrossRef]
- Adachi, K.; Miratsu, M.; Asahi, T. Absorption and scattering of near-infrared light by dispersed lanthanum hexaboride nanoparticles for solar control filters. J. Mater. Res. 2010, 25, 510–521. [Google Scholar] [CrossRef]
- Sato, Y.; Terauchi, M.; Mukai, M.; Kaneyama, T.; Adachi, K. High energy-resolution electron energy-loss spectroscopy study of the dielectric properties of bulk and nanoparticle LaB6 in the near-infrared region. Ultramicroscopy 2011, 111, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; He, W.J.; Tolochko, O.V.; Polzik, L.; Min, G.H. Morphology Characterization and Optical Properties Analysis for Nanostructured Lanthanum Hexaboride Powders. Adv. Mater. Res. 2009, 79–82, 107–110. [Google Scholar] [CrossRef]
- Hang, C.-L.; Yang, L.-X.; Wang, F.; Xu, Y.-B.; Yi, C.-Y. Melt-assisted synthesis to lanthanum hexaboride nanoparticles and cubes. Bull. Mater. Sci. 2017, 40, 1241–1245. [Google Scholar] [CrossRef]
- Mattox, T.M.; Groome, C.; Doran, A.; Beavers, C.M.; Urban, J.J. Anion-mediated negative thermal expansion in lanthanum hexaboride. Solid State Commun. 2017, 265, 47–51. [Google Scholar] [CrossRef]
- Zhang, M.; Yuan, L.; Wang, X.; Fan, H.; Wang, X.; Wu, X.; Wang, H.; Qian, Y. A low-temperature route for the synthesis of nanocrystalline LaB6. J. Solid State Chem. 2008, 181, 294–297. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, L.; Liang, L.; He, K.; Liu, R.; Min, G. A solid-state reaction route to prepare LaB6 nanocrystals in vacuum. Ceram. Int. 2011, 37, 2891–2896. [Google Scholar] [CrossRef]
- Brewer, J.R.; Deo, N.; Wang, Y.M.; Cheung, C.L. Lanthanum Hexaboride Nanoobelisks. Chem. Mater. 2007, 19, 6379–6381. [Google Scholar] [CrossRef]
- Zhang, H.; Shang, Q.; Tang, J.; Qin, L.-C. Single-crystalline LaB6 nanowires. J. Am. Chem. Soc. 2005, 127, 2862–2863. [Google Scholar] [CrossRef] [PubMed]
- Mattox, T.M.; Chockkalingam, S.; Roh, I.; Urban, J.J. Evolution of Vibrational Properties in Lanthanum Hexaboride Nanocrystals. J. Phys. Chem. C 2016, 120, 5188–5195. [Google Scholar] [CrossRef]
- Groome, C.; Roh, I.; Mattox, T.M.; Urban, J.J. Effects of size and structural defects on the vibrational perperties of lanthanum hexaboride nanocrystals. ACS Omega 2017, 2, 2248–2254. [Google Scholar] [CrossRef]
- Etourneau, J. Critical survey of rare-earth borides: Occurence, crystal chemistry and physical properties. J. Less-Common Met. 1985, 110, 267–281. [Google Scholar] [CrossRef]
- Cahill, J.T.; Alberga, M.; Bahena, J.; Pisano, C.; Borja-Urby, R.; Vasquez, V.R.; Edwards, D.; Misture, S.T.; Graeve, O.A. Phase Stability of Mixed-Cation Alkaline-Earth Hexaborides. Cryst. Growth Des. 2017, 17, 3450–3461. [Google Scholar] [CrossRef]
- Kasai, H.; Nishibori, E. Spatial distribution of electrons near the Fermi level in the metallic LaB6 through accurate X-ray charge density study. Sci. Rep. 2017, 7, 41375. [Google Scholar] [CrossRef]
- Etourneau, J.; Hagenmuller, P. Structure and physical features of the rare-earth borides. Philos. Mag. B 1985, 52, 589–610. [Google Scholar] [CrossRef]
- Chao, L.; Bao, L.; Wei, W.; Tegus, O. Optical properties of Yb-doped LaB6 from first-principles calculation. Mod. Phys. Lett. B 2016, 30, 1650091. [Google Scholar] [CrossRef]
