Pressure Effect of the Vibrational and Thermodynamic Properties of Chalcopyrite-Type Compound AgGaS2: A First-Principles Investigation
Abstract
1. Introduction
2. Computational Scheme
3. Results and Discussions
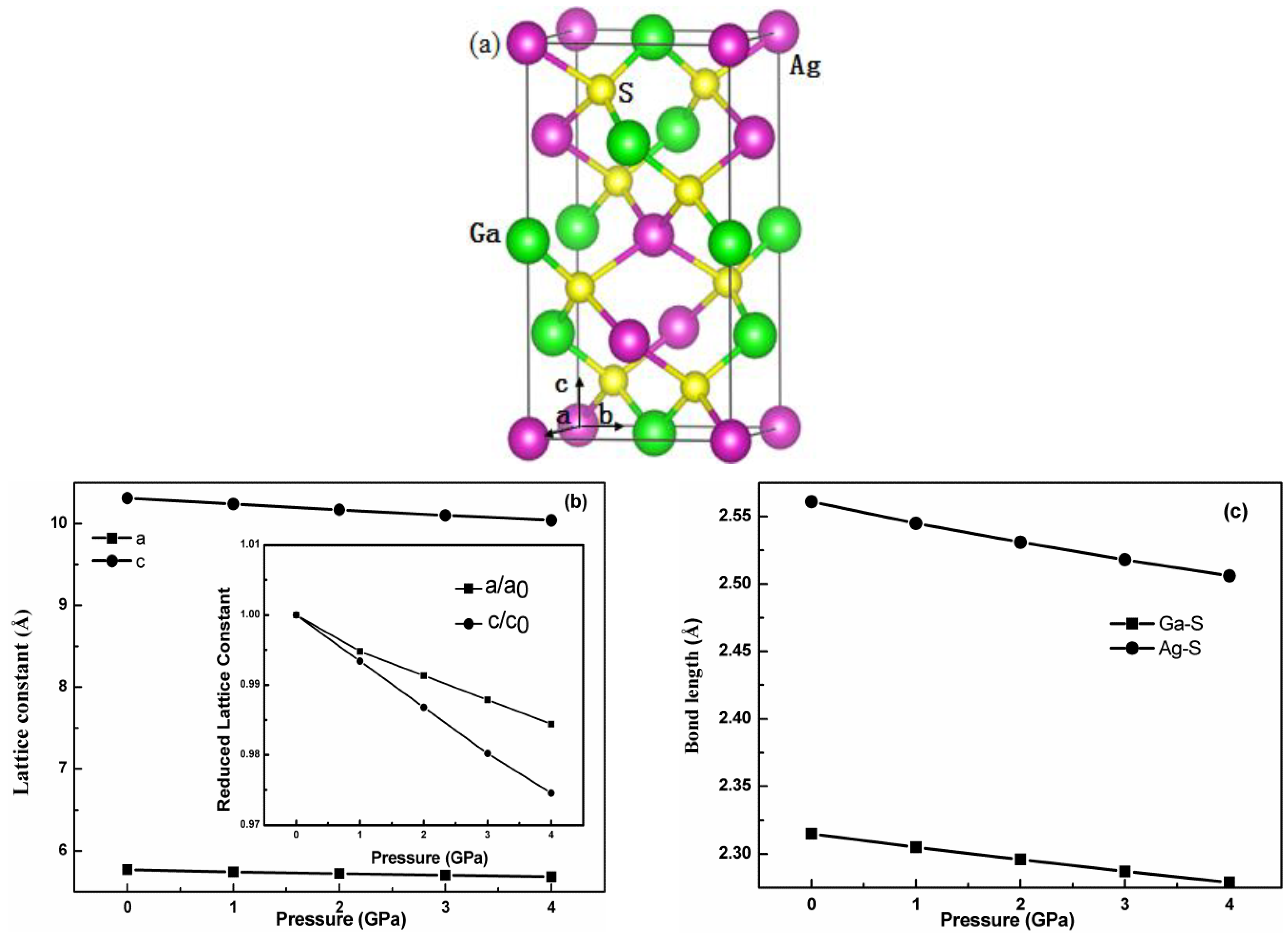
3.1. Structural Optimization
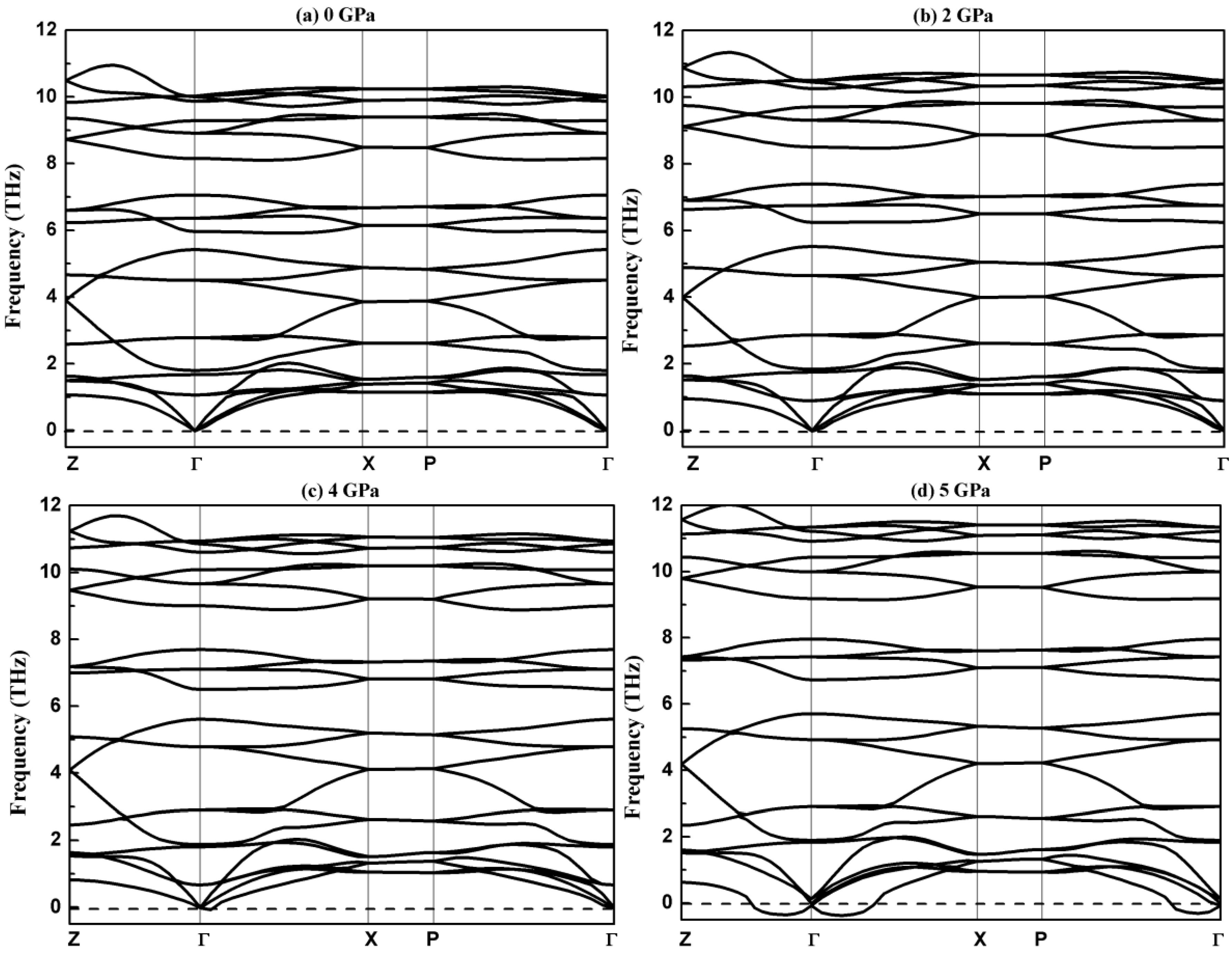
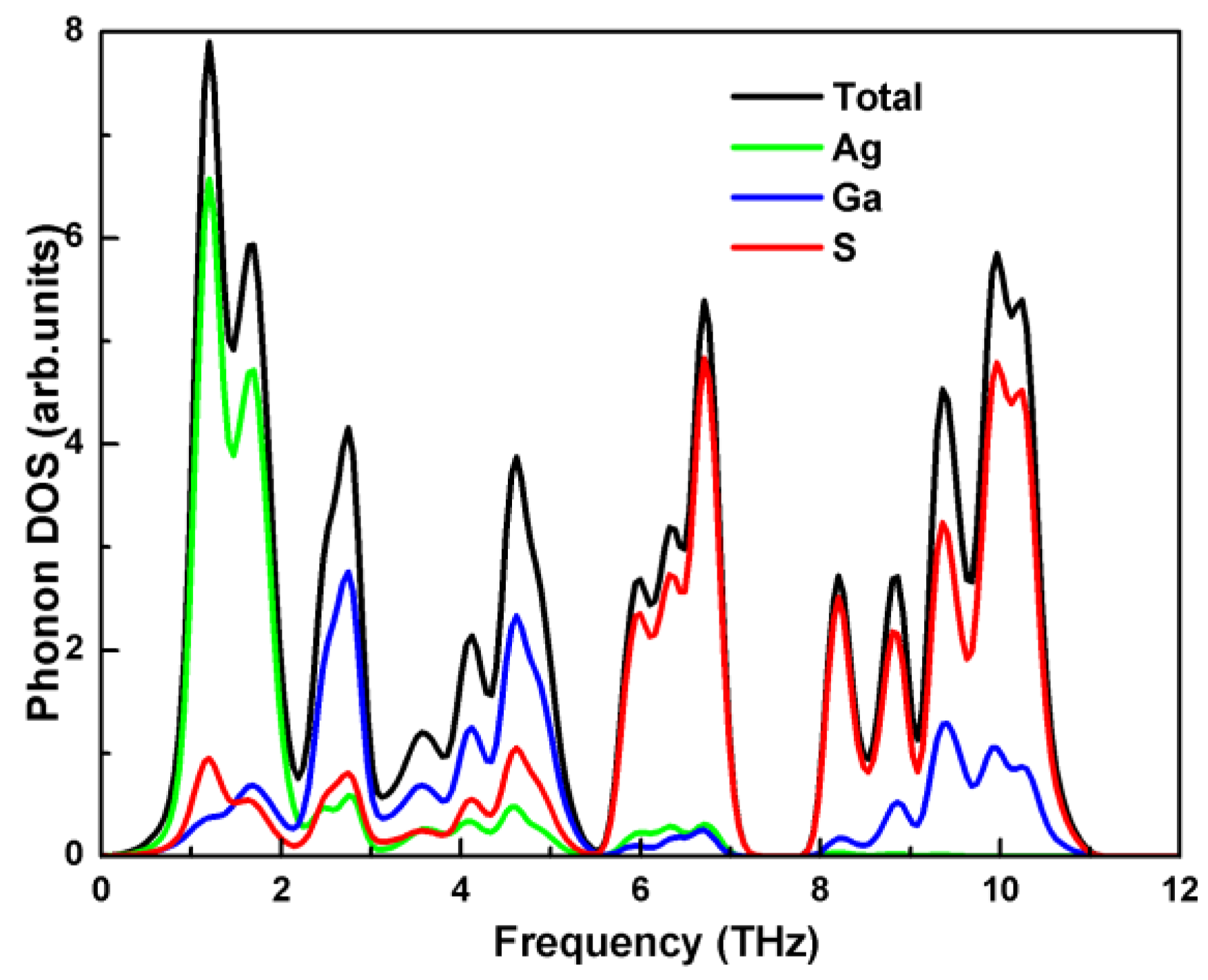
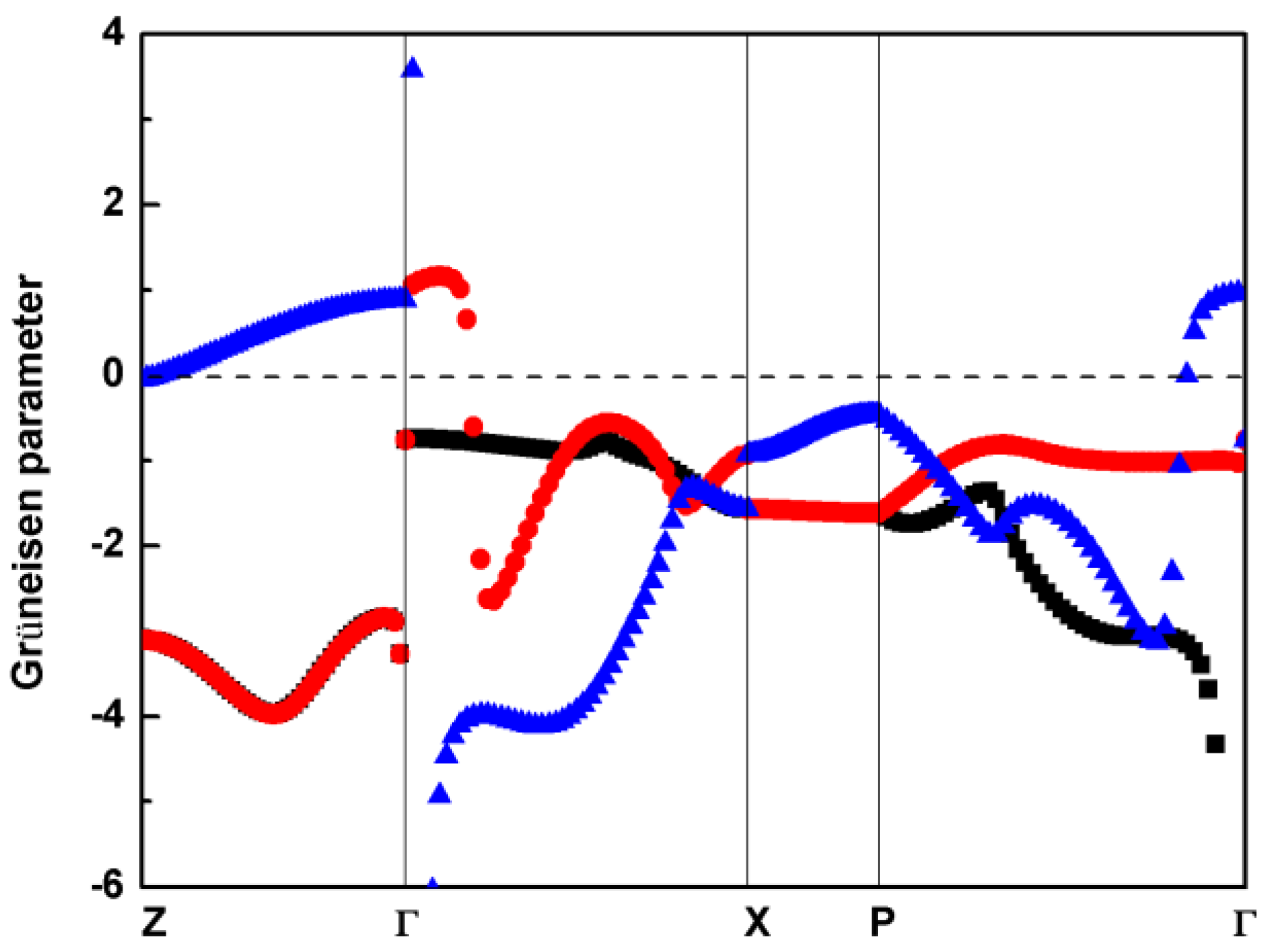
3.2. Vibrational Properties
3.3. Thermodynamic Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, W.; Li, Y.; Xu, Y.; Lu, J.; Wang, P.; Du, J.; Leng, Y. Measurements of nonlinear refraction in the mid-infrared materials ZnGeP2 and AgGaS2. Appl. Phys. B 2017, 123. [Google Scholar] [CrossRef]
- Xue, D.; Betzler, K.; Hesse, H. Dielectric properties of I-III-VI2-type chalcopyrite semiconductors. Phys. Rev. B 2000, 62, 13546–13551. [Google Scholar] [CrossRef]
- Hörig, W.; Neumann, H.; Sobotta, H.; Schumann, B.; Kühn, G. The optical properties of CuInSe2 thin films. Thin Solid Films 1978, 48, 67–72. [Google Scholar] [CrossRef]
- Feng, W.; Xiao, D.; Ding, J.; Yao, Y. Three-Dimensional Topological Insulators in I-III-VI2 and II-IV-V2 Chalcopyrite Semiconductors. Phys. Rev. Lett. 2011, 106, 16402. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Wang, G.; Zhou, X.; Wen, W. Substitution defect enhancing thermoelectric properties in CuInTe2. Mater. Res. Bull. 2018, 101, 184–189. [Google Scholar] [CrossRef]
- Wang, K.; Qin, P.; Ge, Z.; Feng, J. Highly enhanced thermoelectric properties of p-type CuInSe2 alloys by the Vacancy Doping. Scr. Mater. 2018, 149, 88–92. [Google Scholar] [CrossRef]
