Synthesis and Characterization of a Dual-Cation Organomontmorillonite Nanocomposite
Abstract
1. Introduction
2. Experimental Part
2.1. Materials
2.2. Adsorption of Organic Modifiers into Mt and Preparation of OMt
2.3. Desorption of Organic Modifiers from OMt
2.4. Characterization
3. Results and Discussion
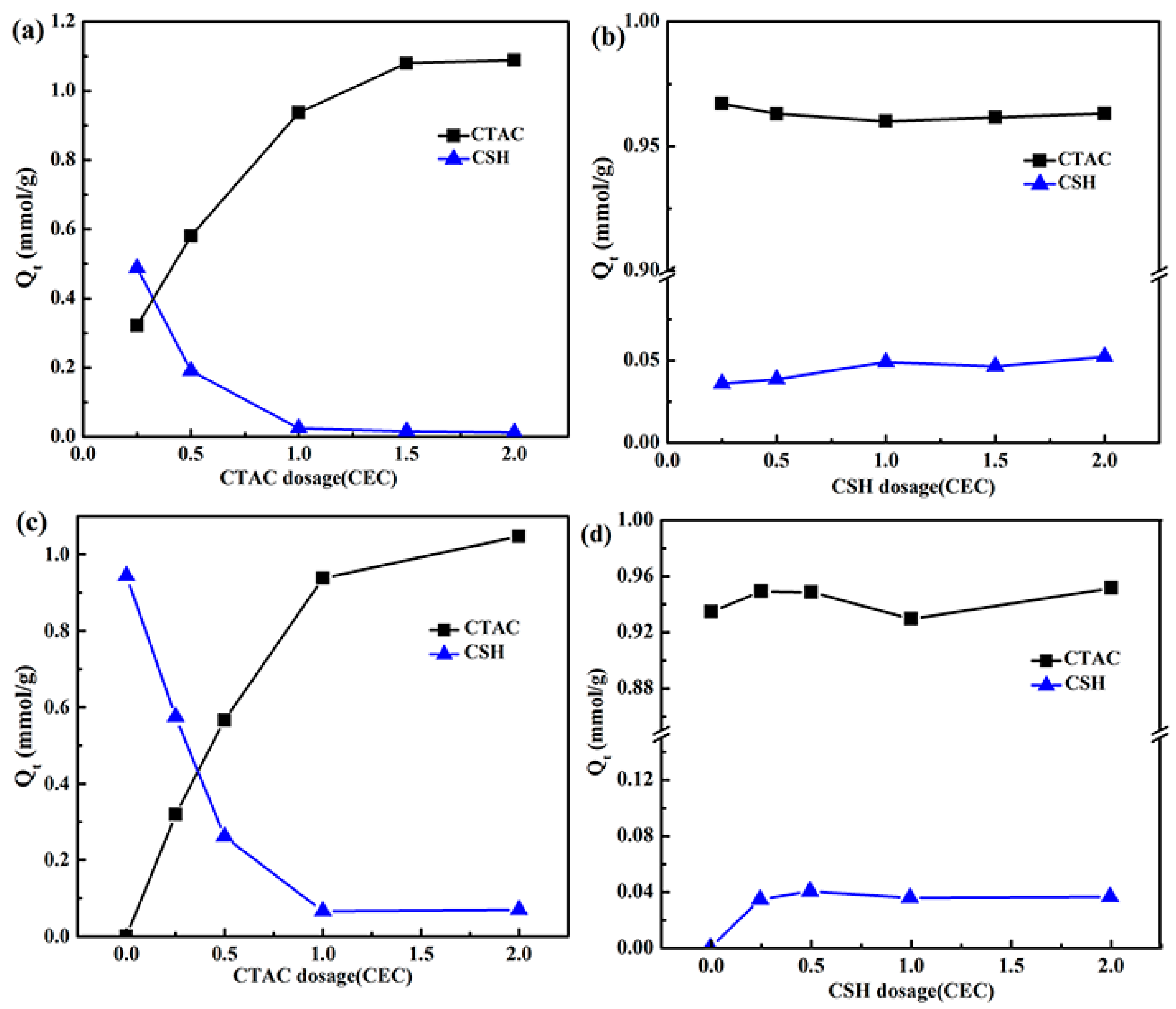
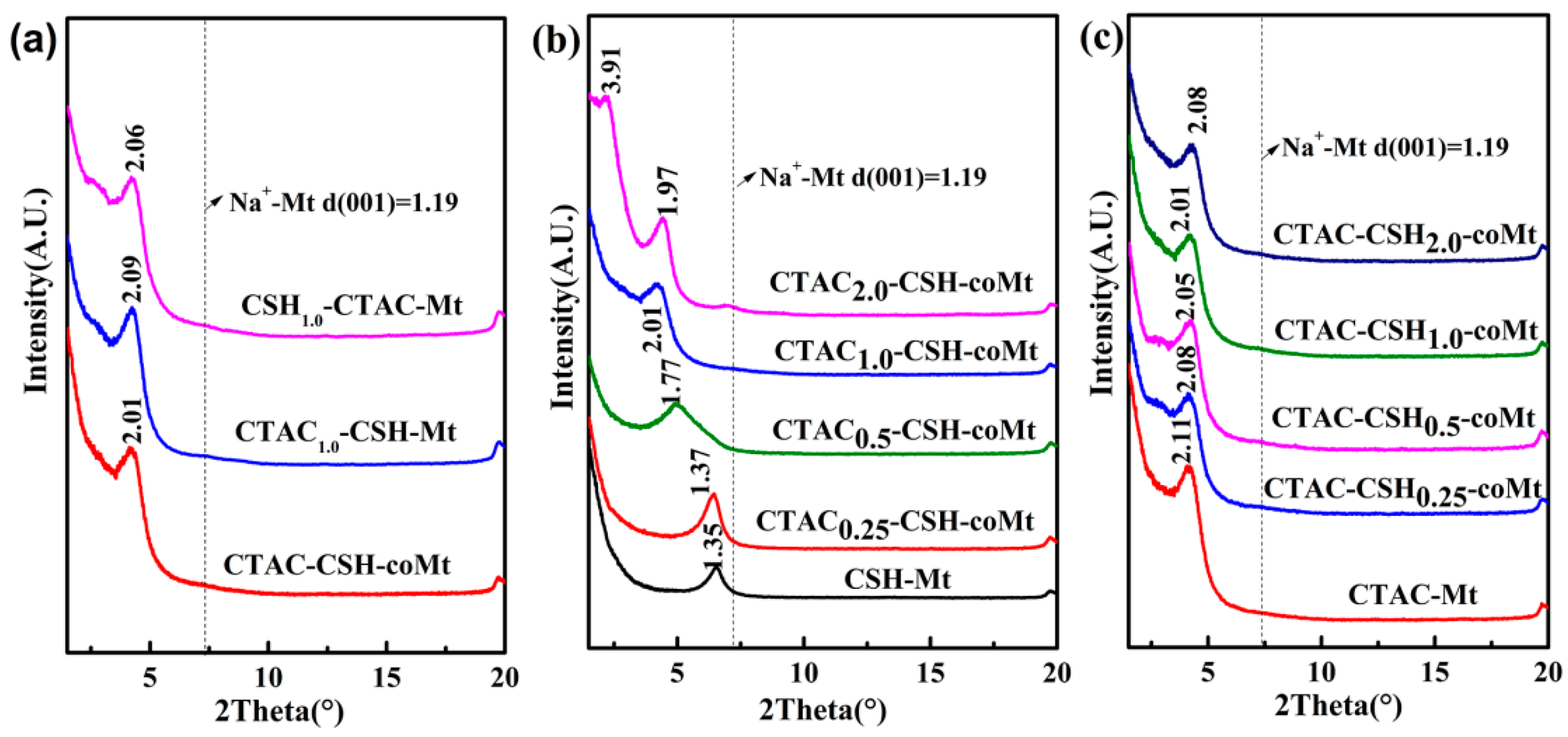
3.1. Effect of the Order of Addition and Amount of Added Modifiers on Their Adsorption by Mt
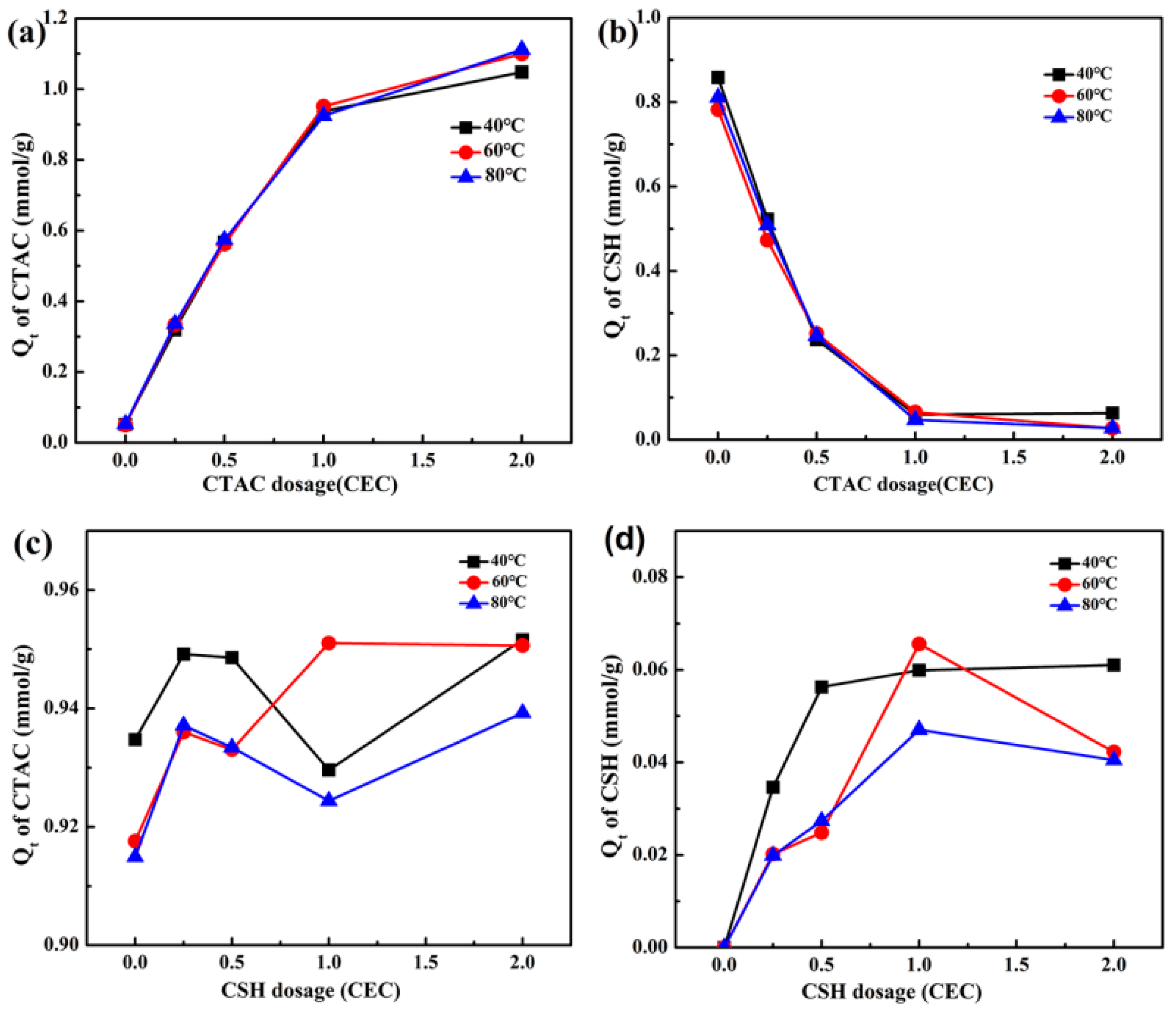
3.2. Effect of Modification Temperature and Time on Adsorption of Organic Modifiers by Mt
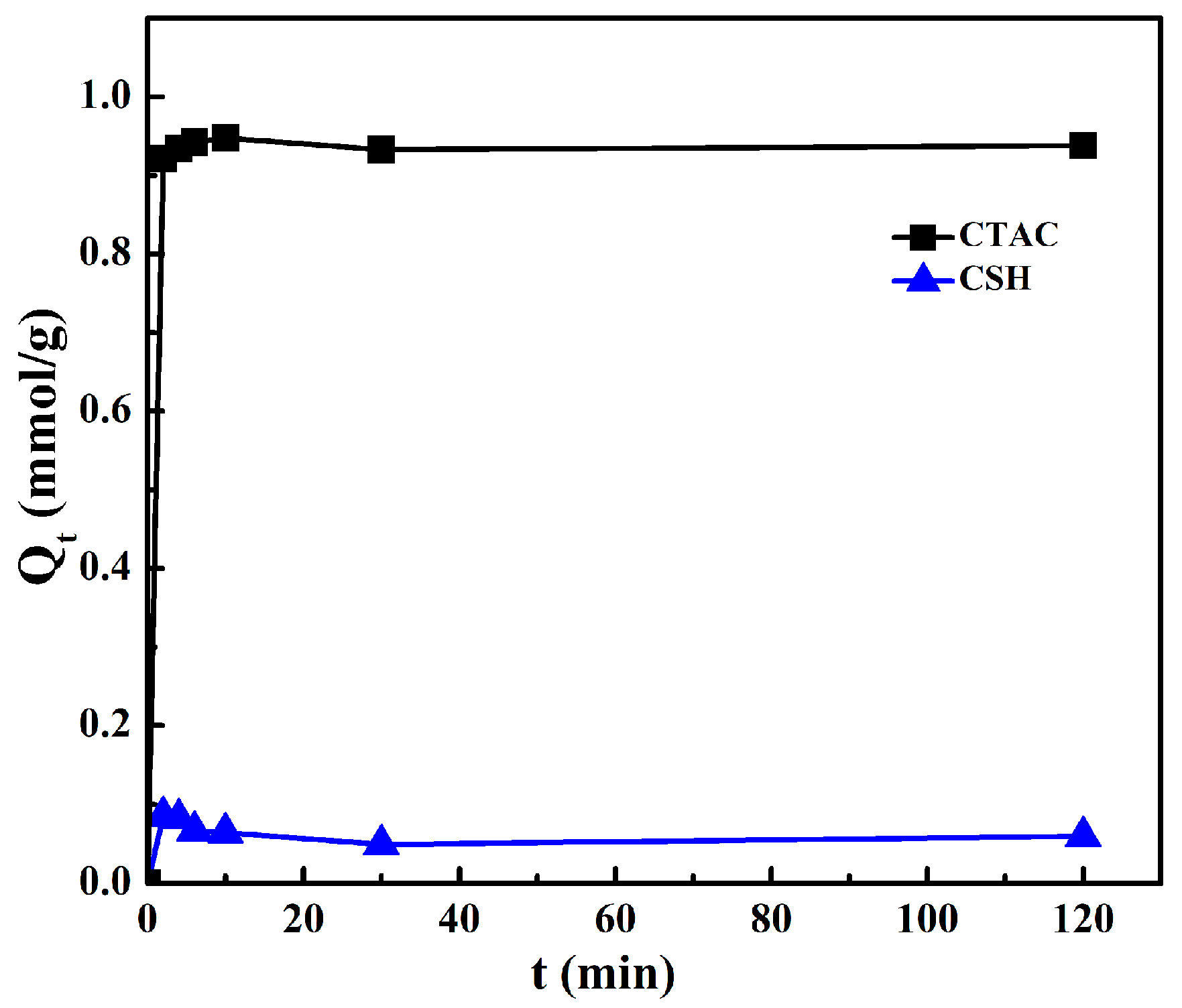
3.3. Adsorption Kinetics
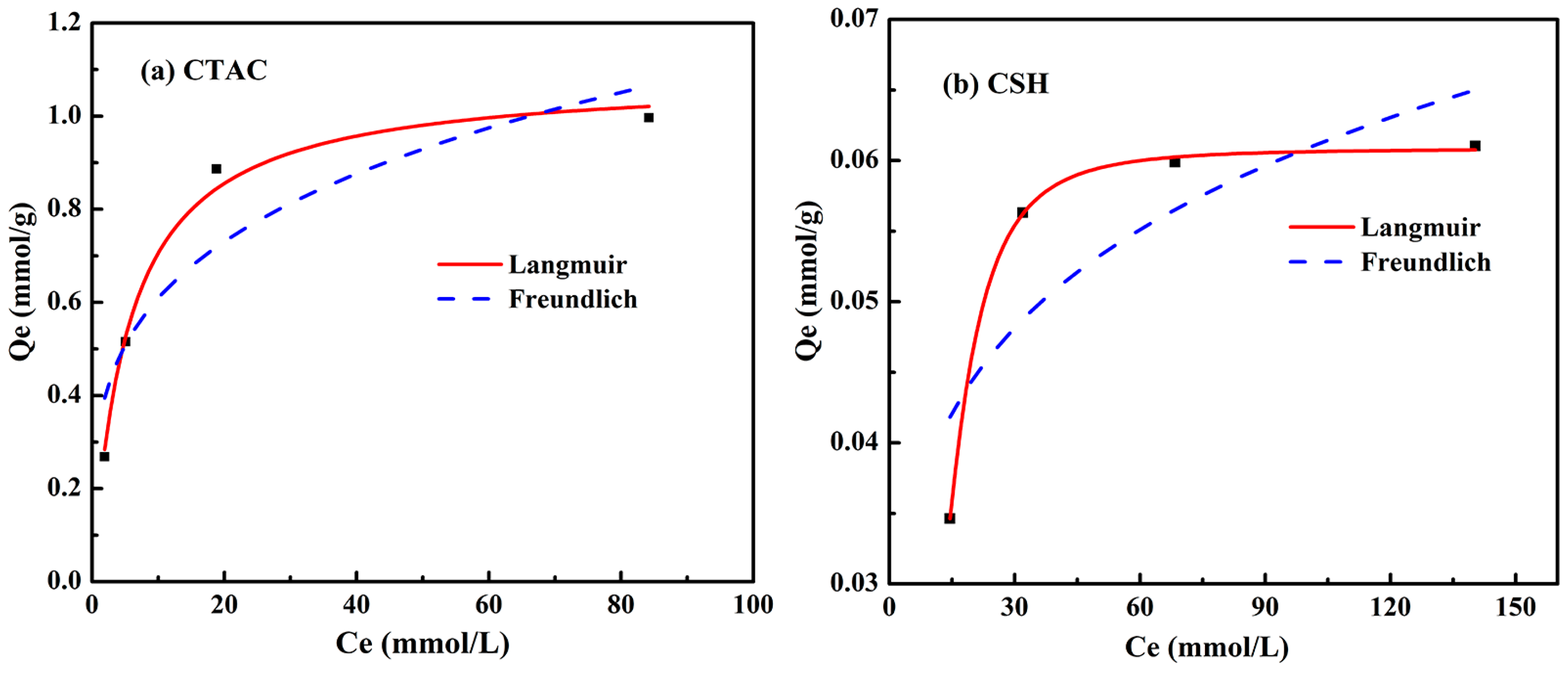
3.4. Adsorption Isotherms
3.5. Desorption Studies of Modified Mt
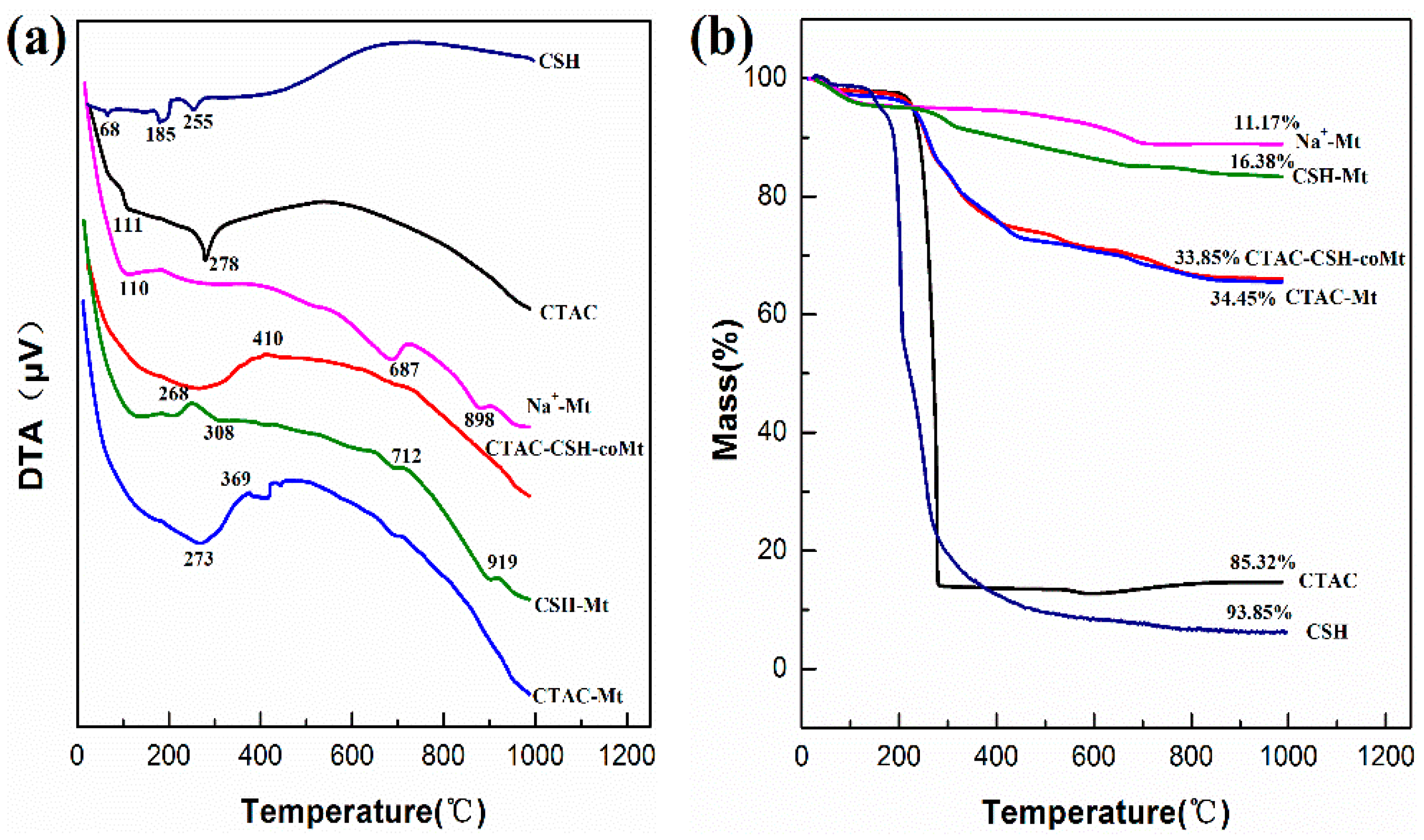
3.6. Characterization Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, L.; Wang, L.; Xu, Y. Chitosan and surfactant co-modified montmorillonite: A multifunctional adsorbent for contaminant removal. Appl. Clay Sci. 2017, 146, 35–42. [Google Scholar] [CrossRef]
- Yang, S.; Wu, P.; Yang, Q.; Zhu, N.; Lu, G.; Dang, Z. Regeneration of iron-montmorillonite adsorbent as an efficient heterogeneous fenton catalytic for degradation of bisphenol a: Structure, performance and mechanism. Chem. Eng. J. 2017, 328, 737–747. [Google Scholar] [CrossRef]
