Fast Microwave-Assisted Hydrothermal Synthesis of Pure Layered δ-MnO2 for Multivalent Ion Intercalation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental
2.2. Characterization Techniques
3. Results & Discussion
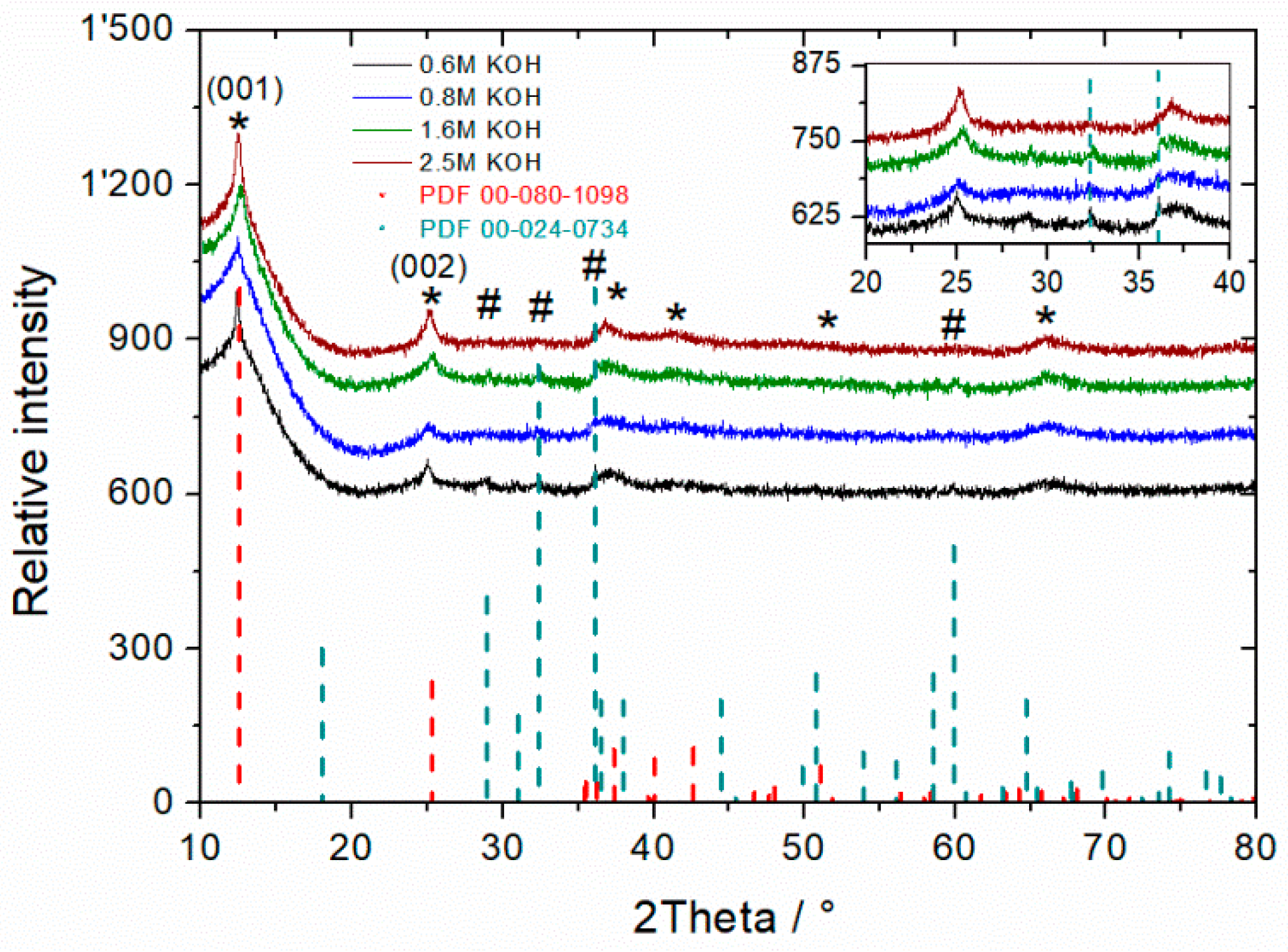
3.1. Influence of KOH Concentration on MnO2 Powder Synthesis
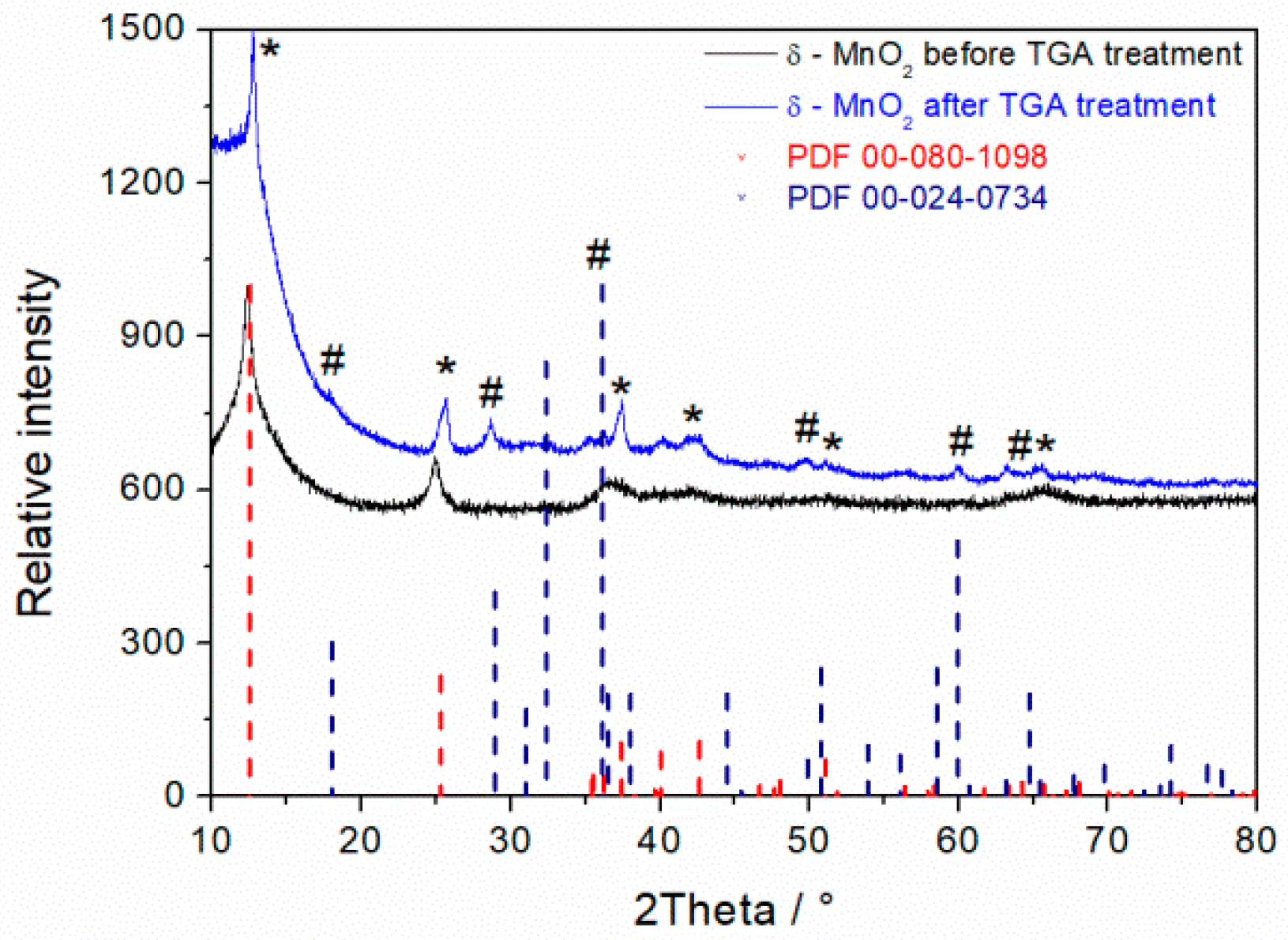
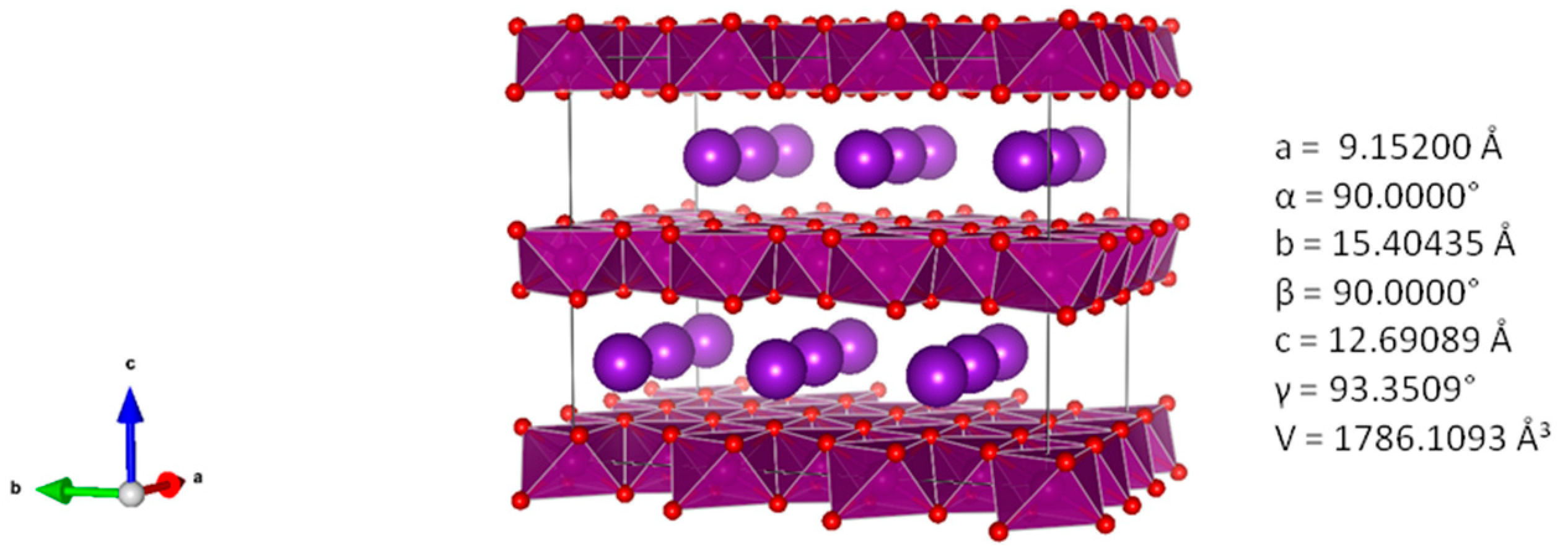
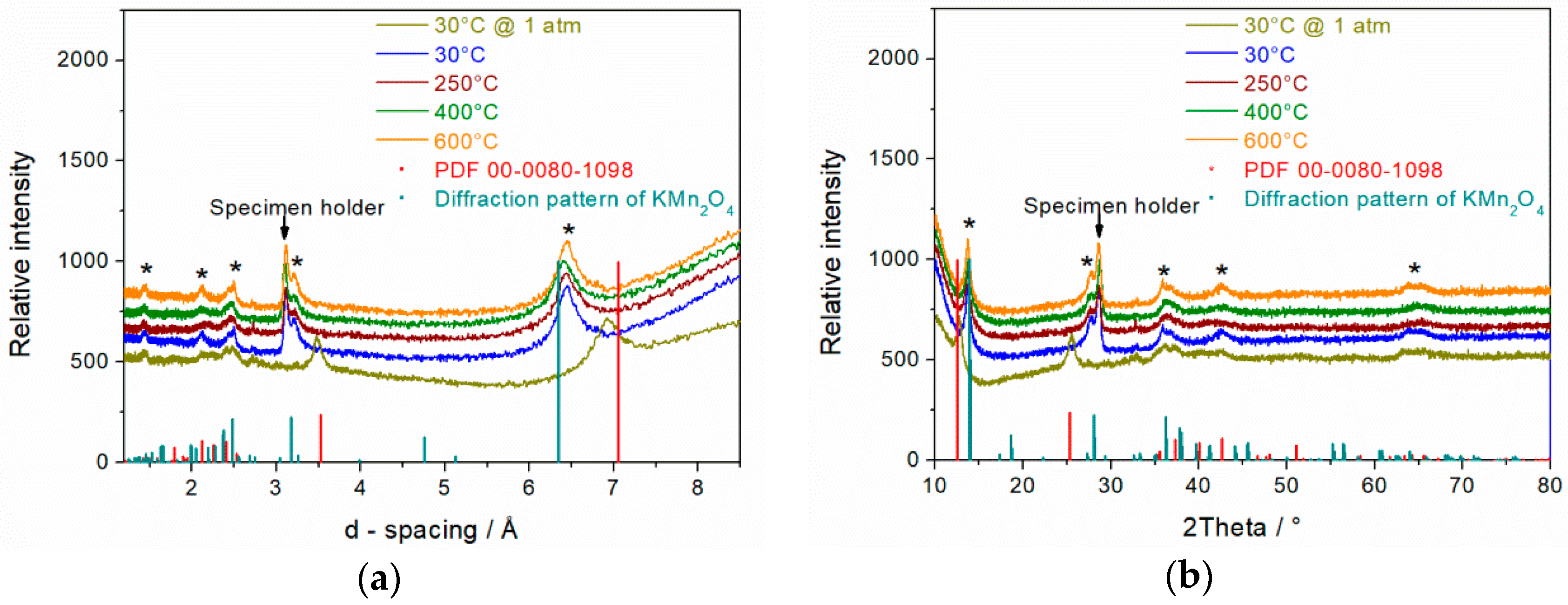
3.2. XRD Spectra of MnO2 Powder Synthesized in 1.6 M KOH for 14 h
3.3. Influence of Hydroxide Concentration on Reaction Kinetics
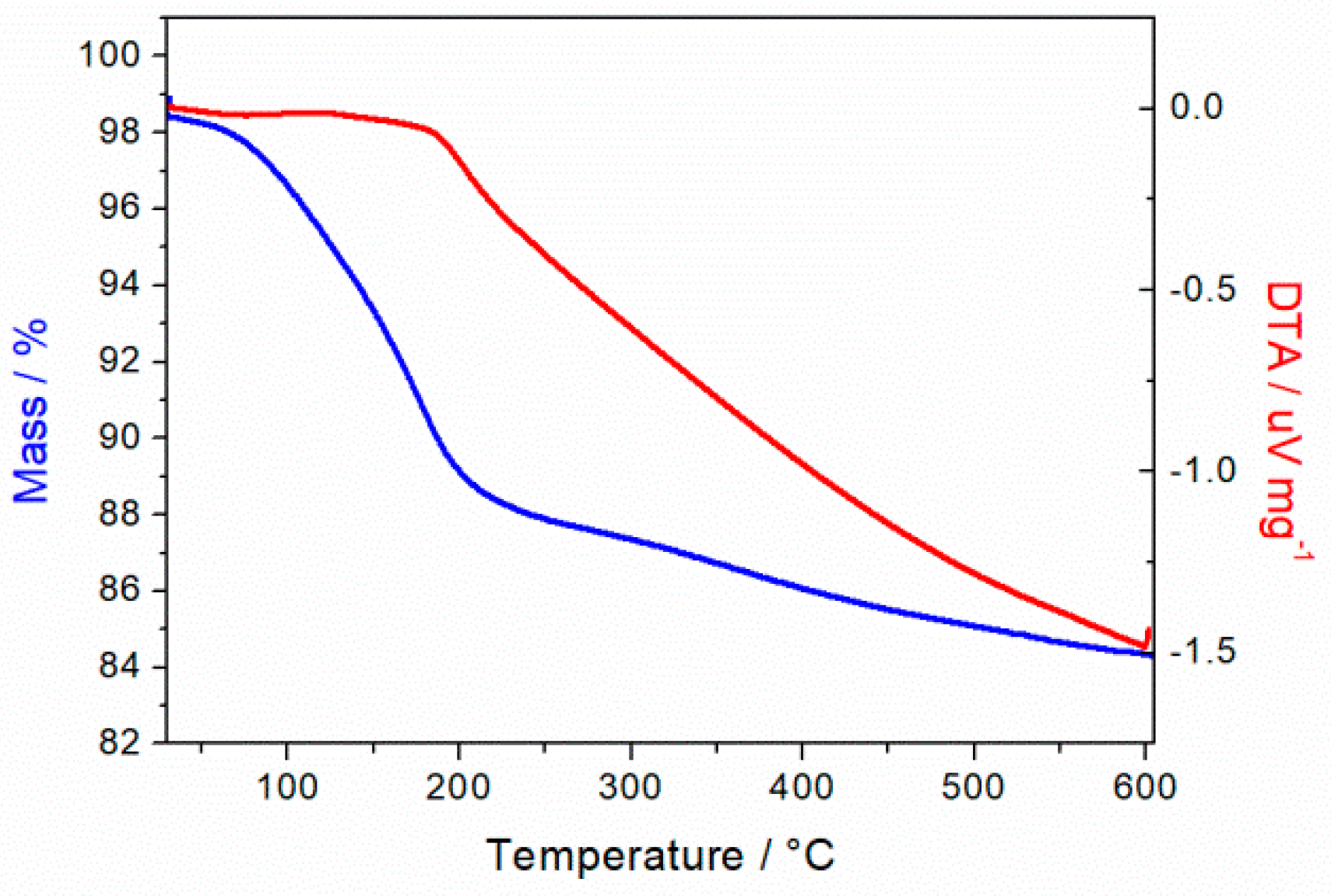
3.4. TGA Measurements
3.5. In-Situ XRD Spectra
3.6. BET Analysis
3.7. Electrochemical Measurements
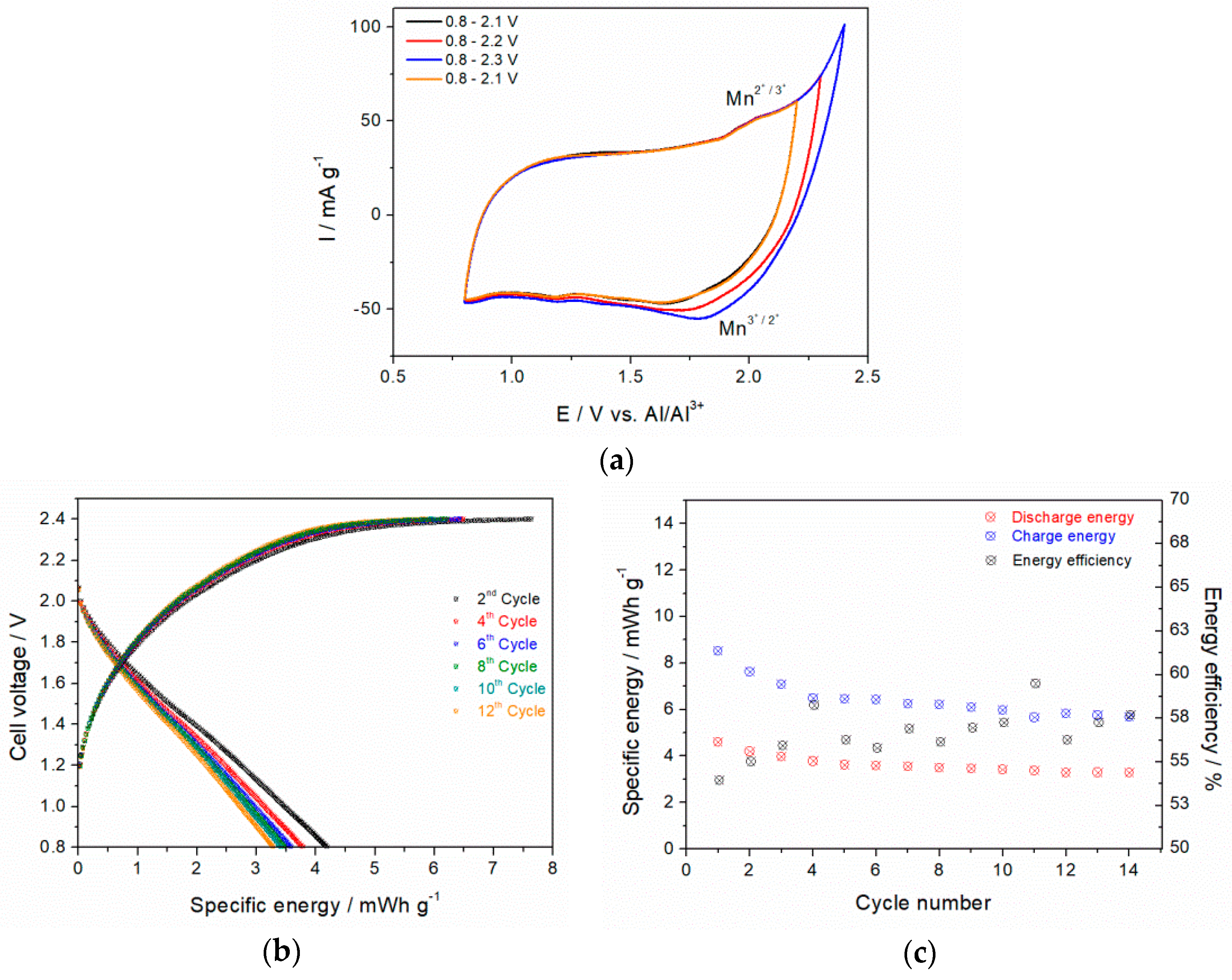
3.7.1. Aluminum-Ion Battery
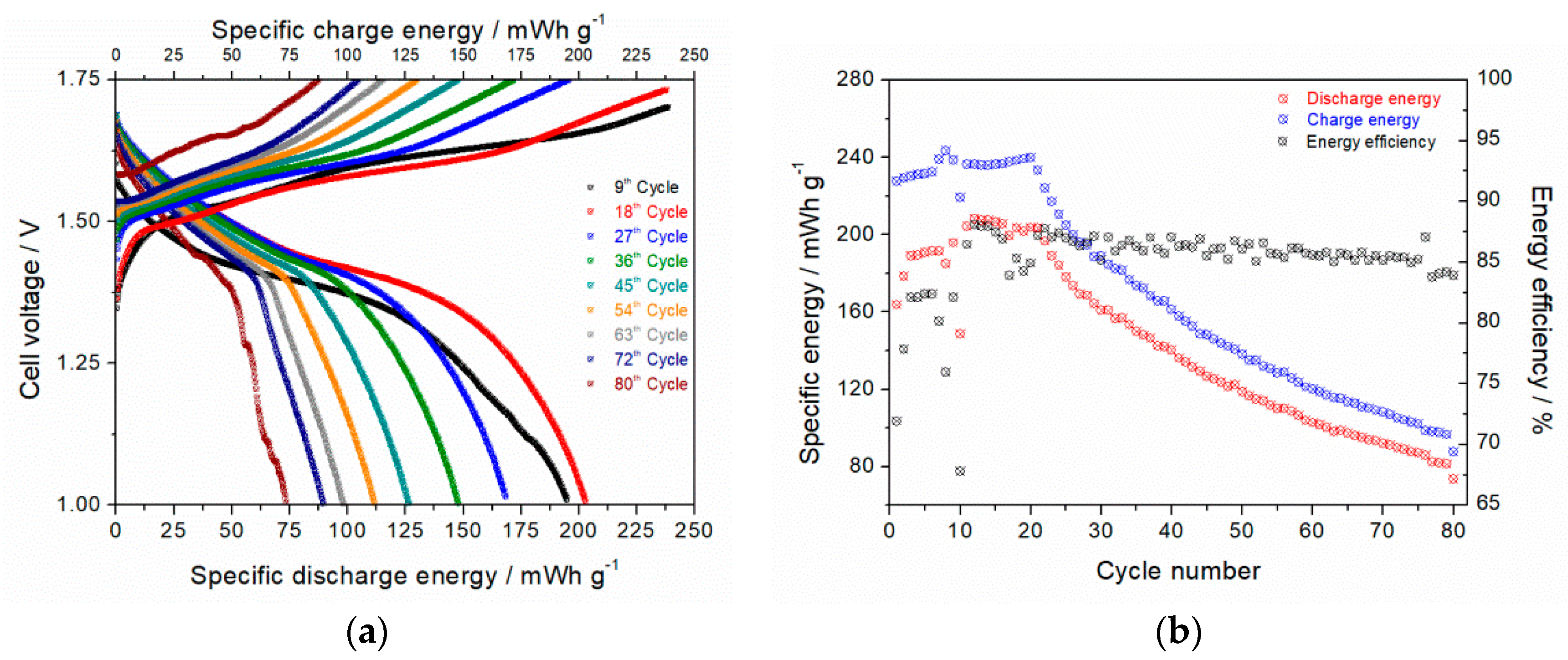
3.7.2. Zinc-Ion Battery
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Glossary
| Interlayer distance = d-spacing: | distance from the manganese atom in the center of one oxide layer to the manganese atom of the next oxide layer |
| Gallery height = diffusion diameter: | Distance or height of the gap between two oxide layers |
Appendix A
| Substance | Formula | Oxidation State |
|---|---|---|
| Birnessite | KxMn(III)xMn(IV)1−xO4 * y H2O | +III & + IV |
| Hausmannite | Mn3O4 | +III & +II |
| Feitknechtite | MnOOH | +III |
| Permanganate | MnO4− | +VII |
| Manganate | MnO42− | +VI |
| Hypomanganate | MnO43− | +V |
| Manganese salt | Mn2+ | +II |
Appendix B

References
