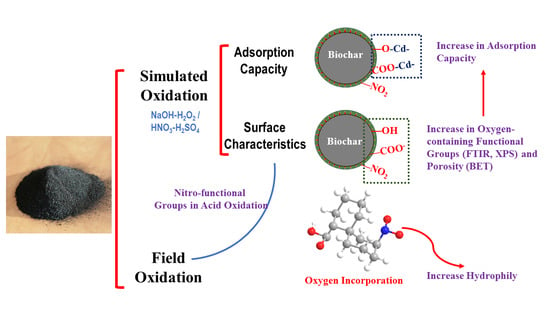
Effects of Wet Oxidation Process on Biochar Surface in Acid and Alkaline Soil Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Analytical Methods
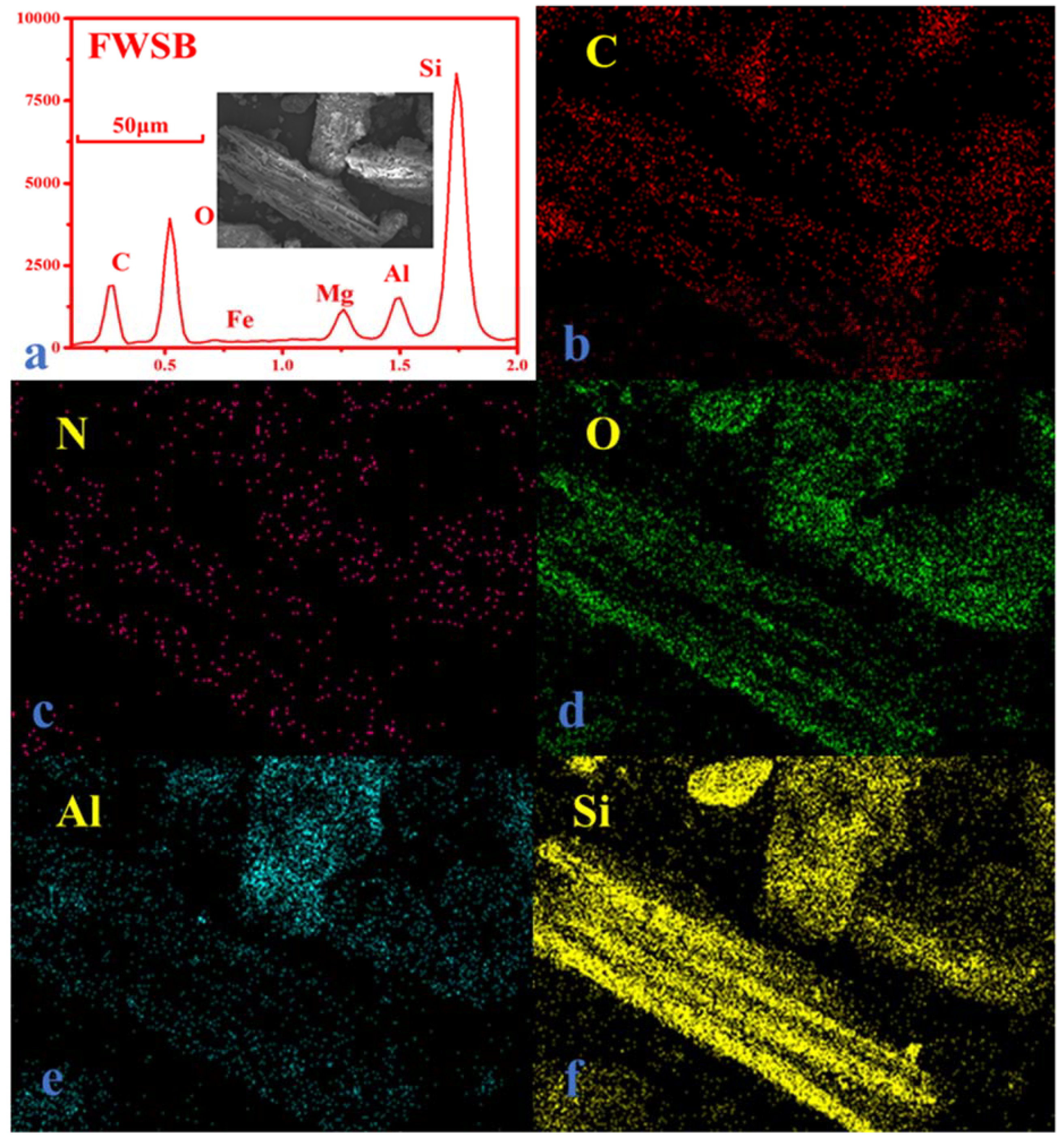
3. Results and Discussion
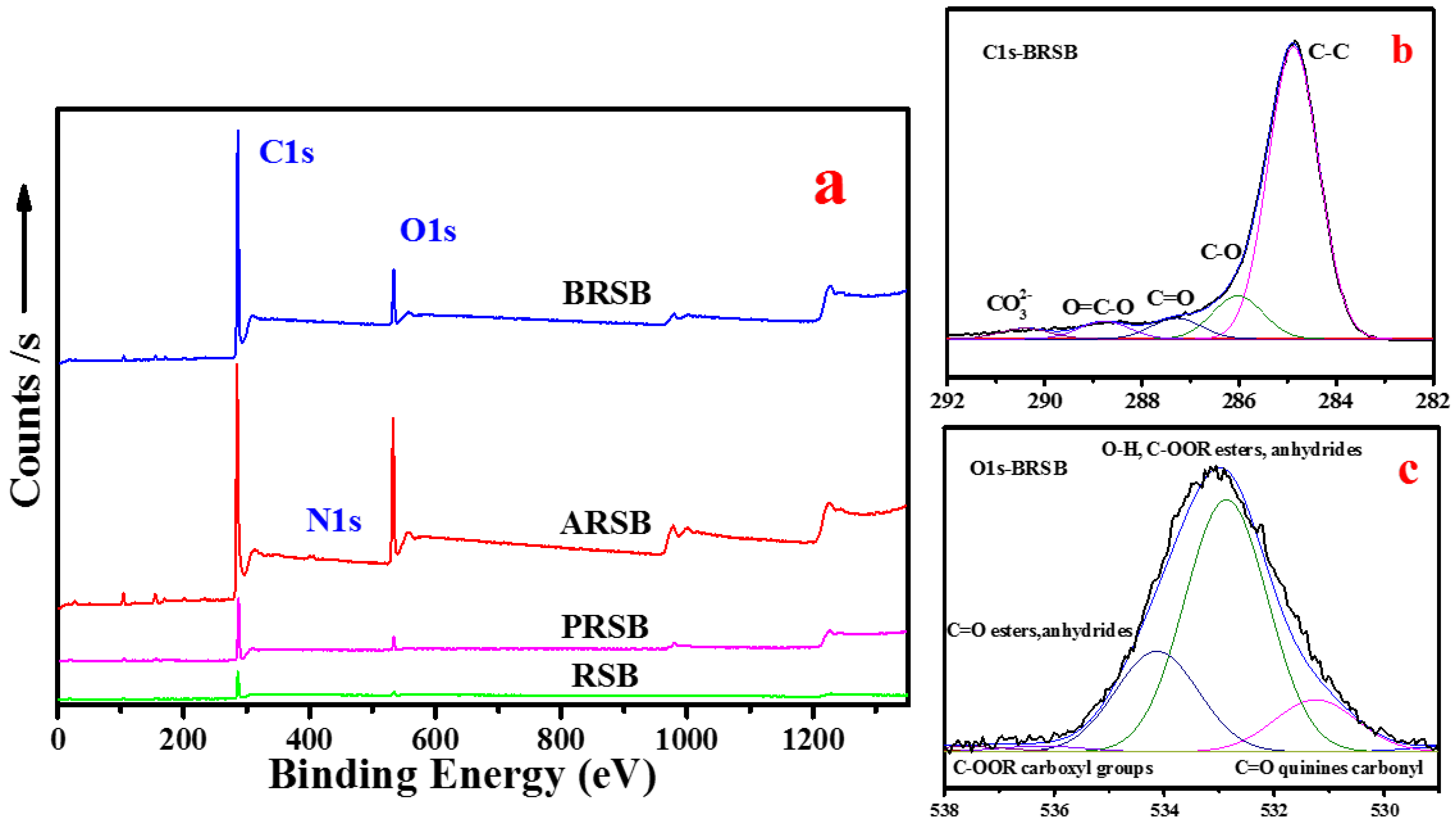
3.1. Comparison of Bulk and Surface Element Composition
3.2. Oxygen-Containing Functional Groups on the Surface
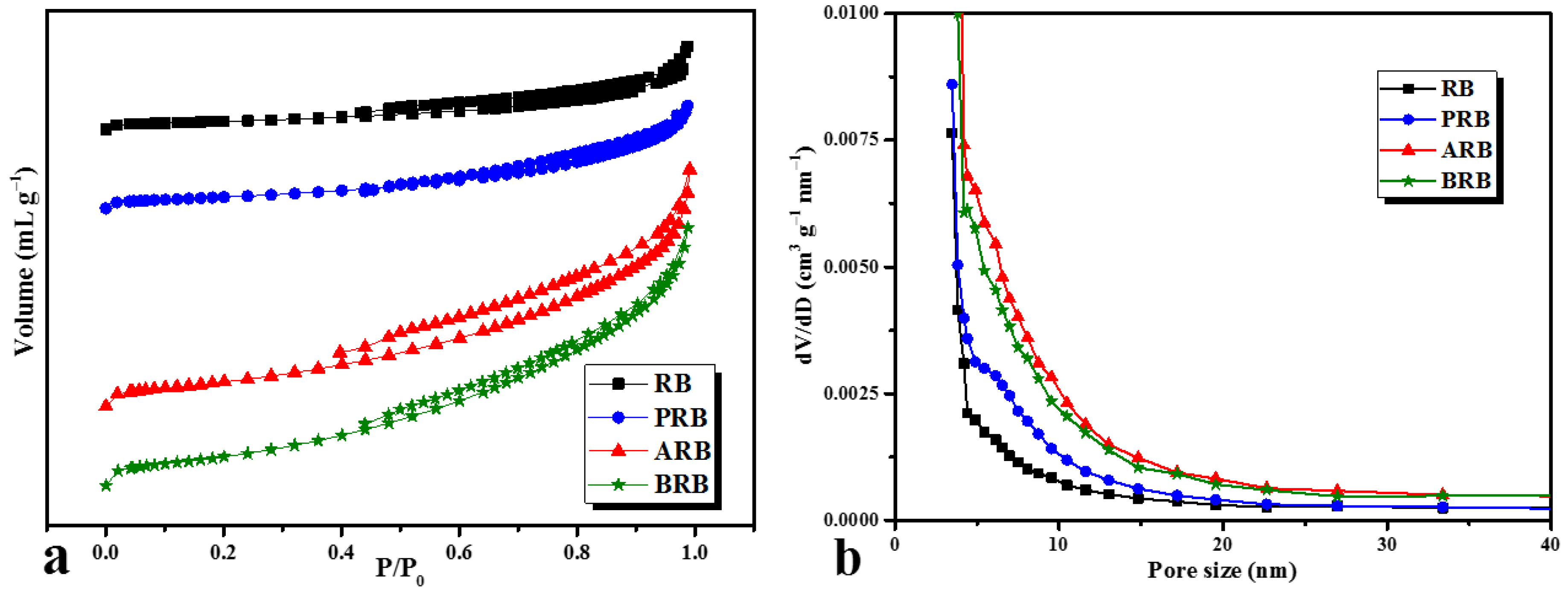
3.3. Surface Structure of Biochars
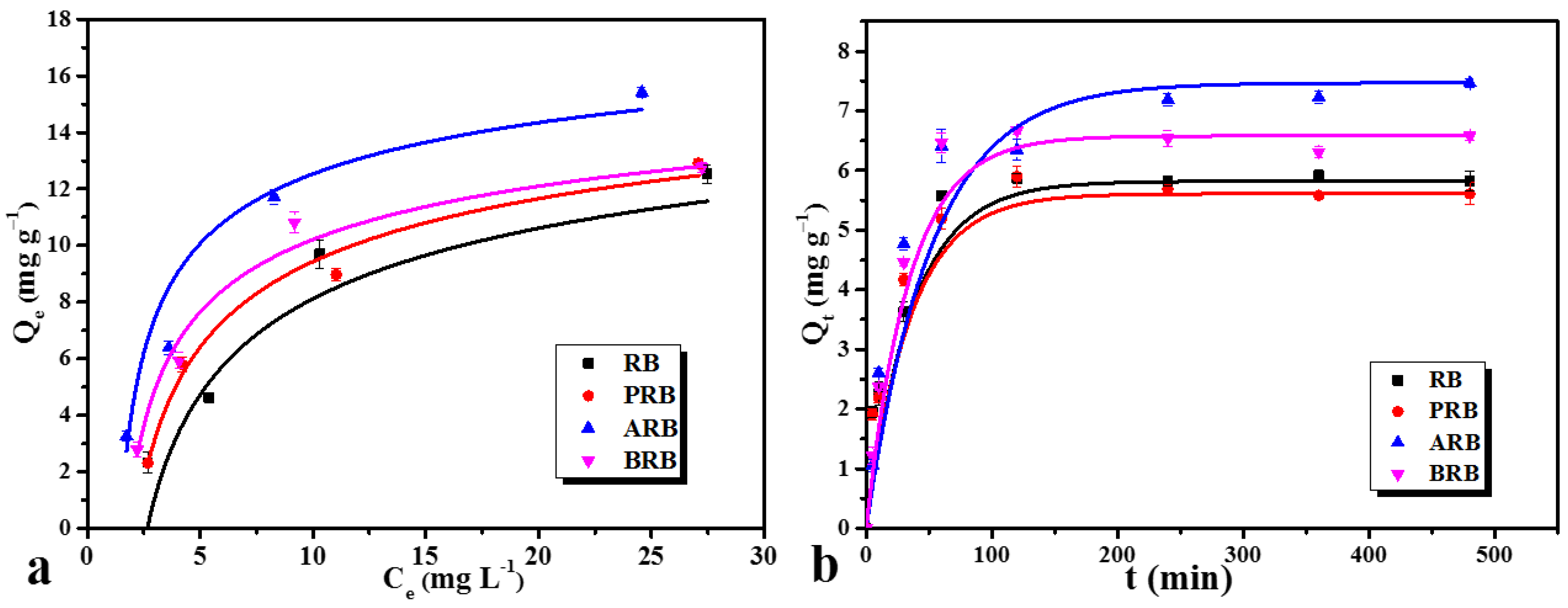
3.4. Effects of the Aging Process on Biochar Adsorption
3.5. Aging Process of Biochar in the Field
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hagemann, N.; Joseph, S.; Schmidt, H.P.; Kammann, C.I.; Harter, J.; Borch, T.; Young, R.B.; Varga, K.; Taherymoosavi, S.; Elliott, K.W.; et al. Organic coating on biochar explains its nutrient retention and stimulation of soil fertility. Nat. Commun. 2017, 8, 1089. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, H.; Cai, L.; Guo, J.; Wang, Y.; Ji, L.; Song, W. Preparation and characterization of macroalgae biochar nanomaterials with highly efficient adsorption and photodegradation ability. Materials 2018, 11, 1709. [Google Scholar] [CrossRef] [PubMed]
