Characterization of MgCl2·6H2O-Based Eutectic/Expanded Perlite Composite Phase Change Material with Low Thermal Conductivity
Abstract
:1. Introduction
2. Experiment
2.1. Material Preparation
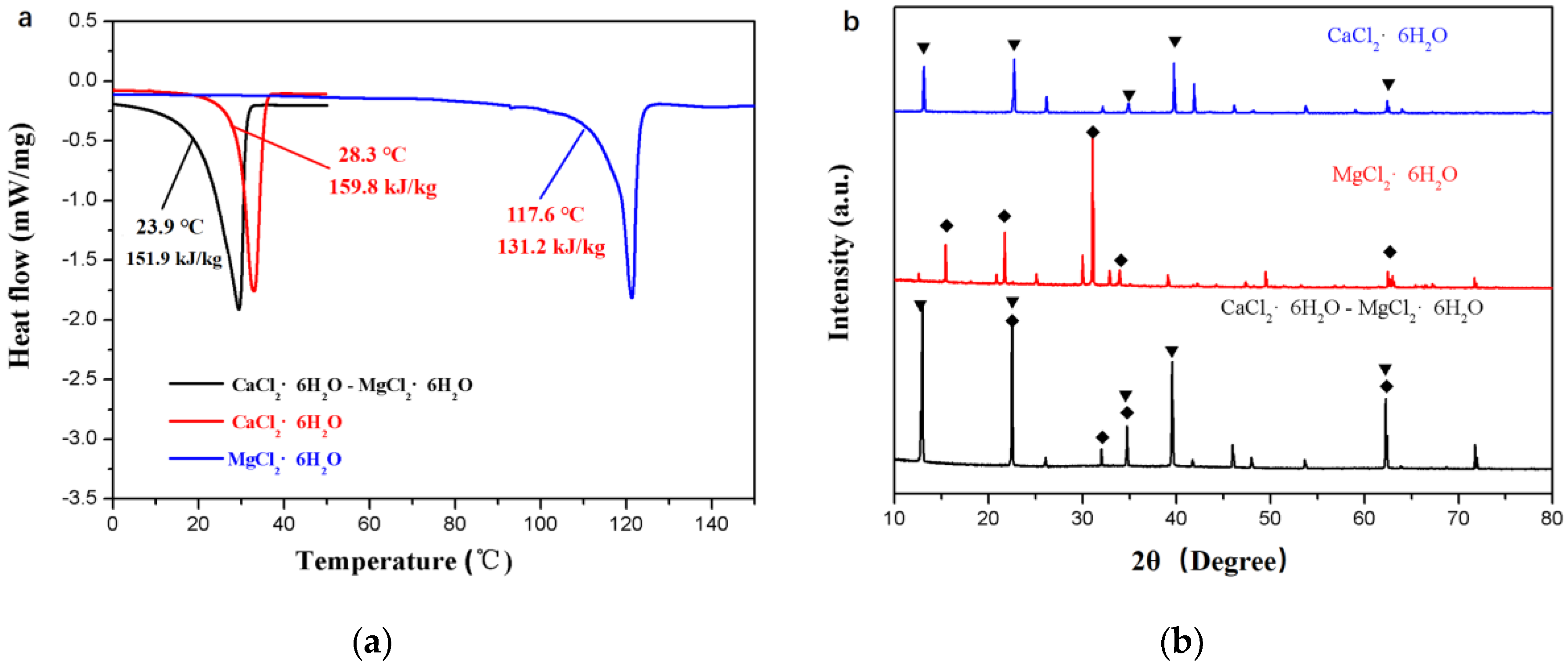
2.1.1. Preparation of the Eutectic
2.1.2. Preparation of the Composite PCM
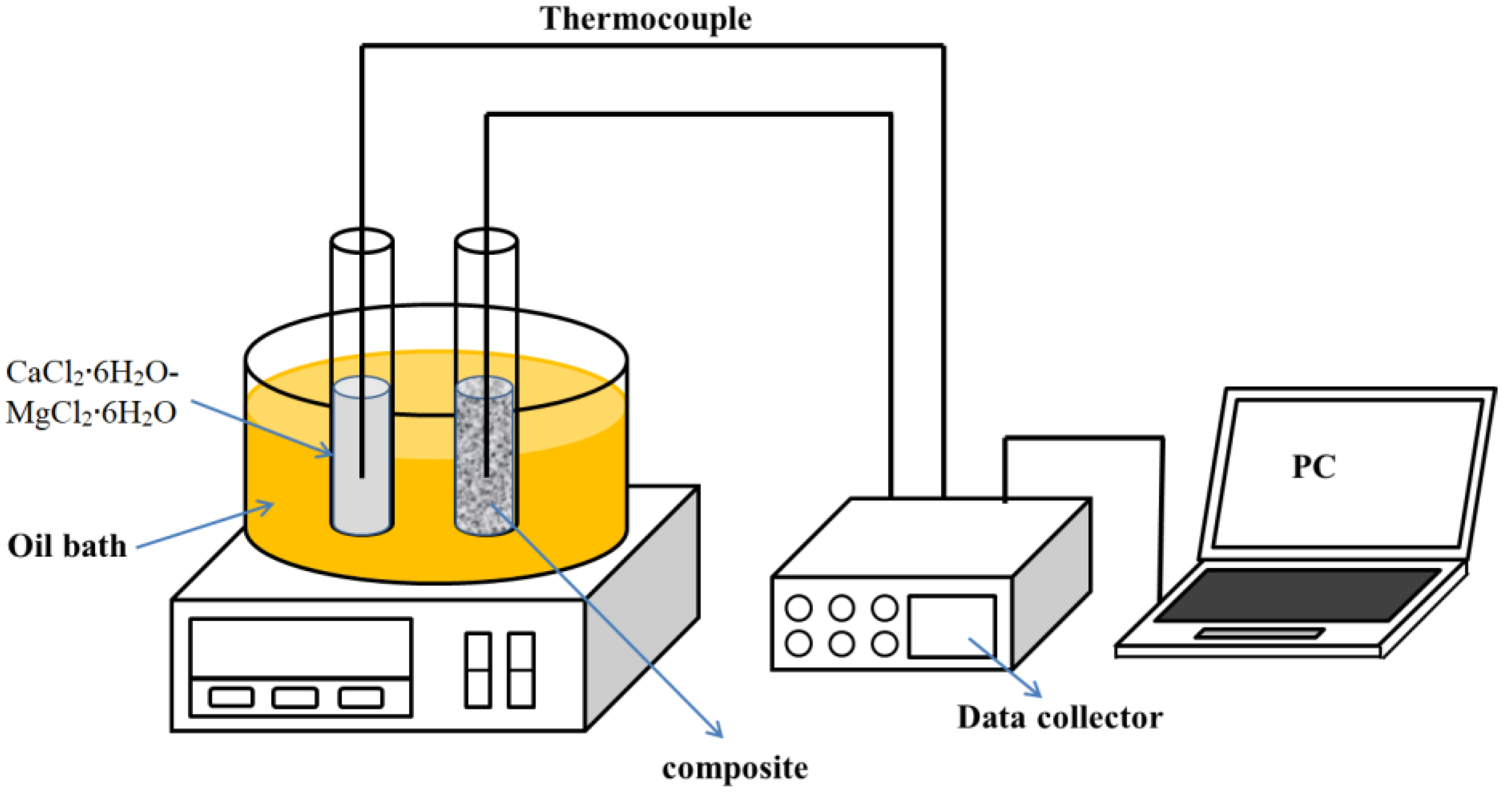
2.2. Characterization
3. Results and Discussion
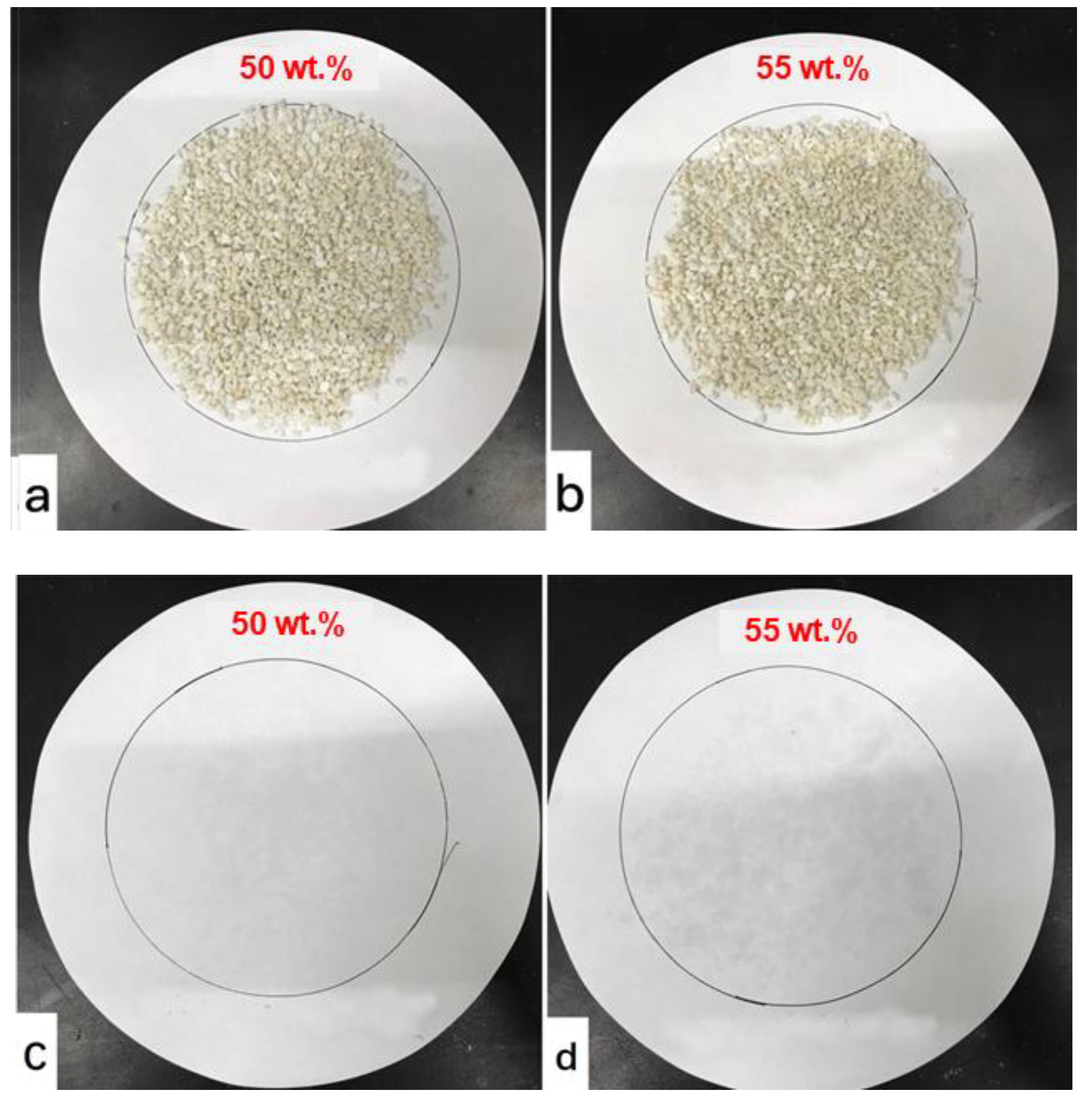
3.1. Determining the Suitable Mass Fraction of MgCl2∙6H2O
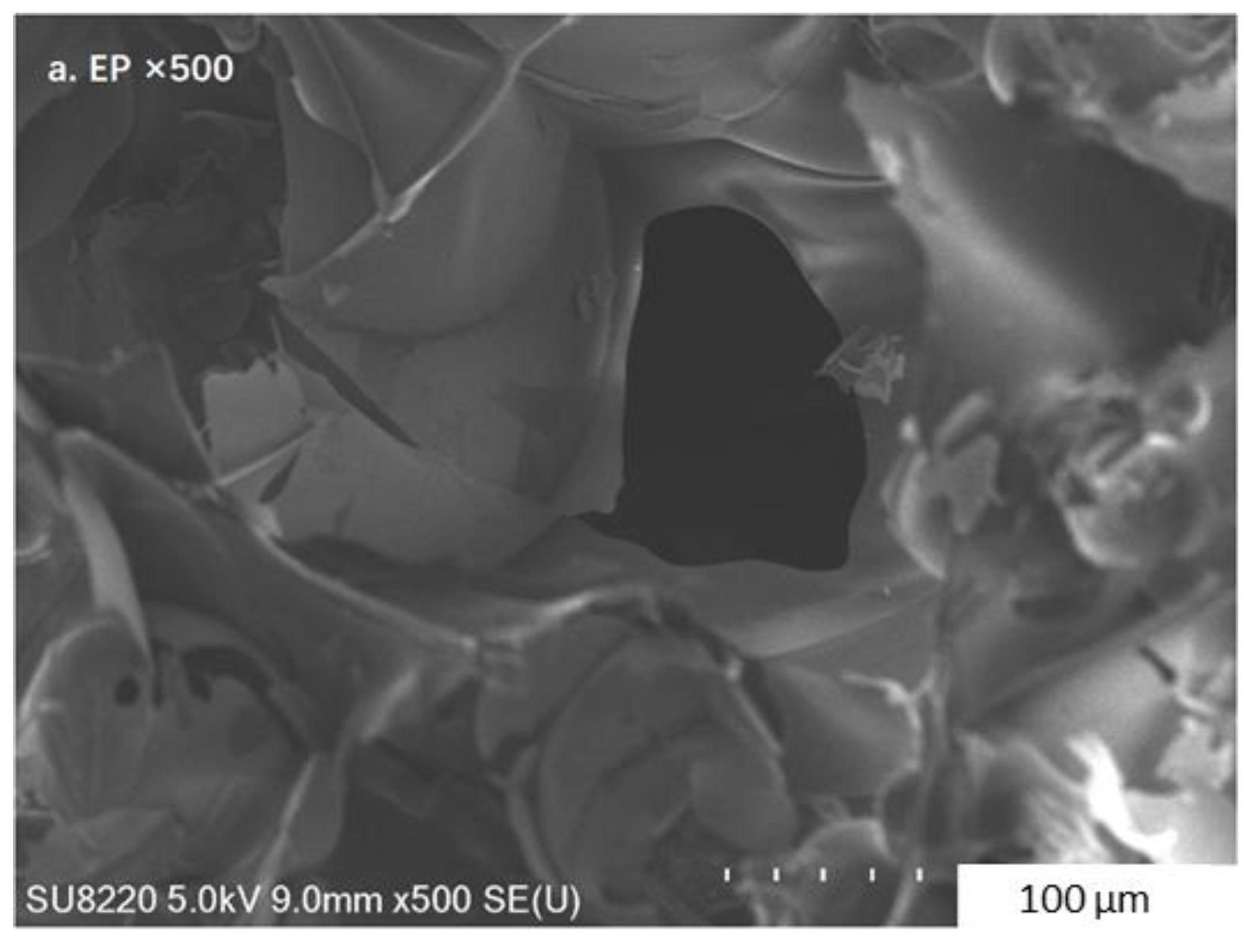
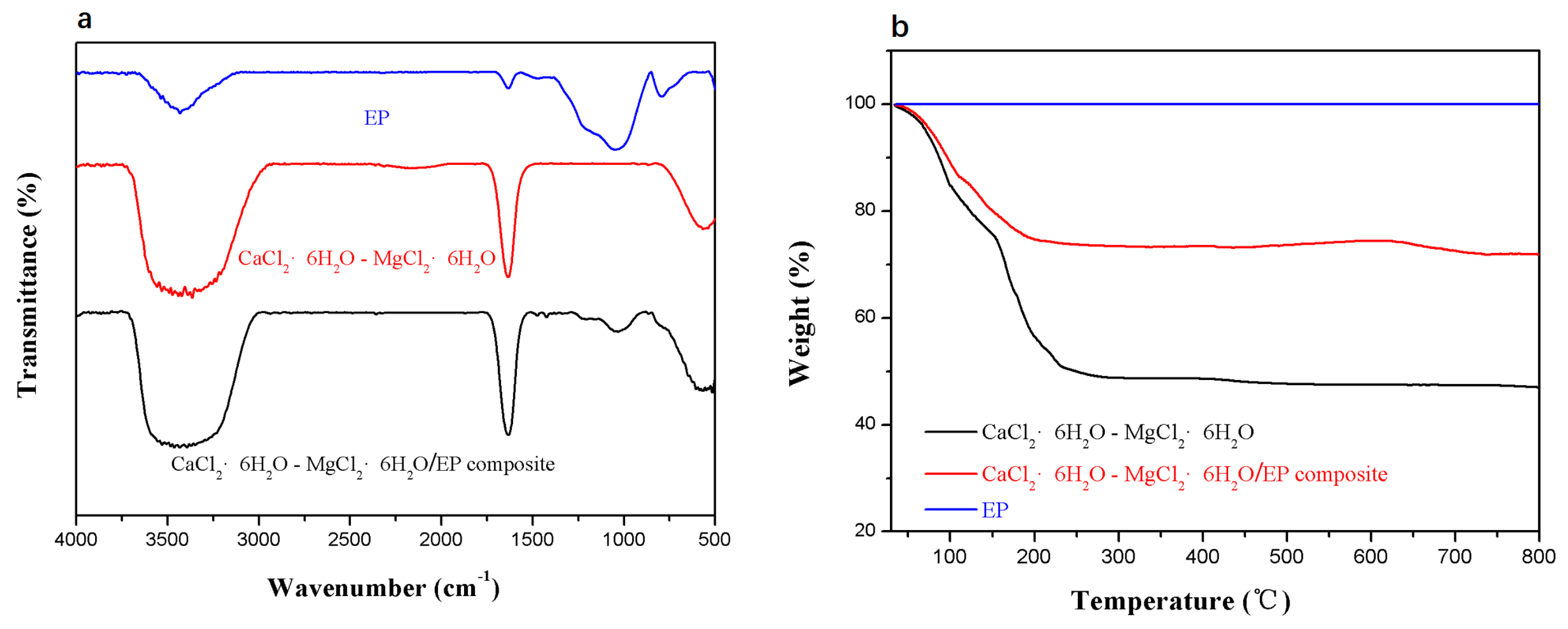
3.2. Characteristics of the Composite PCM
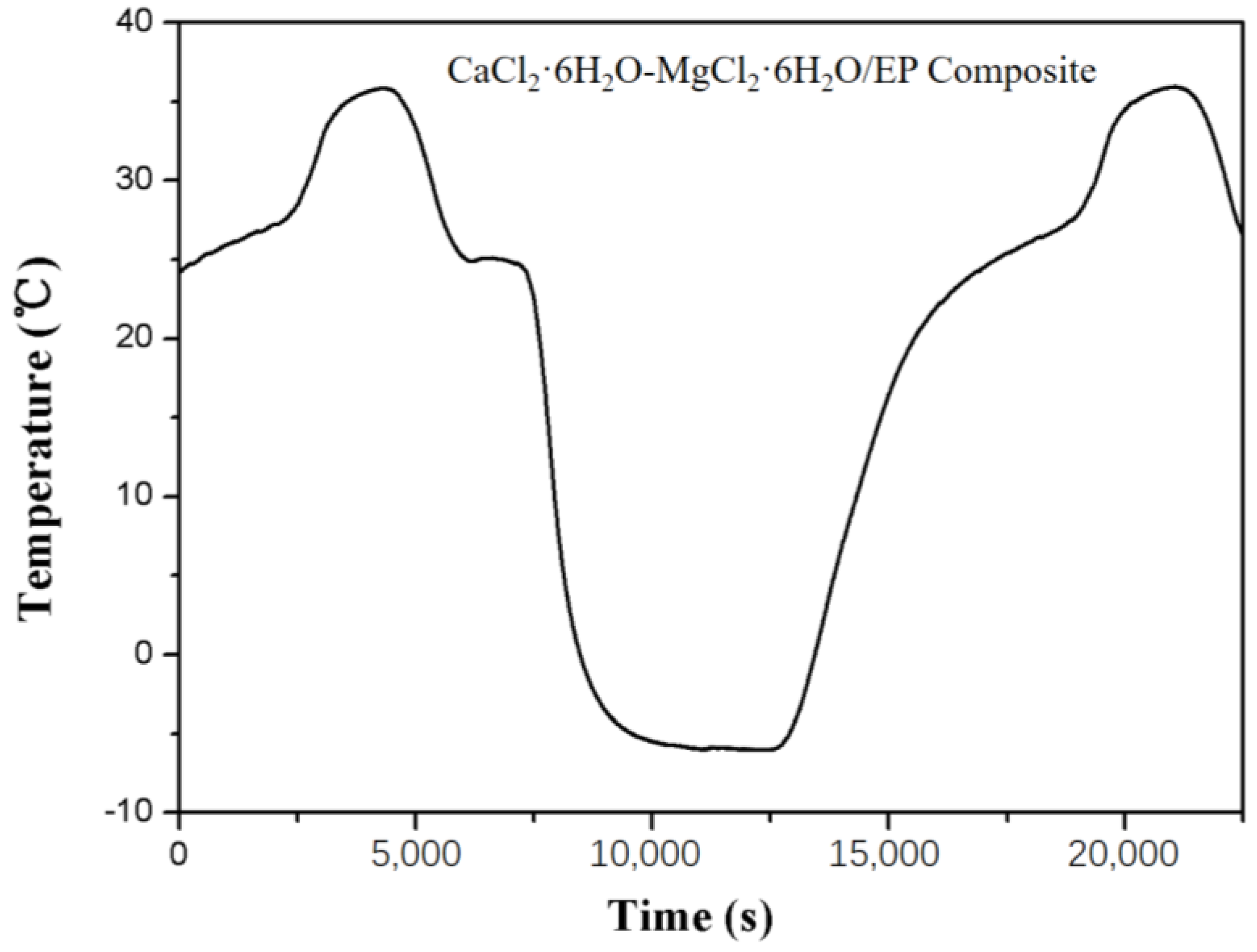
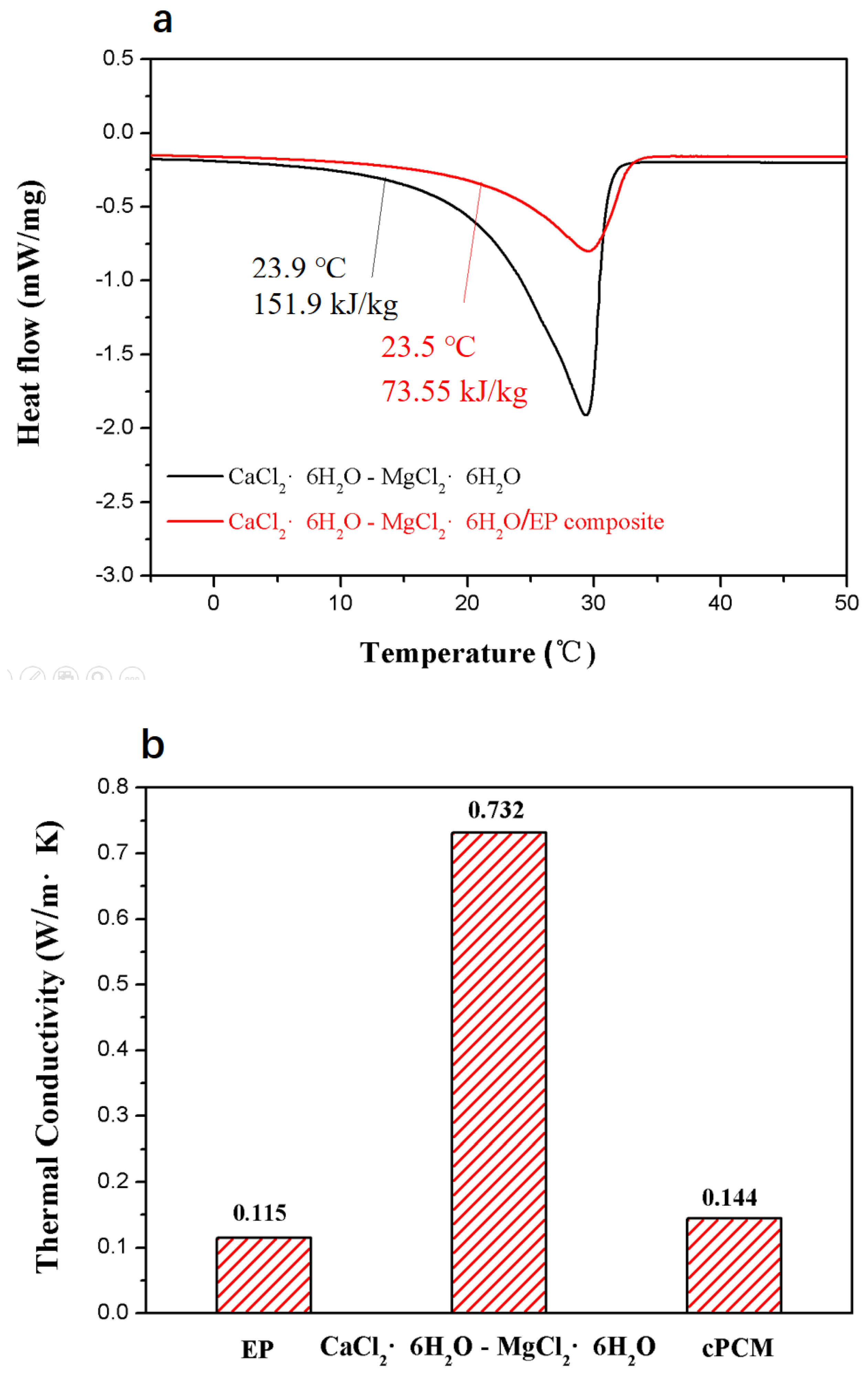
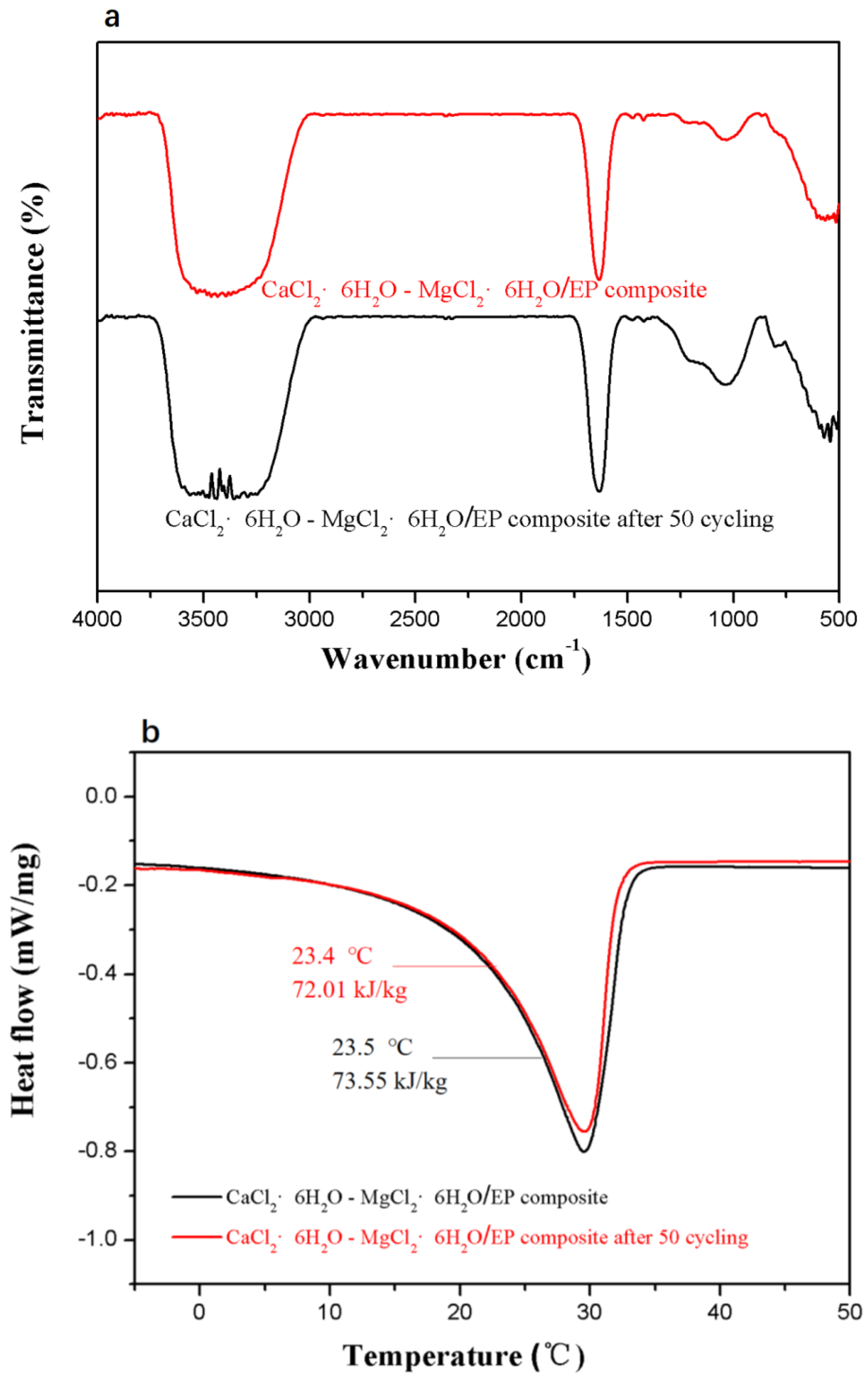
3.3. Thermal Reliability of the Composite PCM