- Yang, J.H.; Fan, Q.; Cheng, X.L. Prediction for electronic, vibrational and thermoelectric properties of chalcopyrite AgX(X = In, Ga)Te2: PBE + U approach. R. Soc. Open Sci. 2017, 4, 170750. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, J.A.; Banger, K.K.; Jin, M.H.C.; Harris, J.D.; Cowen, J.E.; Bohannan, E.W.; Switzer, J.A.; Buhro, W.E.; Hepp, A.F. Single source precursors for fabrication of I–III–VI2 thin-film solar cells via spray CVD. Thin Solid Films 2003, 431–432, 63–67. [Google Scholar] [CrossRef]
- Termsaithong, P.; Tubtimtae, A. Boron-doped CuInTe2 semiconductor-sensitized liquid-junction solar cells. Mater. Lett. 2015, 138, 41–44. [Google Scholar] [CrossRef]
- Ghosh, A.; Thangavel, R.; Rajagopalan, M. Electronic and optical modeling of solar cell compound CuXY2 (X = In, Ga, Al; Y = S, Se, Te): First-principles study via Tran–Blaha-modified Becke–Johnson exchange potential approach. J. Mater. Sci. 2015, 50, 1710–1717. [Google Scholar] [CrossRef]
- Paderick, S.; Kessler, M.; Hurlburt, T.J.; Hughes, S.M. Synthesis and characterization of AgGaS2 nanoparticles: A study of growth and fluorescence. Chem. Commun. 2018, 54, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Migal, E.A.; Potemkin, F.V.; Gordienko, V.M. Highly efficient optical parametric amplifier tunable from near- to mid-IR for driving extreme nonlinear optics in solids. Opt. Lett. 2017, 42, 5218–5221. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Regulacio, M.D.; Ye, C.; Lim, S.H.; Zheng, Y.; Xu, Q.; Xu, A.; Han, M. Colloidal synthesis and photocatalytic properties of orthorhombic AgGaS2 nanocrystals. Chem. Commun. 2014, 50, 7128–7131. [Google Scholar] [CrossRef] [PubMed]
- Zawilski, K.T.; Schunemann, P.G.; Pollak, T.C.; Zelmon, D.E.; Fernelius, N.C.; Kenneth Hopkins, F. Growth and characterization of large CdSiP2 single crystals. J. Cryst. Growth 2010, 312, 1127–1132. [Google Scholar] [CrossRef]
- Holah, G.D.; Webb, J.S.; Montgomery, H. Lattice dynamics of AgGaS2. J. Phys. C Solid State Phys. 1974, 7, 3875. [Google Scholar] [CrossRef]