- Kasak, K.; Truu, J.; Ostonen, I.; Sarjas, J.; Oopkaup, K.; Paiste, P.; Koiv-Vainik, M.; Mander, U.; Truu, M. Biochar enhances plant growth and nutrient removal in horizontal subsurface flow constructed wetlands. Sci. Total Environ. 2018, 639, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Munera-Echeverri, J.L.; Martinsen, V.; Strand, L.T.; Zivanovic, V.; Cornelissen, G.; Mulder, J. Cation exchange capacity of biochar: An urgent method modification. Sci. Total Environ. 2018, 642, 190–197. [Google Scholar] [CrossRef] [PubMed]
- El-Banna, M.F.; Mosa, A.; Gao, B.; Yin, X.; Ahmad, Z.; Wang, H. Sorption of lead ions onto oxidized bagasse-biochar mitigates Pb-induced oxidative stress on hydroponically grown chicory: Experimental observations and mechanisms. Chemosphere 2018, 208, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Rozari, P.D.; Greenway, M.; Hanandeh, A.E. Nitrogen removal from sewage and septage in constructed wetland mesocosms using sand media amended with biochar. Ecol. Eng. 2018, 111, 1–10. [Google Scholar] [CrossRef]
- Gascó, G.; Paz-Ferreiro, J.; Cely, P.; Plaza, C.; Méndez, A. Influence of pig manure and its biochar on soil CO 2 emissions and soil enzymes. Ecol. Eng. 2016, 95, 19–24. [Google Scholar] [CrossRef]
- Karhu, K.; Mattila, T.; Bergström, I.; Regina, K. Biochar addition to agricultural soil increased CH4 uptake and water holding capacity—Results from a short-term pilot field study. Agr. Ecosyst. Environ. 2011, 140, 309–313. [Google Scholar] [CrossRef]
- Cui, L.; Yan, J.; Li, L.; Quan, G.; Ding, C.; Chen, T.; Yin, C.; Gao, J.; Hussain, Q. Does biochar alter the speciation of Cd and Pb in aqueous solution? Bioresources 2014, 10, 88–104. [Google Scholar] [CrossRef]