- Almasri, D.A.; Rhadfi, T.; Atieh, M.A.; Mckay, G.; Ahzi, S. High performance hydroxyiron modified montmorillonite nanoclay adsorbent for arsenite removal. Chem. Eng. J. 2017, 335, 1–12. [Google Scholar] [CrossRef]
- Chen, H.H.; Thirumavalavan, M.; He, R.Z.; Shi, Y.T.; Lee, J.F. Feasible preparation and characterization of tunable novel montmorillonite/block-copolymers based composites as potential dual adsorbent candidates. Appl. Clay Sci. 2017, 137, 192–202. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Aranda, P.; Darder, M.; Rytwo, G. Hybrid materials based on clays for environmental and biomedical applications. J. Mater. Chem. 2011, 42, 9306–9321. [Google Scholar] [CrossRef]
- Adebajo, M.O.; Frost, R.L.; Kloprogge, J.T.; Carmody, O.; Kokot, S. Porous materials for oil spill cleanup: A review of synthesis and absorbing properties. J. Porous Mater. 2003, 10, 159–170. [Google Scholar] [CrossRef]
- Shen, Y.H. Phenol sorption by organoclays having different charge characteristics. Colloids Surf. A 2004, 232, 143–149. [Google Scholar] [CrossRef]
- Park, Y.; Ayoko, G.A.; Horváth, E.; Kurdi, R.; Kristof, J.; Frost, R.L. Structural characterisation and environmental application of organoclays for the removal of phenolic compounds. J. Colloid Interface Sci. 2013, 393, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, B.; Megharaj, M.; Xi, Y.; Naidu, R. Surface charge characteristics of organo-palygorskites and adsorption of p-nitrophenol in flow-through reactor system. Indian Pediatr. 2004, 41, 559. [Google Scholar] [CrossRef]
- Alkaram, U.F.; Mukhlis, A.A.; Aldujaili, A.H. The removal of phenol from aqueous solutions by adsorption using surfactant-modified bentonite and kaolinite. J. Hazard. Mater. 2009, 169, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Nourmoradi, H.; Khiadani, M.; Nikaeen, M. Multi-component adsorption of benzene, toluene, ethylbenzene, and xylene from aqueous solutions by montmorillonite modified with tetradecyl trimethyl ammonium bromide. J. Chem. 2013, 2013, 89–94. [Google Scholar] [CrossRef]
- Dentel, S.K.; Jamrah, A.I.; Sparks, D.L. Sorption and cosorption of 1,2,4-trichlorobenzene and tannic acid by organo-clays. Water Res. 1998, 32, 3689–3697. [Google Scholar] [CrossRef]