- Chao, L.; Bao, L.; Shi, J.; Wei, W.; Tegus, O.; Zhang, Z. The effect of Sm-doping on optical properties of LaB6 nanoparticles. J. Alloys Compd. 2015, 622 (Suppl. C), 618–621. [Google Scholar] [CrossRef]
- Mattox, T.M.; Coffman, D.K.; Roh, I.; Sims, C.; Urban, J.J. Moving the Plasmon of LaB6 from IR to Near-IR via Eu-Doping. Materials 2018, 11, 226. [Google Scholar] [CrossRef] [PubMed]
- Noguez, C. Surface Plasmons on Metal Nanoparticles: The Influence of Shape and Physical Environment. J. Phys. Chem. C 2007, 111, 3806–3819. [Google Scholar] [CrossRef]
- Hong, Y.; Zhang, X.; Li, B.; Li, M.; Shi, Q.; Wang, Y.; Li, L. Size dependent optical properties of LaB6 nanoparticles enhanced by localized surface plasmon resonance. J. Rare Earth 2013, 31, 1096–1101. [Google Scholar] [CrossRef]
- Mattox, T.M.; Agrawal, A.; Milliron, D.J. Low Temperature Synthesis and Surface Plasmon Resonance of Colloidal Lanthanum Hexaboride (LaB6) Nanocrystals. Chem. Mater. 2015, 27, 6620–6624. [Google Scholar] [CrossRef]
- Chen, C.-J.; Chen, D.-H. Preparation of LaB6 nanoparticles as a novel and effective near-infrared photothermal conversion material. Chem. Eng. J. 2012, 180, 337–342. [Google Scholar] [CrossRef]
- Rivera, V.A.G.; Ferri, A.F.; Marega, E., Jr. Localized Surface Plasmon Resonances: Noble Metal Nanoparticle Interaction with Rare-Earth Ions. In Plasmonics: Principles and Applications; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar]
- Kanakala, R.; Rojas-George, G.; Graeve, O.A. Unique Preparation of Hexaboride Nanocubes: A First Example of Boride Formation by Combustion Synthesis. J. Am. Ceram. Soc. 2010, 93, 3136–3141. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, J.; Yuan, J.; Ma, J.; Shinya, N.; Nakajima, K.; Murakami, H.; Ohkubo, T.; Qin, L.-C. Nanostructured LaB6 Field Emitter with Lowest Apical Work Function. Nano Lett. 2010, 10, 3539–3544. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Hou, G.; Li, H.; Zhai, T.; Dong, B.; Yan, H.; Wang, Y.; Yu, B.; Bando, Y.; Golberg, D. Fabrication of vertically aligned single-crystalline lanthanum hexaboride nanowire arrays and investigation of their field emission. NPG Asia Mater. 2013, 5, e53. [Google Scholar] [CrossRef]
- Xu, J.Q.; Zhao, Y.M.; Zhang, Q.Y. Enhanced electron field emission from single-crystalline LaB6 nanowires with ambient temperature. J. Appl. Phys. 2008, 104, 124306. [Google Scholar] [CrossRef]
- Machida, K.; Adachi, K. Particle shape inhomogeneity and plasmon-band broadening of solar-control LaB6 nanoparticles. J. Appl. Phys. 2015, 118, 013103. [Google Scholar] [CrossRef]
- Chao, L.; Bao, L.; Wei, W.; Tegus, O.; Zhang, Z. Effects of nanoparticle shape and size on optical properties of LaB6. Plasmonics 2016, 11, 697–701. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Jiang, F.; Leong, Y.; Saunders, M.; Martyniuk, M.; Faraone, L.; Keating, A.; Dell, J. Uniform Dispersion of Lanthanum Hexaboride Nanoparticles in a Silica Thin Film: Synthesis and Optical Properties. ACS Appl. Mater. Interfaces 2012, 4, 5833–5838. [Google Scholar] [CrossRef]