- Diao, Z.H.; Du, J.J.; Jiang, D.; Kong, L.J.; Huo, W.Y.; Liu, C.M.; Wu, Q.H.; Xu, X.R. Insights into the simultaneous removal of Cr(6+) and Pb(2+) by a novel sewage sludge-derived biochar immobilized nanoscale zero valent iron: Coexistence effect and mechanism. Sci. Total Environ. 2018, 642, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Kaudal, B.B.; Weatherley, A.J. Agronomic effectiveness of urban biochar aged through co-composting with food waste. Waste Manage. 2018, 77, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Paetsch, L.; Mueller, C.W.; Kogel-Knabner, I.; von Lutzow, M.; Girardin, C.; Rumpel, C. Effect of in-situ aged and fresh biochar on soil hydraulic conditions and microbial C use under drought conditions. Sci. Rep. 2018, 8, 6852. [Google Scholar] [CrossRef] [PubMed]
- Mia, S.; Dijkstra, F.A.; Singh, B. Enhanced biological nitrogen fixation and competitive advantage of legumes in mixed pastures diminish with biochar aging. Plant Soil 2018, 424, 639–651. [Google Scholar] [CrossRef]
- Cho, H.H.; Wepasnick, K.; Smith, B.A.; Bangash, F.K.; Fairbrother, D.H.; Ball, W.P. Sorption of aqueous Zn[II] and Cd[II] by multiwall carbon nanotubes: the relative roles of oxygen-containing functional groups and graphenic carbon. Langmuir 2010, 26, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Uchimiya, M.; Bannon, D.I.; Wartelle, L.H. Retention of heavy metals by carboxyl functional groups of biochars in small arms range soil. J. Agr. Food Chem. 2012, 60, 1798. