Formation of Nb(C,N) Carbonitride in Cast Austenitic Heat-Resistant Steel during Directional Solidification under Different Withdraw Rates
Abstract
1. Introduction
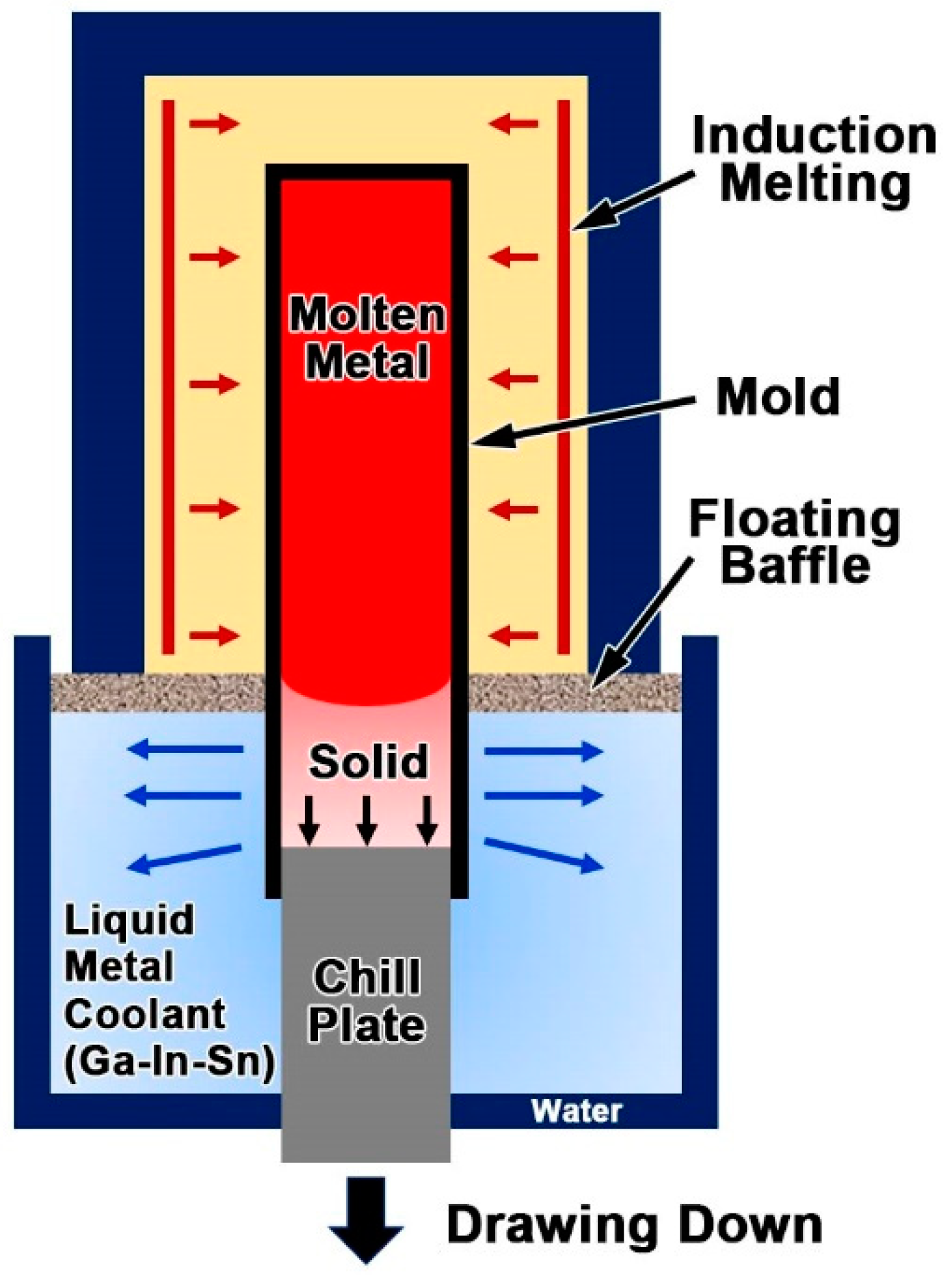
2. Materials and Methods
3. Results