- Wang, G.; Miao, Y.; Sun, Z.; Zheng, S. Simultaneous adsorption of aflatoxin B1 and zearalenone by mono-and di-alkyl cationic surfactants modified montmorillonites. J. Colloid Interface Sci. 2018, 511, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Celis, R.; Hermosín, M.C.; Cornejo, J. Heavy metal adsorption by functionalized clays. Environ. Sci. Technol. 2000, 34, 4593–4599. [Google Scholar] [CrossRef]
- Xue, G.; Gao, M.; Gu, Z.; Luo, Z.; Hu, Z. The removal of p-nitrophenol from aqueous solutions by adsorption using gemini surfactants modified montmorillonites. Chem. Eng. J. 2013, 218, 223–231. [Google Scholar] [CrossRef]
- Moraru, V.N. Structure formation of alkylammonium montmorillonites in organic media. Appl. Clay Sci. 2001, 19, 11–26. [Google Scholar] [CrossRef]
- Khatib, K.; Pons, C.H.; Bottero, J.Y.; François, M.; Baudin, I. Study of the structure of dimethyldioctadecylammonium-montmorillonite by small angle x-ray scattering. J. Colloid Interface Sci. 1995, 172, 317–323. [Google Scholar] [CrossRef]
- Açışlı, Ö.; Karaca, S.; Gürses, A. Investigation of the alkyl chain lengths of surfactants on their adsorption by montmorillonite (Mt) from aqueous solutions. Appl. Clay Sci. 2016, 142, 90–99. [Google Scholar] [CrossRef]
- Yang, R.; Li, J.; Chen, K.; Fu, G. Investigation of adsorption behavior of layered silicate montmorillonite. J. Lanzhou Univ. Technol. 2007, 33, 17–20. [Google Scholar]