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Sun, J.; Chu, L.; Cui, L.; Quan, G.; Yan, J.; Hussain, Q.; Iqbal, M. Effects of chemical oxidation on surface oxygen-containing functional groups and adsorption behavior of biochar. Chemosphere 2018, 207, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Golchin, A.; Oades, J.M.; Skjemstad, J.O.; Clarke, P. Study of free and occluded particulate organic matter in soils by solid state 13C Cp/MAS NMR spectroscopy and scanning electron microscopy. Soil Res. 1994, 32, 285–309. [Google Scholar] [CrossRef]
- Wang, J.; He, Y.; Peng, Z.; Ling, X.; Wang, S. Estimation of hydrophilicity of coals by using the quantum chemistry calculation. Int. J. Miner. Process. 2017, 167, 9–15. [Google Scholar] [CrossRef]
- Mukome, F.N.; Zhang, X.; Silva, L.C.; Six, J.; Parikh, S.J. Use of chemical and physical characteristics to investigate trends in biochar feedstocks. J. Agr. Food Chem. 2013, 61, 2196–2204. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Chen, B. Interactions of aluminum with biochars and oxidized biochars: implications for the biochar aging process. J. Agr. Food Chem. 2014, 62, 373–380. [Google Scholar] [CrossRef] [PubMed]


| Sample | Bulk Element Composition (%) | Surface Element Composition (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | C | H | S | Ash | O | O/C | N | C | O | O/C | |