- Liu, M.; Rong, Z.; Malik, R.; Canepa, P.; Jain, A.; Ceder, G.; Persson, K.A. Spinel compounds as multivalent battery cathodes: A systematic evaluation based on ab initio calculations. Energy Environ. Sci. 2015, 8, 964–974. [Google Scholar] [CrossRef]
- Elia, G.A.; Marquardt, K.; Hoeppner, K.; Fantini, S.; Lin, R.; Knipping, E.; Peters, W.; Drillet, J.F.; Passerini, S.; Hahn, R. An Overview and Future Perspectives of Aluminum Batteries. Adv. Mater. 2016, 28, 7564–7579. [Google Scholar] [CrossRef] [PubMed]
- El Abedin, S.Z.; Moustafa, E.M.; Hempelmann, R.; Natter, H.; Endres, F. Additive free electrodeposition of nanocrystalline aluminium in a water and air stable ionic liquid. Electrochem. Commun. 2005, 7, 1111–1116. [Google Scholar] [CrossRef]
- Liu, Q.X.; el Abedin, S.Z.; Endres, F. Electroplating of mild steel by aluminium in a first generation ionic liquid: A green alternative to commercial Al-plating in organic solvents. Surf. Coat. Technol. 2006, 201, 1352–1356. [Google Scholar] [CrossRef]
- Giridhar, P.; el Abedin, S.Z.; Endres, F. Electrodeposition of aluminium from 1-butyl-1-methylpyrrolidinium chloride/AlCl3 and mixtures with 1-ethyl-3-methylimidazolium chloride/AlCl3. Electrochim. Acta 2012, 70, 210–214. [Google Scholar] [CrossRef]
- Abbott, A.P.; Harris, R.C.; Hsieh, Y.-T.; Ryder, K.S.; Sun, I.-W. Aluminium electrodeposition under ambient conditions. Phys. Chem. Chem. Phys. 2014, 16, 14675–14681. [Google Scholar] [CrossRef] [PubMed]