- Neumann, H.; Nowak, E. High temperature heat capacity and Grüneisen functions in AgGaS2. J. Less Common Met. 1989, 146, L7–L10. [Google Scholar] [CrossRef]
- Łażewski, J.; Parlinski, K. Lattice dynamics and elasticity of silver thiogallate (AgGaS2) from ab initio calculations. J. Chem. Phys. 2001, 114, 6734–6738. [Google Scholar] [CrossRef]
- Wei, L.; Wang, X.P.; Liu, B.; Zhang, Y.Y.; Lv, X.S.; Yang, Y.G.; Zhang, H.J.; Zhao, X. The role of acoustic phonon anharmonicity in determining thermal conductivity of CdSiP2 and AgGaS2: First principles calculations. AIP Adv. 2015, 5, 127236. [Google Scholar] [CrossRef]
- Kushwaha, A.K.; Khenata, R.; Bouhemadou, A.; Bin-Omran, S.; Haddadi, K. Lattice Dynamical Properties and Elastic Constants of the Ternary Chalcopyrite Compounds CuAlS2, CuGaS2, CuInS2, and AgGaS2. J. Electron. Mater. 2017, 46, 4109–4118. [Google Scholar] [CrossRef]
- Sist, M.; Zhang, J.; Brummerstedt Iversen, B. Crystal structure and phase transition of thermoelectric SnSe. Acta Cryst. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tao, Y.; Peng, F. Pressure and temperature induced phase transition in WB4: A first principles study. J. Alloys Compd. 2016, 687, 579–585. [Google Scholar] [CrossRef]
- Javaid, S.; Javed Akhtar, M.; Ahmad, I.; Younas, M.; Shah, S.H.; Ahmad, I. Pressure driven spin crossover and isostructural phase transition in LaFeO3. J. Appl. Phys. 2013, 114, 243712. [Google Scholar] [CrossRef]
- Reddy, P.V.S.; Kanchana, V. Enhanced superconductivity in SnSb under pressure: A first principles study. J. Phys. Condens. Matter 2017, 29, 405502. [Google Scholar] [CrossRef] [PubMed]