- Wang, G.; Zhang, S.; Hua, Y.; Su, X.; Ma, S.; Wang, J.; Tao, Q.; Wang, Y.; Komarneni, S. Phenol and/or Zn2+ adsorption by single- or dual-cation organomontmorillonites. Appl. Clay Sci. 2017, 140, 1–9. [Google Scholar] [CrossRef]
- Zhu, R.L.; Wang, T.; Ge, F.; Chen, W.X.; You, Z.M. Intercalation of both CTMAB and Al13 into montmorillonite. J. Colloid Interface Sci. 2009, 335, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.G.; Yang, K.; Shan, R.R.; Yan, T.; Wei, J.; Yu, S.J.; Yu, H.Q.; Du, B. Kinetic, isotherm and thermodynamic investigations of phosphate adsorption onto core-shell Fe3O4@LDHs composites with easy magnetic separation assistance. J. Colloid Interface Sci. 2015, 448, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S. Adsorption of Heavy Metals from Waste Streams by Peat. Ph.D. Thesis, The University of Brimingham, Brimingham, UK, 1995; pp. 681–689. [Google Scholar]
- Langmuir, I. Constitution and Fundamental Properties of Solids and Liquids: I, Solids. J. Am. Chem. Soc. 1916, 183, 102–105. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Chem. Phys. 2015, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Über die adsorption in Lösungen. Z. Phys. Chem. 1906, 62, 121–125. [Google Scholar] [CrossRef]