- Seeboth, A.; Ruhmann, R.; Mühling, O. Thermotropic and Thermochromic Polymer Based Materials for Adaptive Solar Control. Materials 2010, 3, 5143–5168. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, L.; Hu, L.; Wang, W.; Min, G. Size effect of added LaB6 particles on optical properties of LaB6/Polymer composites. J. Solid State Chem. 2011, 184, 3364–3367. [Google Scholar] [CrossRef]
- Takeda, H.; Kuno, H.; Adachi, K. Solar Control Dispersions and Coatings with Rare-Earth Hexaboride Nanoparticles. J. Am. Ceram. Soc. 2008, 91, 2897–2902. [Google Scholar] [CrossRef]
- Lai, B.H.; Chen, D.H. LaB6 nanoparticles with carbon-doped silica coating for fluorescence imaging and near-IR photothermal therapy of cancer cells. Acta Biomater. 2013, 9, 7556–7563. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-C.; Lin, Z.-W.; Ling, M.-H. Near-Infrared Light-Activatable Microneedle System for Treating Superficial Tumors by Combination of Chemotherapy and Photothermal Therapy. ACS Nano 2016, 10, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.-Y.; Chen, C.-T.; Yeh, C.-S. Comparative efficiencies of photothermal destruction of malignant cells using antibody-coated silica@Au nanoshells, hollow Au/Ag nanospheres and Au nanorods. Nanotechnology 2009, 20, 425104. [Google Scholar] [CrossRef]
- Lai, B.-H.; Chen, D.-H. Vancomycin-modified LaB6@SiO2/Fe3O4 composite nanoparticles for near-infrared photothermal ablation of bacteria. Acta Biomater. 2013, 9, 7573–7579. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, N.; Liao, Q.; Fu, Q.; Huang, Y.; Zhu, X.; Li, Q. A biomaterial doped with LaB6 nanoparticles as photothermal media for enhancing biofilm growth and hydrogen production in photosynthetic bacteria. Int. J. Hydrogen Energy 2017, 42, 5794–5803. [Google Scholar]
- Szépvölgyi, J.; Mohai, I.; Károly, Z.; Gál, L. Synthesis of nanosized ceramic powders in a radiofrequency thermal plasma reactor. J. Eur. Ceram. Soc. 2008, 28, 895–899. [Google Scholar] [CrossRef]
- Selvan, R.K.; Genish, I.; Perelshtein, I.; Calderon Moreno, J.M.; Gedanken, A. Single Step, Low-Temperature Synthesis of Submicron-Sized Rare Earth Hexaborides. J. Phys. Chem. C 2008, 112, 1795–1802. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, S.; Li, W.; Chen, Z. Low temperature synthesis of LaB6 nanoparticles by a molten salt route. Powder Technol. 2018, 323, 203–207. [Google Scholar] [CrossRef]
- Wang, L.; Xu, L.; Ju, Z.; Qian, Y. A versatile route for the convenient synthesis of rare-earth and alkaline-earth hexaborides at mild temperatures. CrystEngComm 2010, 12, 3923–3928. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mattox, T.M.; Urban, J.J. Tuning the Surface Plasmon Resonance of Lanthanum Hexaboride to Absorb Solar Heat: A Review. Materials 2018, 11, 2473. https://doi.org/10.3390/ma11122473
Mattox TM, Urban JJ. Tuning the Surface Plasmon Resonance of Lanthanum Hexaboride to Absorb Solar Heat: A Review. Materials. 2018; 11(12):2473. https://doi.org/10.3390/ma11122473
Chicago/Turabian StyleMattox, Tracy M., and Jeffrey J. Urban. 2018. "Tuning the Surface Plasmon Resonance of Lanthanum Hexaboride to Absorb Solar Heat: A Review" Materials 11, no. 12: 2473. https://doi.org/10.3390/ma11122473
APA StyleMattox, T. M., & Urban, J. J. (2018). Tuning the Surface Plasmon Resonance of Lanthanum Hexaboride to Absorb Solar Heat: A Review. Materials, 11(12), 2473. https://doi.org/10.3390/ma11122473