- Chong, X.; Jiang, Y.; Zhou, R.; Feng, J. Pressure dependence of electronic structure and superconductivity of the MnX (X = N, P, As, Sb). Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Borinaga, M.; Iba Nez-Azpiroz, J.; Bergara, A.; Errea, I. Strong Electron-Phonon and Band Structure Effects in the Optical Properties of High Pressure Metallic Hydrogen. Phys. Rev. Lett. 2018, 120, 57402. [Google Scholar] [CrossRef] [PubMed]
- Javed, Y.; Rafiq, M.A.; Ahmed, N. Pressure-induced changes in the electronic structure and enhancement of the thermoelectric performance of SnS2: A first principles study. RSC Adv. 2017, 7, 38834–38843. [Google Scholar] [CrossRef]
- Hu, J.; Shi, L.; Qin, Y.; Jin, F.; Duan, Y.; Qiu, L.; Chen, L. Phase transitions, band structures, elastic and lattice dynamic properties of CdSnV2 (V = P, As, Sb) under pressure from first principles. Mater. Sci. Semicond. Proc. 2015, 35, 149–161. [Google Scholar] [CrossRef]
- Li, Y.; Lin, J.; Fang, Z.; Qiu, M.; Huang, X.; Ding, K.; Chen, W.; Zhang, Y. Pressure-tuning the nonlinear-optical properties of AgGaS2 crystal: A first-principle study. Opt. Mater. Express 2015, 5, 1738–1751. [Google Scholar] [CrossRef]
- Carlone, C.; Olego, D.; Jayaraman, A.; Cardona, M. Pressure dependence of the Raman modes and pressure-induced phase changes in CuGaS2 and AgGaS2. Phys. Rev. B 1980, 22, 3877–3885. [Google Scholar] [CrossRef]
- Kitahara Eba, H.; Ishizawa, N.; Marumo, F.; Noda, Y. Synchrotron X-ray study of the monoclinic high-pressure structure of AgGaS2. Phys. Rev. B 2000, 61, 3310–3316. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B Condens. Matter 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Togo, A.; Tanaka, I. First principles phonon calculations in materials science. Scr. Mater. 2015, 108, 1–5. [Google Scholar] [CrossRef]
- Chahed, A.; Benhelal, O.; Rozale, H.; Laksari, S.; Abbouni, N. Structural and electronic properties of chalcopyrite semiconductors AgXY2 (X = In, Ga; Y = S, Se, Te) under pressure. Phys. Status Solidi 2007, 244, 629–634. [Google Scholar] [CrossRef]