- Lagaly, G. Layer charge heterogeneity in vermiculites. Clays Clay Miner. 1982, 30, 215–222. [Google Scholar] [CrossRef]
- Schampera, B.; Tunega, D.; Šolc, R.; Woche, S.K.; Mikutta, R.; Wirth, R.; Dultz, S.; Guggenberger, G. External surface structure of organoclays analyzed by transmission electron microscopy and X-ray photoelectron spectroscopy in combination with molecular dynamics simulations. Colloid Interface Sci. 2016, 478, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Kung, K.H.S.; Hayes, K.F. Fourier transform infrared spectroscopic study of the adsorption of cetyltrimethylammonium bromide and cetylpyridinium chloride on silica. Langmuir 1933, 9, 263–267. [Google Scholar] [CrossRef]
- Xue, Y.B.; Tang, Z. Density Functional Study of the Structure of SnO2 (110) Surface and the Property of Oxygen Adsorption. Chin. J. Sens. Actuators 2007, 11, 2364–2368. [Google Scholar]
- Xue, W.; He, H.; Zhu, J.; Yuan, P. FTIR investigation of CTAB-Al-montmorillonite complexes. Spectrochim. Acta Part A 2007, 67, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.Z.; Johnston, C.T.; Parker, P.; Agnew, S.F. Infrared study of water sorption on Na-, Li-, Ca-, and Mg-exchanged (swy-1 and saz-1) montmorillonite. Clays Clay Miner. 2000, 48, 120–131. [Google Scholar] [CrossRef]