- Angell, M.; Pan, C.-J.; Rong, Y.; Yuan, C.; Lin, M.-C.; Hwang, B.-J.; Dai, H. High Coulombic efficiency aluminum-ion battery using an AlCl3-urea ionic liquid analog electrolyte. Proc. Natl. Acad. Sci. USA 2017, 114, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-C.; Gong, M.; Lu, B.; Wu, Y.; Wang, D.-Y.; Guan, M.; Angell, M.; Chen, C.; Yang, J.; Hwang, B.-J.; et al. An ultrafast rechargeable aluminium-ion battery. Nature 2015, 520, 324–328. [Google Scholar] [CrossRef] [PubMed]
- He, Y.J.; Peng, J.F.; Chu, W.; Li, Y.Z.; Tong, D.G. Black mesoporous anatase TiO2 nanoleaves: A high capacity and high rate anode for aqueous Al-ion batteries. J. Mater. Chem. A 2014, 2, 1721–1731. [Google Scholar] [CrossRef]
- Holland, A.; Mckerracher, R.D.; Cruden, A.; Wills, R.G.A. An aluminium battery operating with an aqueous electrolyte. J. Appl. Electrochem. 2018, 48, 243–250. [Google Scholar] [CrossRef]
- Liu, S.; Pan, G.L.; Li, G.R.; Gao, X.P. Copper hexacyanoferrate nanoparticles as cathode material for aqueous Al-ion batteries. J. Mater. Chem. A 2015, 3, 959–962. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, B.; Xiong, W.; Sun, H.; Lin, Z.; Hu, L.; Tu, J.; Hou, J.; Zhu, H.; Jiao, S. A new cathode material for super-valent battery based on aluminium ion intercalation and deintercalation. Sci. Rep. 2013, 3, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.M.; Paranthaman, M.P.; Dai, S.; Dudney, N.J.; Manthiram, A.; McIntyre, T.J.; Sun, X.-G. High Energy Density Aluminum Battery. U.S. Patent Application No. US2012/0082904 A1, 5 April 2012. [Google Scholar]