| WSB | 1.12 | 65.75 | 1.41 | 0.44 | 28.39 | 2.89 | 0.04 | 0.87 | 91.92 | 7.21 | 0.08 |
| RSB | 1.31 | 68.15 | 2.09 | 0.51 | 26.15 | 1.79 | 0.03 | 0.02 | 87.25 | 0.05 | 0.00 |
| RB | 1.22 | 69.27 | 3.15 | 0.38 | 22.88 | 3.1 | 0.04 | 0.84 | 90.38 | 0.12 | 0.00 |
| PWSB | 1.42 | 56.38 | 1.98 | 0.33 | 25.93 | 7.96 | 0.13 | 1.45 | 89.83 | 8.38 | 0.09 |
| PRSB | 1.06 | 52.90 | 1.41 | 0.41 | 25.04 | 9.18 | 0.15 | 0.01 | 86.78 | 0.05 | 0.00 |
| PRB | 1.23 | 69.23 | 3.34 | 0.27 | 21.46 | 4.47 | 0.06 | 0.95 | 89.88 | 0.31 | 0.00 |
| AWSB | 2.84 | 54.06 | 1.84 | 0.74 | 20.72 | 19.8 | 0.37 | 3.38 | 78.91 | 16.64 | 0.21 |
| ARSB | 1.51 | 46.33 | 1.58 | 1.61 | 21.56 | 27.41 | 0.59 | 2.55 | 77.21 | 19.3 | 0.25 |
| ARB | 3.50 | 52.01 | 2.58 | 0.67 | 18.41 | 22.83 | 0.44 | 1.07 | 90.18 | 8.75 | 0.10 |
| BWSB | 1.47 | 60.57 | 1.60 | 0.93 | 24.27 | 11.16 | 0.18 | 1.92 | 85.65 | 10.91 | 0.13 |
| BRSB | 1.35 | 60.28 | 1.44 | 0.64 | 23.58 | 12.71 | 0.21 | 0.91 | 84.19 | 6.35 | 0.08 |
| BRB | 1.72 | 66.33 | 3.10 | 0.38 | 21.06 | 7.41 | 0.11 | 0.57 | 90.23 | 3.69 | 0.04 |
| Sample | C1s | O1s | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C–C | C–O | C=O | O=C–O | CO32− | C=O (Carbonyl) | O–H (Esters) | C=O (Esters) | C–OOR (Carboxyl) | |
| WSB | 0.72 | 0.12 | 0.07 | 0.05 | 0.04 | 0.12 | 0.62 | 0.24 | 0.02 |
| RSB | 0.75 | 0.1 | 0.07 | 0.05 | 0.03 | 0.09 | 0.65 | 0.22 | 0.04 |