- Rathnayake, S.I.; Xi, Y.; Frost, R.L.; Ayoko, G.A. Structural and thermal properties of inorganic–organic montmorillonite: Implications for their potential environmental applications. J. Colloid Interface Sci. 2015, 459, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Frost, R.L.; He, H.; Kloprogge, T.; Bostrom, T. Modification of Wyoming montmorillonite surfaces using a cationic surfactant. Langmuir 2005, 21, 8675–8680. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Ayoko, G.A.; Frost, R.L. Application of organoclays for the adsorpon of recalcitrant organic molecules from aqueous media. J. Colloid Interface Sci. 2011, 354, 292–305. [Google Scholar] [CrossRef] [PubMed]
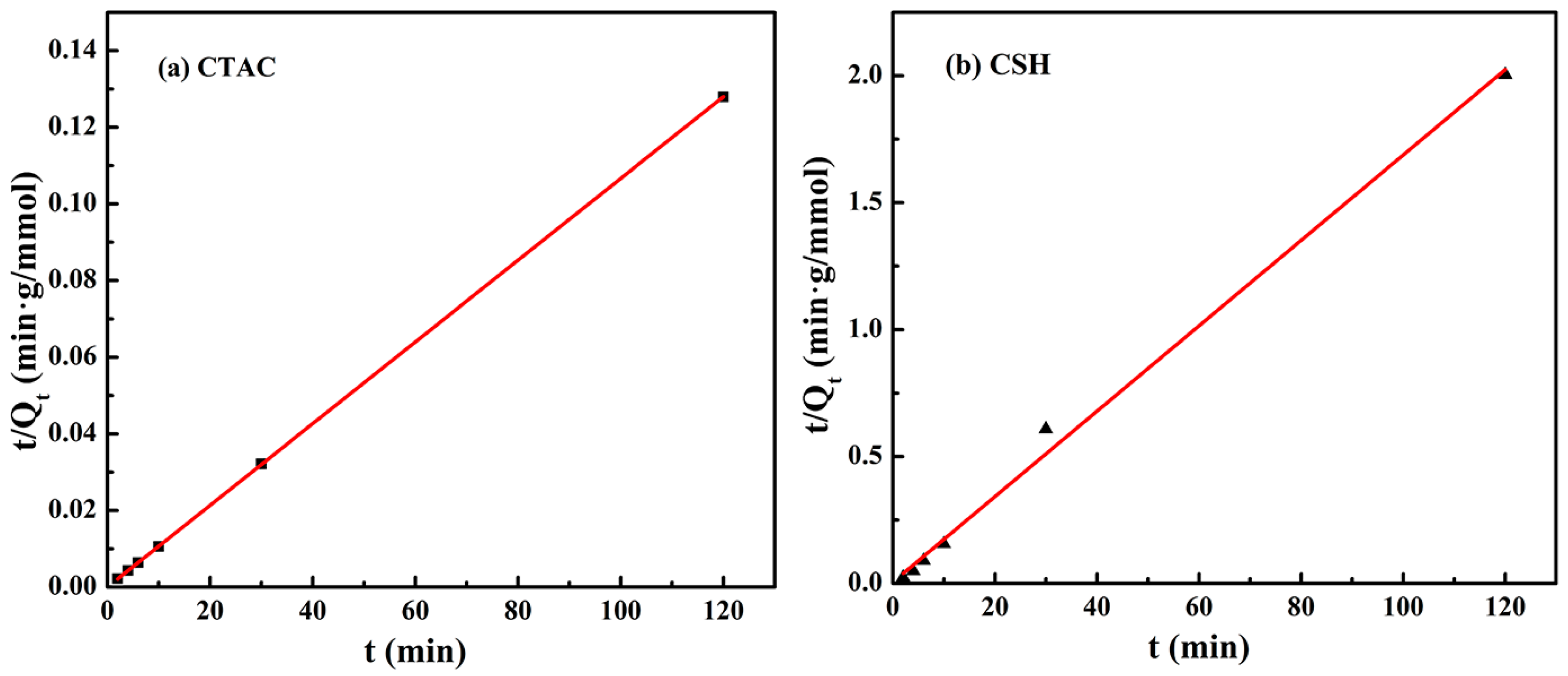

| Organic Modifiers | qe,exp/mmol·g−1 | qe,cal/mmol·g−1 | k2/g·mol−1·min−1 | R2 |
|---|---|---|---|---|
| CSH | 0.05989 | 0.05945 | 0.04748 | 0.995 |
| CTAC | 0.93772 | 0.93758 | 0.13285 | 0.999 |
| Modifiers | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| Qm/mmol·g−1 | b/L·mmol−1 | R2 | KF | n | R2 | |
| CTAC | 1.0862 | 0.1855 | 0.99 | 0.33281 | 3.8104 | 0.86 |