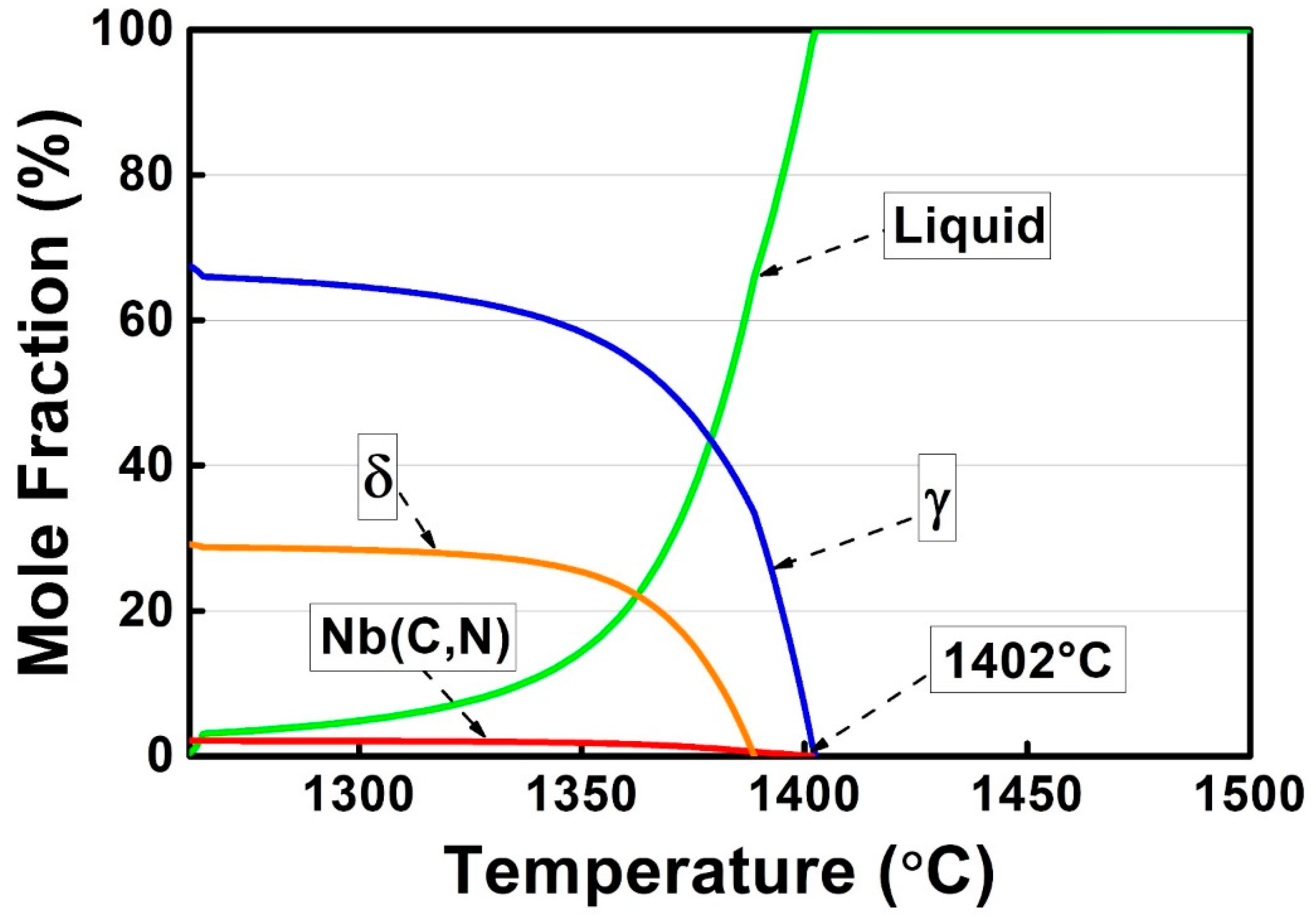
3.1. Thermodynamic Calculations
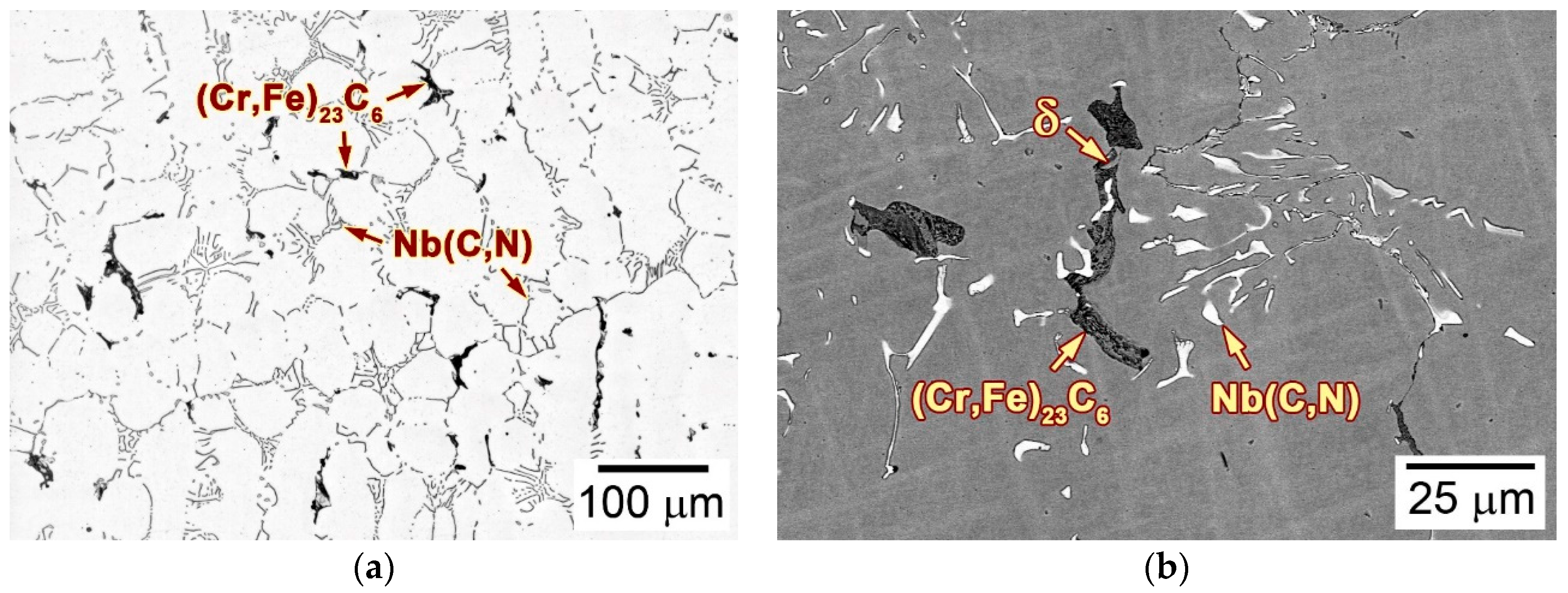
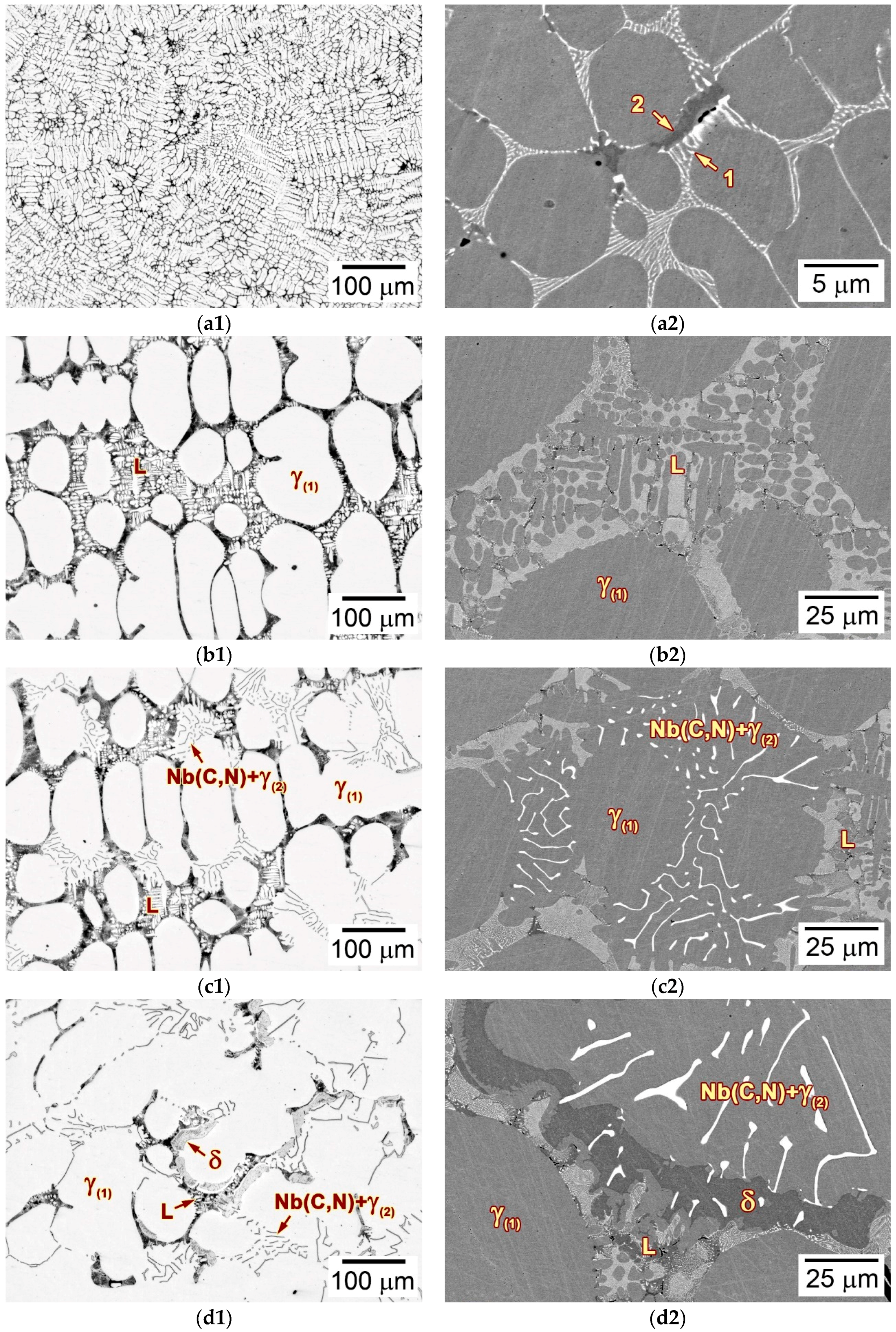
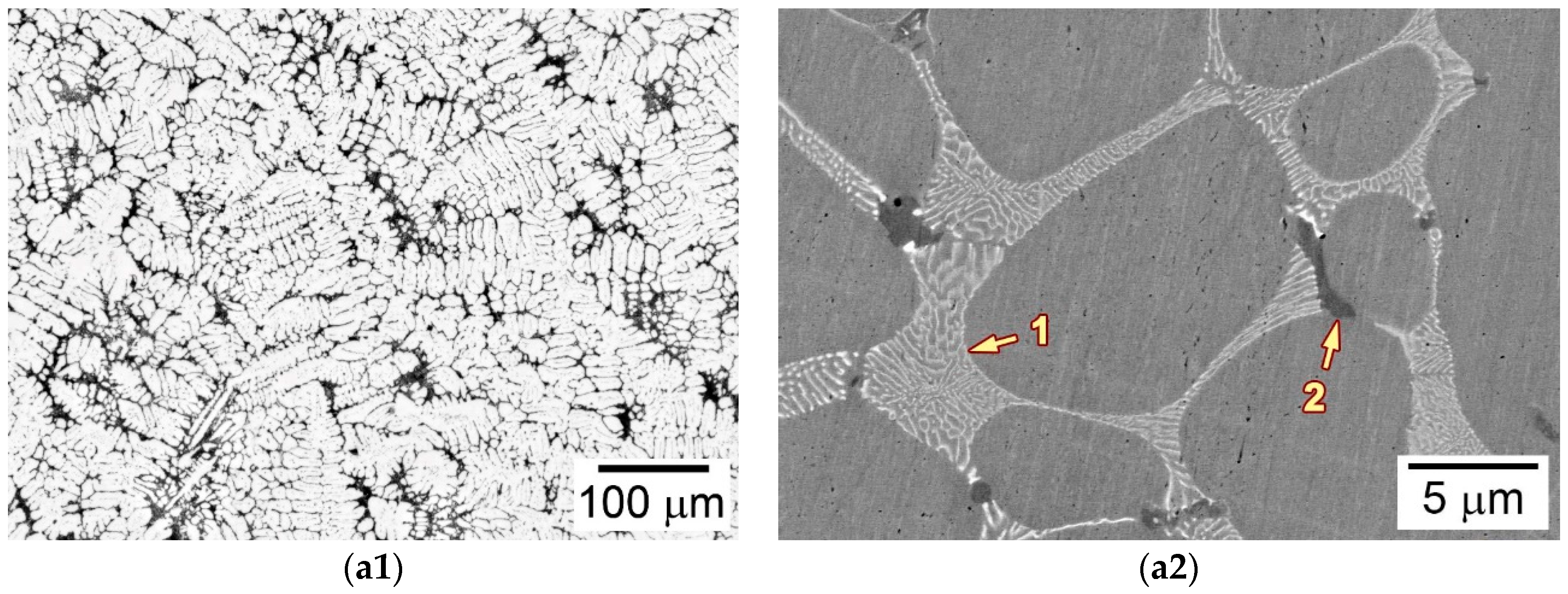
3.2. Microstructure of the as-Cast Ingot
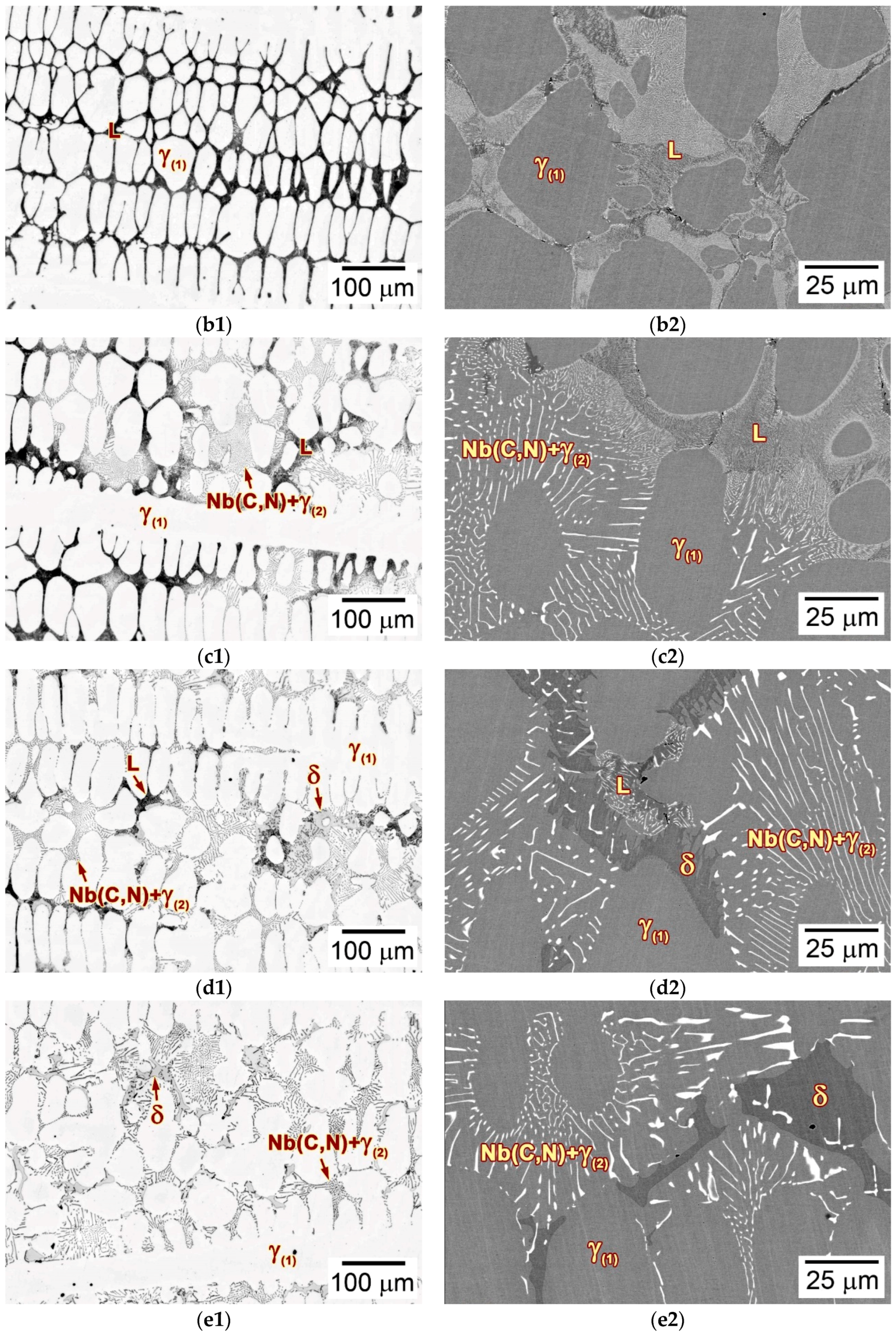