| RB | 0.78 | 0.11 | 0.06 | 0.03 | 0.02 | 0.13 | 0.65 | 0.2 | 0.02 |
| PWSB | 0.7 | 0.12 | 0.08 | 0.06 | 0.04 | 0.07 | 0.68 | 0.2 | 0.05 |
| PRSB | 0.74 | 0.1 | 0.07 | 0.06 | 0.03 | 0.09 | 0.65 | 0.21 | 0.05 |
| PRB | 0.76 | 0.12 | 0.07 | 0.03 | 0.02 | 0.15 | 0.64 | 0.17 | 0.04 |
| AWSB | 0.65 | 0.16 | 0.09 | 0.09 | 0.01 | 0.16 | 0.59 | 0.19 | 0.06 |
| ARSB | 0.69 | 0.14 | 0.08 | 0.07 | 0.02 | 0.14 | 0.6 | 0.18 | 0.08 |
| ARB | 0.69 | 0.13 | 0.09 | 0.08 | 0.01 | 0.14 | 0.59 | 0.2 | 0.07 |
| BWSB | 0.69 | 0.13 | 0.07 | 0.08 | 0.03 | 0.14 | 0.61 | 0.21 | 0.04 |
| BRSB | 0.74 | 0.11 | 0.07 | 0.06 | 0.02 | 0.13 | 0.62 | 0.2 | 0.05 |
| BRB | 0.76 | 0.11 | 0.08 | 0.04 | 0.01 | 0.13 | 0.67 | 0.18 | 0.02 |
| Sample | Pseudo-First-Order | Pseudo-Second-Order | Langmuir Isotherm | Freundlich Isotherm | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| K1 | R2 | K2 | R2 | Qmax (mg g−1) | KL | R2 | n | KF | R2 | |
| WSB | 0.026 | 0.903 | 0.031 | 0.958 | 5.57 | 0.25 | 0.92 | 2.93 | 1.51 | 0.91 |
| PWSB | 0.026 | 0.827 | 0.038 | 0.940 | 5.65 | 0.25 | 0.91 | 3.01 | 1.38 | 0.85 |
| AWSB | 0.027 | 0.925 | 0.032 | 0.972 | 6.31 | 0.24 | 0.99 | 2.64 | 1.73 | 0.93 |
| BWSB | 0.026 | 0.909 | 0.036 | 0.955 | 6.02 | 0.26 | 0.94 | 2.13 | 2.06 | 0.83 |
| RSB | 0.028 | 0.894 | 0.032 | 0.942 | 5.52 | 0.24 | 0.94 | 3.18 | 1.43 | 0.87 |
| PRSB | 0.028 | 0.882 | 0.036 | 0.902 | 6.04 | 0.27 | 0.92 | 2.85 | 1.61 | 0.85 |
| ARSB | 0.027 | 0.852 | 0.033 | 0.920 | 6.74 | 0.25 | 0.97 | 3.24 | 1.27 | 0.92 |