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jegadheeswaran, S.; Pohekar, S.D. Performance enhancement in latent heat thermal storage system: A review. Renew. Sustain. Energy Rev. 2009, 13, 2225–2244. [Google Scholar] [CrossRef]
- Agyenim, F.; Hewitt, N.; Eames, P.; Smyth, M. A review of materials, heat transfer and phase change problem formulation for latent heat thermal energy storage systems (LHTESS). Renew. Sustain. Energy Rev. 2010, 14, 615–628. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, Z.J.; Yang, T.; Qin, D.; Li, S.; Zhang, G.; Haghighat, F.; Joybari, M.M. A review on macro-encapsulated phase change material for building envelope applications. Build. Environ. 2018, 144, 281–294. [Google Scholar] [CrossRef]
- Konuklu, Y.; Ostry, M.; Paksoy, H.O.; Charvat, P. Review on using microencapsulated phase change materials (PCM) in building applications. Energy Build. 2015, 106, 134–155. [Google Scholar] [CrossRef]
- Kenisarin, M.M. Thermophysical properties of some organic phase change materials for latent heat storage. A review. Sol. Energy 2014, 107, 553–575. [Google Scholar] [CrossRef]
- Xie, N.; Huang, Z.; Luo, Z.; Gao, X.; Fang, Y.; Zhang, Z. Inorganic salt hydrate for thermal energy storage. Appl. Sci. 2017, 7, 1317. [Google Scholar] [CrossRef]
- Kenisarin, M.; Mahkamov, K. Salt hydrates as latent heat storage materials: Thermophysical properties and costs. Sol. Energy Mater. Sol. Cells 2016, 145, 255–286. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y. Form-stable phase change material based on Na2CO3·10H2O-Na2HPO4·12H2O eutectic hydrated salt/expanded graphite oxide composite: The influence of chemical structures of expanded graphite oxide. Renew. Energy 2018, 115, 734–740. [Google Scholar] [CrossRef]