3.3. Microstructural Developments during Directional Solidification
3.3.1. Macro-Grain Structures
3.3.2. Microstructures
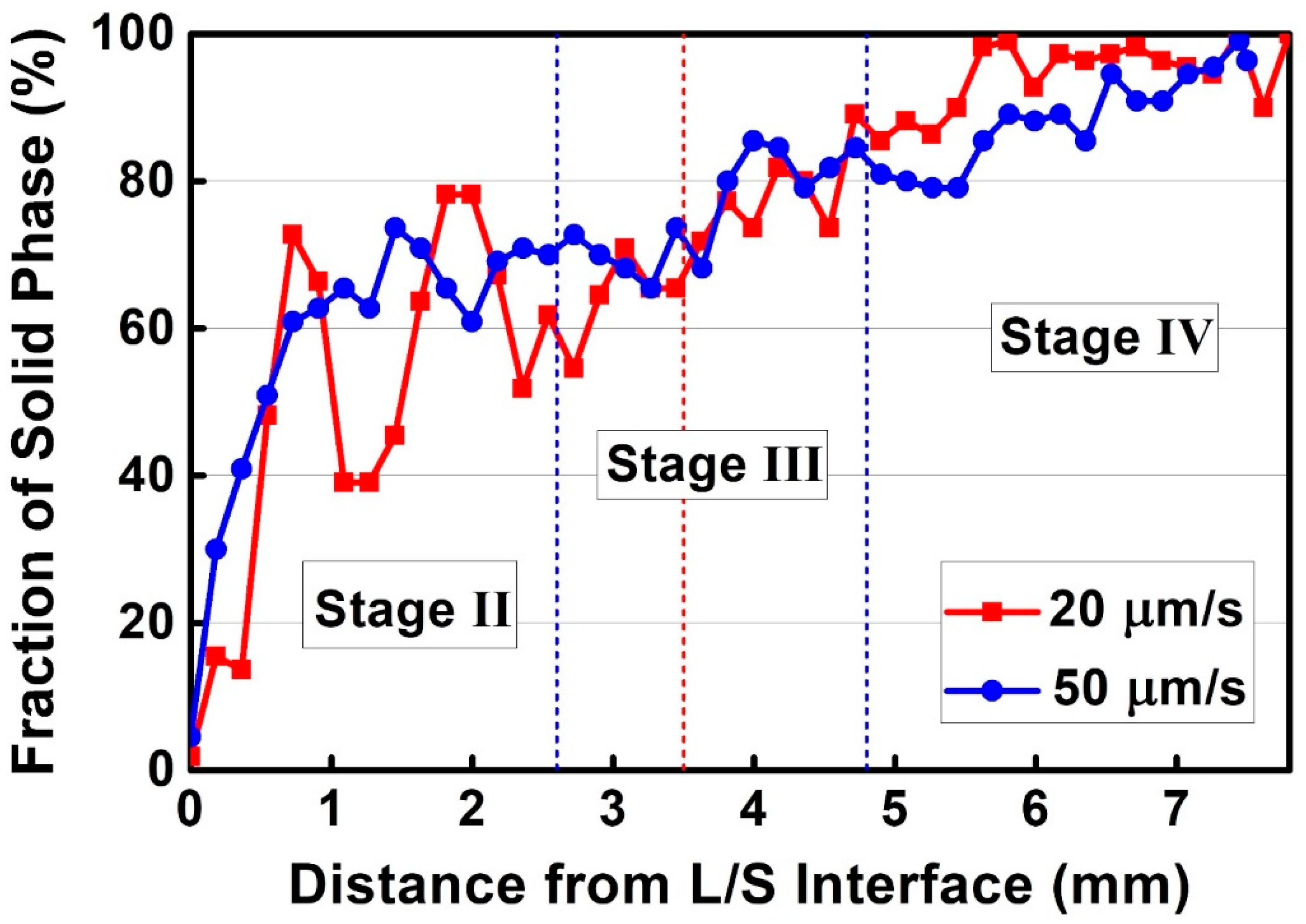
3.3.3. Chemical Composition of the Liquid Phase
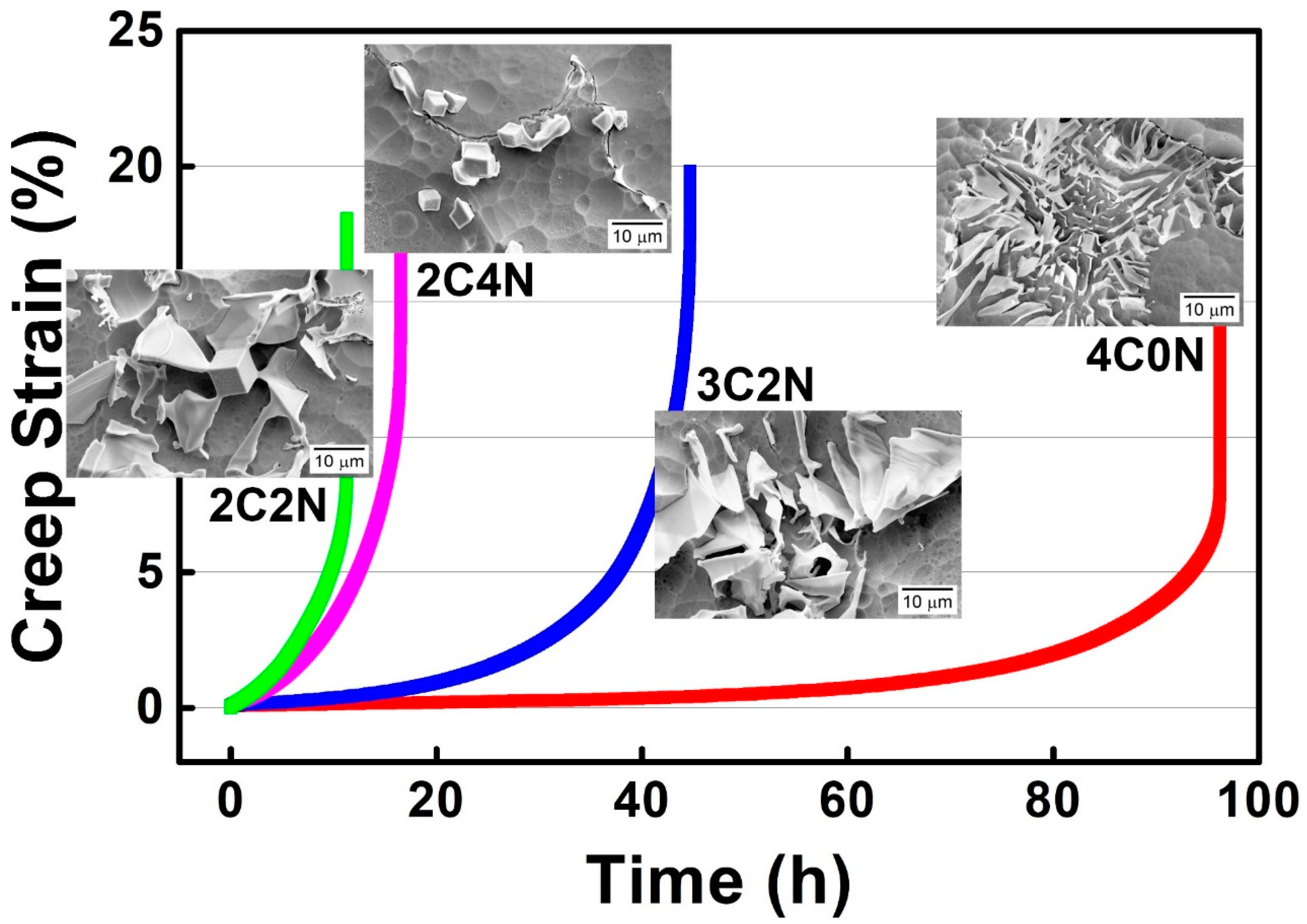
4. Discussion
5. Conclusions
- The solidification path of alloy 3C2N can be described as L → L + γ(1) → L + γ(1) + (Nb(C,N) + γ(2))eutectic → L + γ(1) + (Nb(C,N) + γ(2))eutectic + δ → γ(1) + (Nb(C,N) + γ(2))eutectic + δ, which agrees with the solidification sequence as calculated by thermodynamic methods.
- The PDAS (λ1) and SDAS (λ2) are inversely proportional to the withdraw rate (V) and cooling rate (R), and the relationship can be described as λ1 = 2656 × V−0.73 and λ2 = 24 × R−0.81 = 1005 × V−0.81, respectively.
- The eutectic reaction range increases with an increase in the withdraw rate, which is primarily attributed to the larger undercooling that induces a rapid increase of the solid phase fraction and an accumulation of solutes in liquid phase.