- Kurzweil, P.; Dietlmeier, O.K. Elektrochemische Speicher-Superkondensatoren, Batterien, Elektrolyse-Wasserstoff, Rechtliche Grundlagen; Springer: Wiesbaden, Germany, 2015. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Sun, X.; Ma, C.; Zeng, L.; Wang, Y.; Li, H. A facile method to prepare layered manganese oxides with large interplanar spacing. Mater. Res. Bull. 2002, 37, 331–341. [Google Scholar] [CrossRef]
- Alfaruqi, M.H.; Gim, J.; Kim, S.; Song, J.; Jo, J.; Kim, S.; Mathew, V.; Kim, J. Enhanced reversible divalent zinc storage in a structurally stable α-MnO2 nanorod electrode. J. Power Sources 2015, 288, 320–327. [Google Scholar] [CrossRef]
- Alfaruqi, M.H.; Gim, J.; Kim, S.; Song, J.; Pham, D.T.; Jo, J.; Xiu, Z.; Mathew, V.; Kim, J. Electrochemistry Communications Short communication A layered δ-MnO2 nanoflake cathode with high zinc-storage capacities for eco-friendly battery applications. Electrochem. Commun. 2015, 60, 121–125. [Google Scholar] [CrossRef]
- Xu, C.; Li, B.; Du, H.; Kang, F. Energetic zinc ion chemistry: The rechargeable zinc ion battery. Angew. Chem. 2012, 51, 933–935. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Cui, X.; Chen, W.; Ivey, D.G. Manganese oxide-based materials as electrochemical supercapacitor electrodes. Chem. Soc. Rev. 2011, 40, 1697–1721. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Zhang, S.; Jiang, H.; Qu, F.; Wu, X. Phase-controlled synthesis of polymorphic MnO2 structures for electrochemical energy storage. J. Mater. Chem. A 2015, 3, 5722–5729. [Google Scholar] [CrossRef]