| CSH | 0.06082 | 0.00069 | 0.99 | 0.02484 | 5.14034 | 0.56 |
| Initial pH Value | Desorption Rate of CSH/% | Desorption Rate of CTAC/% |
|---|---|---|
| 3.0 | 0.87 | 1.15 |
| 7.0 | 1.29 | 0.91 |
| 11.0 | 0.85 | 0.72 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Xiao, H.; Zhang, S.; Qiu, J.; Li, H.; Yang, M.; Ma, S.; Komarneni, S. Synthesis and Characterization of a Dual-Cation Organomontmorillonite Nanocomposite. Materials 2018, 11, 2320. https://doi.org/10.3390/ma11112320
Wang G, Xiao H, Zhang S, Qiu J, Li H, Yang M, Ma S, Komarneni S. Synthesis and Characterization of a Dual-Cation Organomontmorillonite Nanocomposite. Materials. 2018; 11(11):2320. https://doi.org/10.3390/ma11112320
Chicago/Turabian StyleWang, Guifang, Huizhen Xiao, Shuai Zhang, Jun Qiu, Hengjun Li, Meijin Yang, Shaojian Ma, and Sridhar Komarneni. 2018. "Synthesis and Characterization of a Dual-Cation Organomontmorillonite Nanocomposite" Materials 11, no. 11: 2320. https://doi.org/10.3390/ma11112320
APA StyleWang, G., Xiao, H., Zhang, S., Qiu, J., Li, H., Yang, M., Ma, S., & Komarneni, S. (2018). Synthesis and Characterization of a Dual-Cation Organomontmorillonite Nanocomposite. Materials, 11(11), 2320. https://doi.org/10.3390/ma11112320