- Abrahams, S.C.; Bernstein, J.L. Crystal structure of piezoelectric nonlinear-optic AgGaS2. J. Chem. Phys. 1973, 59, 1625–1629. [Google Scholar] [CrossRef]
- Xiao, H.; Tahir-Kheli, J.; Goddard, W.A. Accurate Band Gaps for Semiconductors from Density Functional Theory. J. Phys. Chem. Lett. 2011, 2, 212–217. [Google Scholar] [CrossRef]
- Ullah, S.; Din, H.U.; Murtaza, G.; Ouahrani, T.; Khenata, R.; Naeemullah; Bin Omran, S. Structural, electronic and optical properties of AgXY2(X = Al, Ga, In and Y = S, Se, Te). J. Alloys Compd. 2014, 617, 575–583. [Google Scholar] [CrossRef]
- Chen, S.; Gong, X.G.; Wei, S. Band-structure anomalies of the chalcopyrite semiconductors CuGaX2 versus AgGa2(X = S and Se) and their alloys. Phys. Rev. B 2007, 75, 205209. [Google Scholar] [CrossRef]
- Orlova, N.S.; Andreeva, O.E.; Bodnar, I.V. Pressure Effects on the Structural and Elastic Properties of AgGaS2-AgGaSe2 Crystals: An X-ray Diffraction Study. Inorg. Mater. 2001, 37, 548–555. [Google Scholar] [CrossRef]
- Mori, Y.; Takarabe, K.; Iwamoto, S.; Minomura, S.; Niwa, E.; Masumoto, K. High Pressure Structural Study of I–III–VI2 Chalcopyrites. Phys. Status Solidi 1996, 198, 427–431. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, D. High thermoelectric performance from optimization of hole-doped CuInTe2. Phys. Chem. Chem. Phys. 2016, 18, 5925–5931. [Google Scholar] [CrossRef] [PubMed]
- Koschel, W.H.; Bettini, M. Zone-centered phonons in AIBIIIS2 chalcopyrites. Phys. Status Solidi 1975, 72, 729–737. [Google Scholar] [CrossRef]
- Ohrendorf, F.W.; Haeuseler, H. Lattice Dynamics of Chalcopyrite Type Compounds. Part I. Vibrational Frequencies. Cryst. Res. Technol. 1999, 34, 339–349. [Google Scholar] [CrossRef]