| BRSB | 0.028 | 0.942 | 0.037 | 0.974 | 6.21 | 0.26 | 0.87 | 3.06 | 1.34 | 0.89 |
| RB | 0.028 | 0.974 | 0.035 | 0.984 | 6.17 | 0.23 | 0.94 | 3.14 | 1.44 | 0.91 |
| PRB | 0.027 | 0.852 | 0.037 | 0.951 | 6.15 | 0.24 | 0.98 | 3.30 | 1.28 | 0.95 |
| ARB | 0.027 | 0.913 | 0.031 | 0.924 | 7.52 | 0.27 | 0.96 | 2.04 | 3.13 | 0.93 |
| BRB | 0.026 | 0.920 | 0.033 | 0.963 | 6.60 | 0.24 | 0.98 | 3.06 | 1.70 | 0.94 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Q.; Cui, L.; Quan, G.; Wang, S.; Sun, J.; Han, X.; Wang, J.; Yan, J. Effects of Wet Oxidation Process on Biochar Surface in Acid and Alkaline Soil Environments. Materials 2018, 11, 2362. https://doi.org/10.3390/ma11122362
Fan Q, Cui L, Quan G, Wang S, Sun J, Han X, Wang J, Yan J. Effects of Wet Oxidation Process on Biochar Surface in Acid and Alkaline Soil Environments. Materials. 2018; 11(12):2362. https://doi.org/10.3390/ma11122362
Chicago/Turabian StyleFan, Qinya, Liqiang Cui, Guixiang Quan, Sanfei Wang, Jianxiong Sun, Xiangyun Han, Jia Wang, and Jinlong Yan. 2018. "Effects of Wet Oxidation Process on Biochar Surface in Acid and Alkaline Soil Environments" Materials 11, no. 12: 2362. https://doi.org/10.3390/ma11122362
APA StyleFan, Q., Cui, L., Quan, G., Wang, S., Sun, J., Han, X., Wang, J., & Yan, J. (2018). Effects of Wet Oxidation Process on Biochar Surface in Acid and Alkaline Soil Environments. Materials, 11(12), 2362. https://doi.org/10.3390/ma11122362