- Sonnick, S.; Erlbeck, L.; Schlachter, K.; Strischakov, J.; Mai, T.; Mayer, C.; Jakob, K.; Nirschl, H.; Rädle, M. Temperature stabilization using salt hydrate storage system to achieve thermal comfort in prefabricated wooden houses. Energy Build. 2018, 164, 48–60. [Google Scholar] [CrossRef]
- Sun, W.; Huang, R.; Ling, Z.; Fang, X.; Zhang, Z. Two types of composite phase change panels containing a ternary hydrated salt mixture for use in building envelope and ventilation system. Energy Convers. Manag. 2018, 177, 306–314. [Google Scholar] [CrossRef]
- Ye, R.; Zhang, C.; Sun, W.; Fang, X.; Zhang, Z. Novel wall panels containing CaCl2·6H2O-Mg(NO3)2·6H2O/expanded graphite composites with different phase change temperatures for building energy savings. Energy Build. 2018, 176, 407–417. [Google Scholar] [CrossRef]
- Xu, X.; Cui, H.; Memon, S.A.; Yang, H.; Tang, W. Development of novel composite PCM for thermal energy storage using CaCl2·6H2O with graphene oxide and SrCl2·6H2O. Energy Build. 2017, 156, 163–172. [Google Scholar] [CrossRef]
- Li, G.; Zhang, B.; Li, X.; Zhou, Y.; Sun, Q.; Yun, Q. The preparation, characterization and modification of a new phase change material: CaCl2·6H2O-MgCl2·6H2O eutectic hydrate salt. Sol. Energy Mater. Sol. Cells 2014, 126, 51–55. [Google Scholar] [CrossRef]
- He, M.; Yang, L.; Zhang, Z. Experimental studies on cycling stable characteristics of inorganic phase change material CaCl2·6H2O-MgCl2·6H2O modified with SrCl2·6H2O and CMC. IOP Conf. Ser. Earth Environ. Sci. 2018, 108, 022058. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, W.; Ling, Z.; Fang, X.; Zhang, Z. Hydrophilic modification of expanded graphite to prepare a high-performance composite phase change block containing a hydrate salt. Ind. Eng. Chem. Res. 2017, 56, 14799–14806. [Google Scholar] [CrossRef]