- Giovannelli, F.; Autret-Lambert, C.; Mathieu, C.; Chartier, T.; Delorme, F.; Seron, A. Synthesis of manganese spinel nanoparticles at room temperature by coprecipitation. J. Solid State Chem. 2012, 192, 109–112. [Google Scholar] [CrossRef]
- Mery, A.; Ghamouss, F.; Autret, C.; Farhat, D.; Tran-Van, F. Aqueous ultracapacitors using amorphous MnO2 and reduced graphene oxide. J. Power Sources 2016, 305, 37–45. [Google Scholar] [CrossRef]
- Ming, B.; Li, J.; Kang, F.; Pang, G.; Zhang, Y.; Chen, L.; Xu, J.; Wang, X. Microwave-hydrothermal synthesis of birnessite-type MnO2 nanospheres as supercapacitor electrode materials. J. Power Sources 2012, 198, 428–431. [Google Scholar] [CrossRef]
- Boytsova, O.V.; Shekunova, T.O.; Baranchikov, A.E. Nanocrystalline manganese dioxide synthesis by microwave-hydrothermal treatment. Russ. J. Inorg. Chem. 2015, 60, 546–551. [Google Scholar] [CrossRef]
- Korotkov, R.F.; Baranchikov, A.E.; Boytsova, O.V.; Ivanov, V.K. Synthesis of nanocrystalline birnessite and cryptomelane by microwave hydrothermal treatment. Russ. J. Inorg. Chem. 2015, 60, 1299–1303. [Google Scholar] [CrossRef]
- Boumaiza, H.; Coustel, R.; Medjahdi, G.; Ruby, C.; Bergaoui, L. Conditions for the formation of pure birnessite during the oxidation of Mn(II) cations in aqueous alkaline medium. J. Solid State Chem. 2017, 248, 18–25. [Google Scholar] [CrossRef]