- Milman, V.; Perlov, A.; Refson, K.; Clark, S.J.; Gavartin, J.; Winkler, B. Structural, electronic and vibrational properties of tetragonal zirconia under pressure: A density functional theory study. J. Phys. Condens. Matter 2009, 21, 485404. [Google Scholar] [CrossRef] [PubMed]
- Sheremetyeva, N.; Cherniak, D.J.; Watson, E.B.; Meunier, V. Effect of pressure on the Raman-active modes of zircon (ZrSiO4): A first-principles study. Phys. Chem. Miner. 2017. [Google Scholar] [CrossRef]
- Yang, M.; Cheng, X.; Li, Y.; Ren, Y.; Liu, M.; Qi, Z. Anharmonicity of monolayer MoS2, MoSe2, and WSe2: A Raman study under high pressure and elevated temperature. Appl. Phys. Lett. 2017, 110, 93108. [Google Scholar] [CrossRef]
- Shirakata, S. Raman scattering and its hydrostatic pressure dependence in ZnGeP2 crystal. J. Appl. Phys. 1999, 85, 3294–3300. [Google Scholar] [CrossRef]
- Zhang, Y.; Hao, S.; Zhao, L.; Wolverton, C.; Zeng, Z. Pressure induced thermoelectric enhancement in SnSe crystals. J. Mater. Chem. A 2016, 4, 12073–12079. [Google Scholar] [CrossRef]
- Zhang, Y. First-principles Debye–Callaway approach to lattice thermal conductivity. J. Materiomics 2016, 2, 237–247. [Google Scholar] [CrossRef]
- Morelli, D.T.; Heremans, J.P.; Slack, G.A. Estimation of the isotope effect on the lattice thermal conductivity of group IV and group III–V semiconductors. Phys. Rev. B 2002, 66, 195304. [Google Scholar] [CrossRef]
- Neumann, H.; Łażewski, J.; Jochym, P.T.; Parlinski, K. Ab initio heat capacity and atomic temperature factors of chalcopyrites. Phys. Rev. B 2007, 75, 224301. [Google Scholar] [CrossRef]
- Abrahams, S.C.; Hsu, F.S.L. Debye temperatures and cohesive properties. J. Chem. Phys. 1975, 63, 1162–1165. [Google Scholar] [CrossRef]
- Sharma, S.; Verma, A.S.; Jindal, V.K. Ab initio studies of structural, electronic, optical, elastic and thermal properties of silver gallium dichalcogenides (AgGaX2: X = S, Se, Te). Mater. Res. Bull. 2014, 53, 218–233. [Google Scholar] [CrossRef]