- Zhou, S.; Zhou, Y.; Ling, Z.; Zhang, Z.; Fang, X. Modification of expanded graphite and its adsorption for hydrated salt to prepare composite PCMs. Appl. Therm. Eng. 2018, 133, 446–451. [Google Scholar] [CrossRef]
- Ye, R.; Lin, W.; Yuan, K.; Fang, X.; Zhang, Z. Experimental and numerical investigations on the thermal performance of building plane containing CaCl2·6H2O/expanded graphite composite phase change material. Appl. Energy 2017, 193, 325–335. [Google Scholar] [CrossRef]
- Ling, Z.; Liu, J.; Wang, Q.; Lin, W.; Fang, X.; Zhang, Z. MgCl2·6H2O-Mg(NO3)2·6H2O eutectic/SiO2 composite phase change material with improved thermal reliability and enhanced thermal conductivity. Sol. Energy Mater. Sol. Cells 2017, 172, 195–201. [Google Scholar] [CrossRef]
- Fu, L.; Wang, Q.; Ye, R.; Fang, X.; Zhang, Z. A calcium chloride hexahydrate/expanded perlite composite with good heat storage and insulation properties for building energy conservation. Renew. Energy 2017, 114, 733–743. [Google Scholar] [CrossRef]
- Fu, L.; Ling, Z.; Fang, X.; Zhang, Z. Thermal performance of CaCl2·6H2O/expanded perlite composite phase change boards embedded in aluminous gusset plates for building energy conservation. Energy Build. 2017, 155, 484–491. [Google Scholar] [CrossRef]
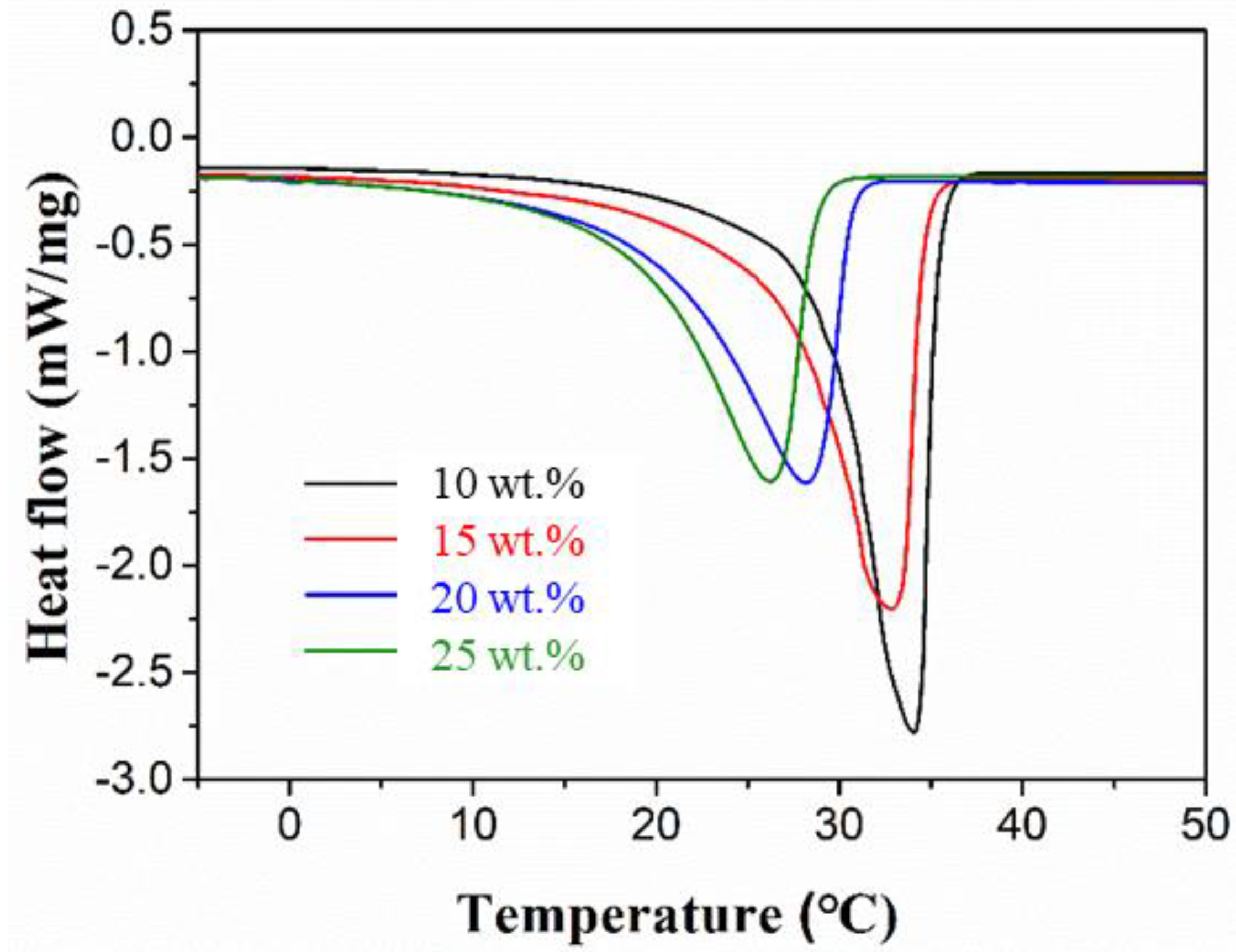

| MgCl2·6H2O (wt.%) | Tm-o (°C) | Tm-p (°C) | Hm (kJ/kg) |
|---|---|---|---|
| 10 | 24.5 | 34.3 | 162.1 |
| 15 | 23.9 | 32.5 | 151.9 |
| 20 | 21.6 | 27.1 | 147.6 |
| 25 | 20.2 | 25.9 | 130.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Zhang, Z.; Ye, R.; Gao, X.; Ling, Z. Characterization of MgCl2·6H2O-Based Eutectic/Expanded Perlite Composite Phase Change Material with Low Thermal Conductivity. Materials 2018, 11, 2369. https://doi.org/10.3390/ma11122369
Zhang C, Zhang Z, Ye R, Gao X, Ling Z. Characterization of MgCl2·6H2O-Based Eutectic/Expanded Perlite Composite Phase Change Material with Low Thermal Conductivity. Materials. 2018; 11(12):2369. https://doi.org/10.3390/ma11122369
Chicago/Turabian StyleZhang, Chao, Zeyu Zhang, Rongda Ye, Xuenong Gao, and Ziye Ling. 2018. "Characterization of MgCl2·6H2O-Based Eutectic/Expanded Perlite Composite Phase Change Material with Low Thermal Conductivity" Materials 11, no. 12: 2369. https://doi.org/10.3390/ma11122369
APA StyleZhang, C., Zhang, Z., Ye, R., Gao, X., & Ling, Z. (2018). Characterization of MgCl2·6H2O-Based Eutectic/Expanded Perlite Composite Phase Change Material with Low Thermal Conductivity. Materials, 11(12), 2369. https://doi.org/10.3390/ma11122369