- Luo, J.; Huang, A.; Park, S.H.; Suib, S.L.; Young, C.O. Crystallization of Sodium - Birnessite and Accompanied Phase Transformation. Chem. Mater. 1998, 10, 1561–1568. [Google Scholar] [CrossRef]
- Luo, J.; Suib, S.L. Preparative parameters, Magnesium effects, and anion effects in the crystallization of Birnessites. J. Phys. Chem. B 1997, 101, 10403–10413. [Google Scholar] [CrossRef]
- Drits, V.A.; Silvester, E.; Gorshkov, A.I.; Manceau, A. Structure of synthetic monoclinic Na-rich birnessite and hexagonal birnessite: I. Results from X-ray diffraction and selected-area electron diffraction. Am. Mineral. 1997, 82, 946–961. [Google Scholar] [CrossRef]
- Persson, K. Materials Data on KMn2O4 (SG:11) by Materials Project. Lawrence Berkeley National Laboratory (LBNL): Berkeley, CA, USA, 2016. Available online: www.materialsproject.org (accessed on 5 September 2018). [CrossRef]
- Dean, J.A. Lange’s Handbook of Chemistry, 15th ed.; McGraw Hill, Inc.: New York, NY, USA, 1999. [Google Scholar]
- Tye, F.L. Manganese dioxide electrode—X. A theoretical treatment based on the concept of two solid solutions in the range γ-MnO2 to δ-MnOOH. Electrochim. Acta 1985, 30, 17–23. [Google Scholar] [CrossRef]
- Carrington, A.; Symons, M.C.R. 655. Structure and reactivity of the oxy-anions of transition metals. Part I. The manganese oxy-anions. J. Chem. Soc. 1956, 3373–3380. [Google Scholar] [CrossRef]
- He, S.; Hu, C.; Hou, H.; Chen, W. Ultrathin MnO2 nanosheets supported on cellulose based carbon papers for high-power supercapacitors. J. Power Sources 2014, 246, 754–761. [Google Scholar] [CrossRef]