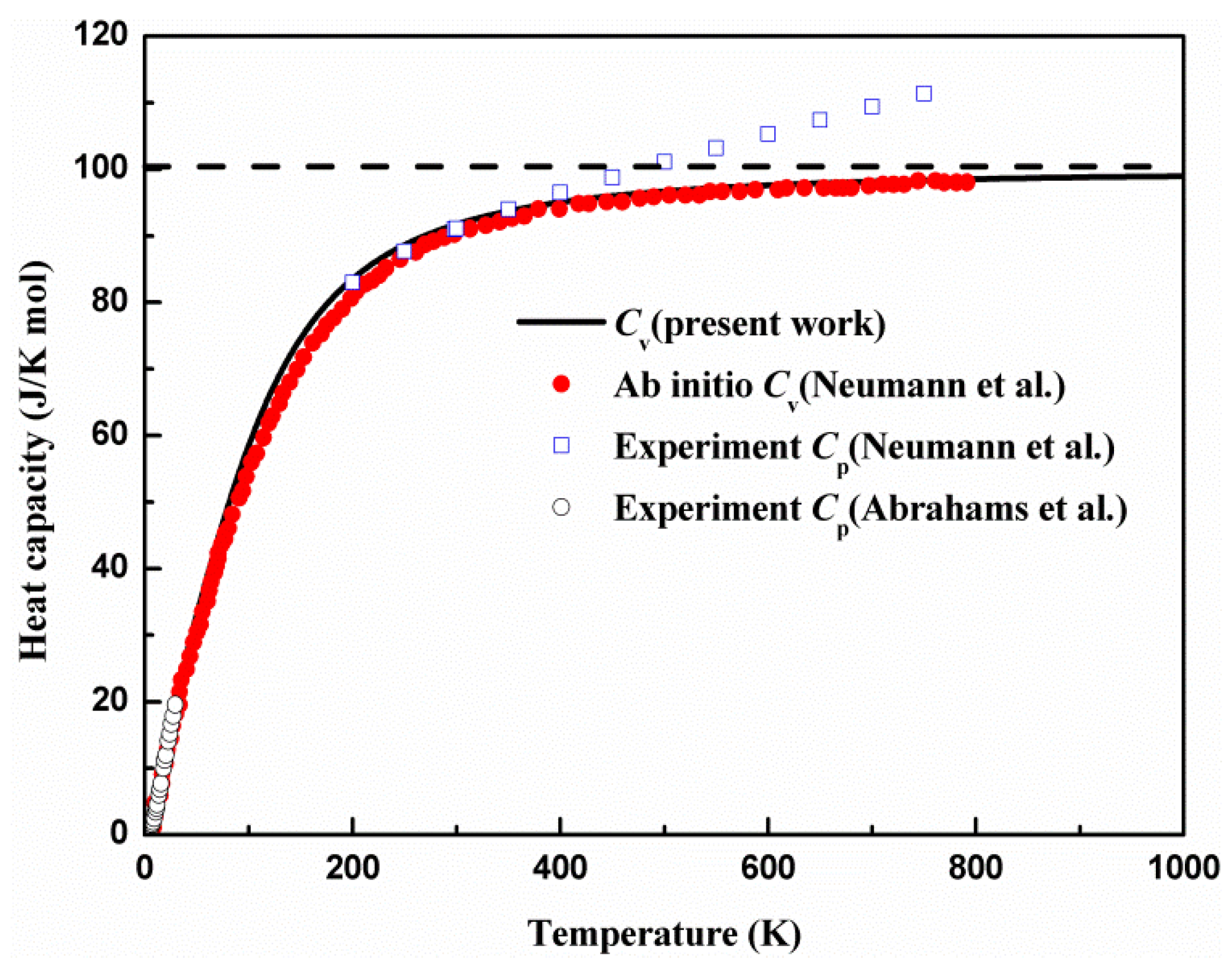
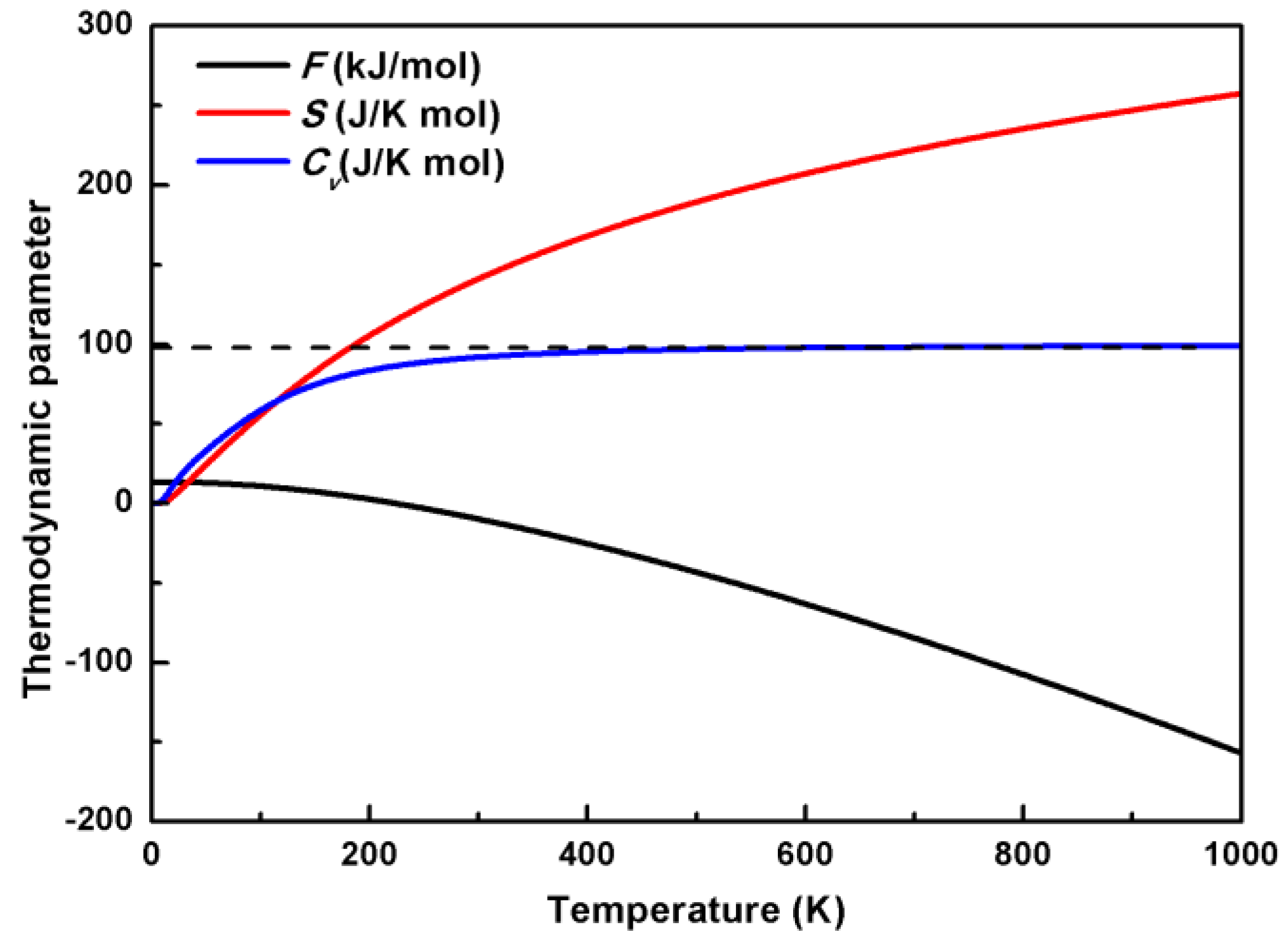
| Irreps | 0 GPa | 1 GPa | 2 GPa | 3 GPa | 4 GPa | ||
|---|---|---|---|---|---|---|---|
| Reference [19] | Reference [41] | ||||||
| A1 | 8.16 | 8.88 | 8.85 | 8.36 | 8.51 | 8.83 | 9.00 |
| A2 | 9.87 | 10.17 | 10.07 | 10.26 | 10.44 | 10.60 | |
| B1 | 7.06 | 6.84 | 7.23 | 7.39 | 7.55 | 7.69 | |
| 9.29 | 10.11 | 10.20 | 9.51 | 9.71 | 9.91 | 10.09 | |
| 5.42 | 5.76 | 5.70 | 5.47 | 5.52 | 5.57 | 5.61 | |
| 1.67 | 1.59 | 1.62 | 1.71 | 1.75 | 1.78 | 1.81 | |
| B2 (LO/TO) | 10.03/10.00 | 12.06/11.07 | 11.94/10.92 | 10.27/10.23 | 10.50/10.45 | 10.74/10.66 | 10.94/10.85 |
| 6.37/5.96 | 7.17/6.42 | 7.14/6.36 | 6.57/6.11 | 6.75/6.24 | 6.94/6.38 | 7.11/6.50 | |
| 1.80/1.80 | 1.95/1.92 | 1.92 | 1.81/1.81 | 1.84/1.84 | 1.86/1.86 | 1.88/1.88 | |
| E (LO/TO) | 10.96/10.61 | 12.06/11.22 | 11.88/11.04 | 11.17/10.83 | 11.37/11.04 | 11.57/11.25 | 11.74/11.43 |
| 9.29/8.91 | 10.56/9.78 | 10.47/9.75 | 9.51/9.12 | 9.71/9.30 | 9.91/9.50 | 10.08/9.66 | |
| 6.64/6.53 | 7.02/6.75 | 6.96/6.78 | 6.76/6.57 | 6.91/6.89 | 7.10/7.00 | 7.27/7.12 | |
| 4.56/4.50 | 4.89/4.47 | 4.80/4.71 | 4.62/4.57 | 4.69/4.65 | 4.76/4.72 | 4.83/4.79 | |
| 2.80/2.77 | 2.77/2.77 | 2.85 | 2.84/2.80 | 2.87/2.86 | 2.90/2.89 | 2.91/2.91 | |
| 1.07/1.07 | 1.07/0.99 | 1.02 | 0.98/0.98 | 0.89/0.89 | 0.79/0.79 | 0.67/0.67 | |
| High-Symmetry Path | 0 GPa | 1 GPa | 2 GPa | 3 GPa | 4 GPa |
|---|---|---|---|---|---|
| Z-Γ | 1.65 | 1.76 | 1.85 | 2.21 | 6.33 |
| Γ-X | 1.39 | 1.61 | 1.73 | 2.98 | 7.86 |
| X-P | 1.19 | 1.18 | 1.15 | 1.18 | 1.79 |
| P-Γ | 1.73 | 1.78 | 1.80 | 1.83 | 5.67 |
| T | P | 0 GPa | 1 GPa | 2 GPa | 3 GPa | 4 GPa |
|---|---|---|---|---|---|---|