- Increasing the withdraw rate facilitates the formation of “Chinese-script” Nb(C,N) carbonitride with small interlamellar spacing, whereas the rate should also be limited for the consideration of preventing fine grains.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fukai, N.; Imamura, T.; Ito, K.; Kuribayashi, Y. Challenges to expand eco-products with high functional materials and components. Hitachi Rev. 2008, 57, 248–252. [Google Scholar]
- Karnik, A.Y.; Shelby, M.H. Effect of exhaust gas temperature limits on the peak power performance of a turbocharged gasoline engine. J. Eng. Gas Turb. Power 2010, 132, 112801–112807. [Google Scholar] [CrossRef]
- Itoh, K.; Hayashi, K.; Souda, T. Development of HERCUNITE®-S NSHR®-A5N for high performance gasoline engines. Hitachi Met. Tech. Rev. 2006, 22, 51–56. [Google Scholar]
- Ike, M.; Akiyama, K.; Ohtsuka, K.; Itoh, K. Development of heat-resistant cast steel for exhaust manifolds. Int. J. Mater. Prod. Technol. 1991, 6, 243–256. [Google Scholar]
- Shingledecker, J.P.; Maziasz, P.J.; Evans, N.D.; Pollard, M.J. Creep behavior of a new cast austenitic alloy. Int. J. Pres. Ves. Pip. 2007, 84, 21–28. [Google Scholar] [CrossRef]
- Maziasz, P.J. Development of creep-resistant and oxidation-resistant austenitic stainless steels for high temperature applications. JOM 2018, 70, 66–75. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Li, M.; Godlewski, L.A.; Zindel, J.W.; Feng, Q. Effective design of new austenitic cast steels for ultra-high temperature automotive exhaust components through combined CALPHAD and experimental approaches. Mater. Sci. Eng. A 2017, 683, 195–206. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Feng, Q. Effects of W on creep behaviors of novel Nb-bearing austenitic heat-resistant cast steels at 1000 °C. Acta Metall. Sin. 2017, 53, 1025–1037. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Li, M.; Godlewski, L.A.; Zindel, J.W.; Feng, Q. Effects of N on creep properties of austenitic heat-resistant cast steels developed for exhaust component applications at 1000 °C. Acta Metall. Sin. 2016, 52, 661–671. [Google Scholar]
- Zhang, Y.H.; Yang, J. Effect of N/C ratio on precipitation behavior of (Cr,Fe)23C6 carbide in novel cast austenitic heat-resistant steels during directional solidification. Metals 2018, 8, 678. [Google Scholar] [CrossRef]
- Zhao, H.L.; Engler-Pinto, C.C.; Zindel, J.; Godlewski, L.; Zhang, Y.H.; Feng, Q.; Li, M. The Effect of metal-carbide morphology on the thermomechanical fatigue (TMF) behavior of cast austenitic alloys for exhaust manifolds. Procedia Eng. 2015, 133, 669–680. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Li, M.; Godlewski, L.A.; Zindel, J.W.; Feng, Q. Effects of W on creep behaviors of novel Nb-bearing high nitrogen austenitic heat-resistant cast steels at 1000 °C. Mater. Charact. 2018, 139, 19–29. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Li, M.; Godlewski, L.A.; Zindel, J.W.; Feng, Q. Creep behavior at 1273 K (1000 °C) in Nb-bearing austenitic heat-resistant cast steels developed for exhaust component applications. Metall. Mater. Trans. A 2016, 47, 3289–3294. [Google Scholar] [CrossRef]
- Zhao, H.L.; Engler-Pinto, C.C.; Tong, J.Y.; Godlewski, L.A.; Zindel, J.W.; Li, L.F.; Li, M.; Feng, Q. Mechanical response and dislocation substructure of a cast austenitic steel under low cycle fatigue at elevated temperatures. Mater. Sci. Eng. A 2017, 703, 422–429. [Google Scholar] [CrossRef]
- Tin, S.; Pollock, T.M.; Murphy, W. Stabilization of thermosolutal convective instabilities in Ni-based single-crystal superalloys: Carbon additions and freckle formation. Metall. Mater. Trans. A 2001, 32, 1743–1753. [Google Scholar] [CrossRef]
- Li, X.W.; Wang, L.; Dong, J.S.; Lou, L.H. Effect of solidification condition and carbon content on the morphology of MC carbide in directionally solidified nickel-base superalloys. J. Mater. Sci. Technol. 2014, 30, 1296–1300. [Google Scholar] [CrossRef]
- Zhang, H.W.; Qin, X.Z.; Li, X.W.; Zhou, L.Z. Effect of minor additions on the microstructures and stress rupture properties of a directionally solidified Ni-based superalloy. Mater. Sci. Eng. A 2018, 711, 303–312. [Google Scholar] [CrossRef]
- Antonov, S.; Chen, W.; Huo, J.J.; Feng, Q.; Isheim, D.; Seidman, D.N.; Sun, E.; Tin, S. MC carbide characterization in high refractory content powder-processed Ni-based superalloys. Metall. Mater. Trans. A 2018, 49, 2340–2351. [Google Scholar] [CrossRef]