- Frías, D.; Nousir, S.; Barrio, I.; Montes, M.; López, T.; Centeno, M.A.; Odriozola, J.A. Synthesis and characterization of cryptomelane- and birnessite-type oxides: Precursor effect. Mater. Charact. 2007, 58, 776–781. [Google Scholar] [CrossRef]
- Donne, S.W.; Hollenkamp, A.F.; Jones, B.C. Structure, morphology and electrochemical behaviour of manganese oxides prepared by controlled decomposition of permanganate. J. Power Sources 2010, 195, 367–373. [Google Scholar] [CrossRef]
- Thapa, A.K.; Pandit, B.; Thapa, R.; Luitel, T.; Paudel, H.S.; Sumanasekera, G.; Sunkara, M.K.; Gunawardhana, N.; Ishihara, T.; Yoshio, M. Synthesis of mesoporous birnessite-MnO2 composite as a cathode electrode for lithium battery. Electrochim. Acta 2014, 116, 188–193. [Google Scholar] [CrossRef]
- Vargas, O.A.; Caballero, A.; Hernán, L.; Morales, J. Improved capacitive properties of layered manganese dioxide grown as nanowires. J. Power Sources 2011, 196, 3350–3354. [Google Scholar] [CrossRef]
- Wang, H.; Bai, Y.; Chen, S.; Luo, X.; Wu, C.; Wu, F.; Lu, J.; Amine, K. Binder-free V2O5 cathode for greener rechargeable aluminum battery. ACS Appl. Mater. Interfaces 2015, 7, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Wang, F.; Hou, S.; Yang, C.; Fan, X.; Ma, Z.; Gao, T.; Han, F.; Hu, R.; Zhu, M.; et al. Zn/MnO2 Battery Chemistry with H+ and Zn2+ Coinsertion. J. Am. Chem. Soc. 2017, 139, 9775–9778. [Google Scholar] [CrossRef] [PubMed]