| 200 | F | 2.60 | 2.92 | 3.24 | 3.55 | 3.78 |
| S | 105.39 | 104.57 | 103.68 | 102.92 | 102.44 | |
| Cv | 83.51 | 82.94 | 82.39 | 81.84 | 81.35 | |
| 400 | F | −25.33 | −24.81 | −24.28 | −23.79 | −23.44 |
| S | 167.98 | 166.91 | 165.78 | 164.77 | 164.07 | |
| Cv | 95.05 | 94.86 | 97.43 | 94.47 | 94.30 | |
| 600 | F | −63.08 | −62.35 | −61.58 | −60.88 | −60.38 |
| S | 207.10 | 205.98 | 204.79 | 203.72 | 202.98 | |
| Cv | 97.61 | 97.52 | 97.43 | 97.34 | 97.25 | |
| 800 | F | −107.46 | −106.49 | −105.49 | 104.57 | −103.92 |
| S | 235.33 | 234.18 | 232.98 | 231.89 | 231.13 | |
| Cv | 98.54 | 98.49 | 98.44 | 98.38 | 98.34 | |
| 1000 | F | −156.81 | −155.61 | −154.36 | −153.23 | −152.43 |
| S | 257.37 | 256.22 | 255.00 | 253.90 | 253.13 | |
| Cv | 98.98 | 98.95 | 98.91 | 98.88 | 98.85 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Fan, Q.; Yu, Y.; Zhang, W. Pressure Effect of the Vibrational and Thermodynamic Properties of Chalcopyrite-Type Compound AgGaS2: A First-Principles Investigation. Materials 2018, 11, 2370. https://doi.org/10.3390/ma11122370
Yang J, Fan Q, Yu Y, Zhang W. Pressure Effect of the Vibrational and Thermodynamic Properties of Chalcopyrite-Type Compound AgGaS2: A First-Principles Investigation. Materials. 2018; 11(12):2370. https://doi.org/10.3390/ma11122370
Chicago/Turabian StyleYang, Jianhui, Qiang Fan, You Yu, and Weibin Zhang. 2018. "Pressure Effect of the Vibrational and Thermodynamic Properties of Chalcopyrite-Type Compound AgGaS2: A First-Principles Investigation" Materials 11, no. 12: 2370. https://doi.org/10.3390/ma11122370
APA StyleYang, J., Fan, Q., Yu, Y., & Zhang, W. (2018). Pressure Effect of the Vibrational and Thermodynamic Properties of Chalcopyrite-Type Compound AgGaS2: A First-Principles Investigation. Materials, 11(12), 2370. https://doi.org/10.3390/ma11122370