- Chen, Q.Z.; Jones, C.N.; Knowles, D.M. Effect of alloying chemistry on MC carbide morphology in modified RR2072 and RR2086 SX superalloys. Scr. Mater. 2002, 47, 669–675. [Google Scholar] [CrossRef]
- Min, K.S.; Nam, S.W. Correlation between characteristics of grain boundary carbides and creep–fatigue properties in AISI 321 stainless steel. J. Nucl. Mater. 2003, 322, 91–97. [Google Scholar] [CrossRef]
- De Almeida, L.H.; Ribeiro, A.F.; Le May, I. Microstructural characterization of modified 25Cr–35Ni centrifugally cast steel furnace tubes. Mater. Charact. 2002, 49, 219–229. [Google Scholar] [CrossRef]
- Piekarski, B. Effect of Nb and Ti additions on microstructure, and identification of precipitates in stabilized Ni-Cr cast austenitic steels. Mater. Charact. 2001, 47, 181–186. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Li, M.; Godlewski, L.A.; Zindel, J.W.; Feng, Q. Effects of N/C ratio on solidification behaviors of novel Nb-bearing austenitic heat-resistant cast steels for exhaust components of gasoline engines. Metall. Mater. Trans. A 2017, 48, 1151–1162. [Google Scholar] [CrossRef]
- Reed, R. Nitrogen in austenitic stainless steels. JOM 1989, 41, 16–21. [Google Scholar] [CrossRef]
- Bhambri, A.K.; Kattamis, T.Z.; Morral, J.E. Cast microstructure of Inconel 713C and its dependence on solidification variables. Metall. Trans. B 1975, 6, 523–537. [Google Scholar] [CrossRef]
- Liu, L.; Sommer, F.; Fu, H.Z. Effect of solidification conditions on MC carbides in a nickel-base superalloy IN 738 LC. Scr. Metall. 1994, 30, 587–591. [Google Scholar] [CrossRef]
- Buchanan, K.G.; Kral, M.V. Crystallography and morphology of niobium carbide in as-cast HP-niobium reformer tubes. Metall. Mater. Trans. A 2012, 43, 1760–1769. [Google Scholar] [CrossRef]
- Buchanan, K.G.; Kral, M.V.; Bishop, C. Crystallography and morphology of MC carbides in niobium-titanium modified as-cast HP alloys. Metall. Mater. Trans. A 2014, 45, 3373–3385. [Google Scholar] [CrossRef]
- Miller, J.D.; Pollock, T.M. Stability of dendrite growth during directional solidification in the presence of a non-axial thermal field. Acta Mater. 2014, 78, 23–36. [Google Scholar] [CrossRef]
- Ding, X.F.; Lin, J.P.; Zhang, L.Q.; Wang, H.L.; Hao, G.J.; Chen, G.L. Microstructure development during directional solidification of Ti–45Al–8Nb alloy. J. Alloys Compd. 2010, 506, 115–119. [Google Scholar] [CrossRef]
- Yu, Y.N.; Yang, P.; Qiang, W.J.; Chen, L. Foundations of Materials Science; High Education Press: Beijing, China, 2006; pp. 659–707. [Google Scholar]
- Hunt, J.D.; Lu, S.Z. Numerical modeling of cellular/dendritic array growth: Spacing and structure predictions. Metall. Mater. Trans. A 1996, 27, 611–623. [Google Scholar] [CrossRef]
- Zheng, X.; Luo, A.; Zhang, C.; Dong, J.; Waldo, R. Directional solidification and microsegregation in a magnesium-aluminum-calcium alloy. Metall. Mater. Trans. A 2012, 43, 3239–3248. [Google Scholar] [CrossRef]
- Wang, Y.N.; Yang, J.; Xin, X.L.; Wang, R.Z.; Xu, L.Y. The effect of cooling conditions on the evolution of non-metallic inclusions in high manganese TWIP steels. Metall. Mater. Trans. B 2016, 47, 1378–1389. [Google Scholar] [CrossRef]





| Alloy | Fe | Cr | Ni | Si | Mn | Nb | S | P | C | N |
|---|---|---|---|---|---|---|---|---|---|---|
| 3C2N | Bal. | 19.68 | 10.12 | 0.80 | 0.93 | 2.09 | 0.008 | 0.013 | 0.29 | 0.15 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yang, J. Formation of Nb(C,N) Carbonitride in Cast Austenitic Heat-Resistant Steel during Directional Solidification under Different Withdraw Rates. Materials 2018, 11, 2397. https://doi.org/10.3390/ma11122397
Zhang Y, Yang J. Formation of Nb(C,N) Carbonitride in Cast Austenitic Heat-Resistant Steel during Directional Solidification under Different Withdraw Rates. Materials. 2018; 11(12):2397. https://doi.org/10.3390/ma11122397
Chicago/Turabian StyleZhang, Yinhui, and Jian Yang. 2018. "Formation of Nb(C,N) Carbonitride in Cast Austenitic Heat-Resistant Steel during Directional Solidification under Different Withdraw Rates" Materials 11, no. 12: 2397. https://doi.org/10.3390/ma11122397
APA StyleZhang, Y., & Yang, J. (2018). Formation of Nb(C,N) Carbonitride in Cast Austenitic Heat-Resistant Steel during Directional Solidification under Different Withdraw Rates. Materials, 11(12), 2397. https://doi.org/10.3390/ma11122397