| Compound | Mn2+ | Mn(OH)2 | MnO4− | MnO42− | MnO43− | MnOOH | MnO2 | Mn3O4 | H2O | OH− |
|---|---|---|---|---|---|---|---|---|---|---|
| ∆rG/kJ mol−1 | −228 | −615 | −447 | −501 | −722 | −548 | −465 | −1283 | −237 | −157 |
| Mnx+ Redox Pair | SHE | Al | Zn ChOAc | Zn ZnSO4 | |
|---|---|---|---|---|---|
| V | V vs. Al/Al3+ | V vs. Zn/Zn2+ | V vs. Zn/Zn2+ | ||
| Mn3+/Mn2+ | Anodic | 0.3–0.4 | 1.96–2.03 | 1.19 | n.d. |
| Cathodic | 0.1–0.2 | 1.75–1.82 | 0.94 | n.d. | |
| Mn4+/Mn3+ | Anodic | 0.8–1.0 | [2.47–2.67 *] | 1.6–1.8 | 1.64 |
| Cathodic | 0.6–0.7 | [2.27–2.37 *] | 1.4–1.5 | 1.28 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eckert, M.; Peters, W.; Drillet, J.-F. Fast Microwave-Assisted Hydrothermal Synthesis of Pure Layered δ-MnO2 for Multivalent Ion Intercalation. Materials 2018, 11, 2399. https://doi.org/10.3390/ma11122399
Eckert M, Peters W, Drillet J-F. Fast Microwave-Assisted Hydrothermal Synthesis of Pure Layered δ-MnO2 for Multivalent Ion Intercalation. Materials. 2018; 11(12):2399. https://doi.org/10.3390/ma11122399
Chicago/Turabian StyleEckert, Martin, Willi Peters, and Jean-Francois Drillet. 2018. "Fast Microwave-Assisted Hydrothermal Synthesis of Pure Layered δ-MnO2 for Multivalent Ion Intercalation" Materials 11, no. 12: 2399. https://doi.org/10.3390/ma11122399
APA StyleEckert, M., Peters, W., & Drillet, J.-F. (2018). Fast Microwave-Assisted Hydrothermal Synthesis of Pure Layered δ-MnO2 for Multivalent Ion Intercalation. Materials, 11(12), 2399. https://doi.org/10.3390/ma11122399






