Selected Physicochemical Properties of Lyophilized Hydrogel with Liposomal Fraction of Calcium Dobesilate
Abstract
:1. Introduction
2. Materials
3. Methods
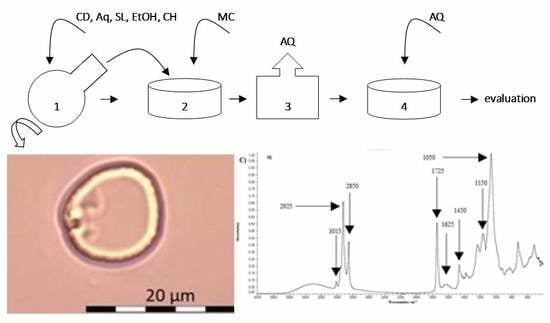
3.1. Preparation of Liposomes (in Reference to the Previous Publication)
3.2. Preparation of Lyophilizates
3.3. DLS—Dynamic Light Scattering
3.4. Optical Microscopy
3.5. FTIR—Fourier-Transform Infrared Spectroscopy
4. Results
4.1. DLS
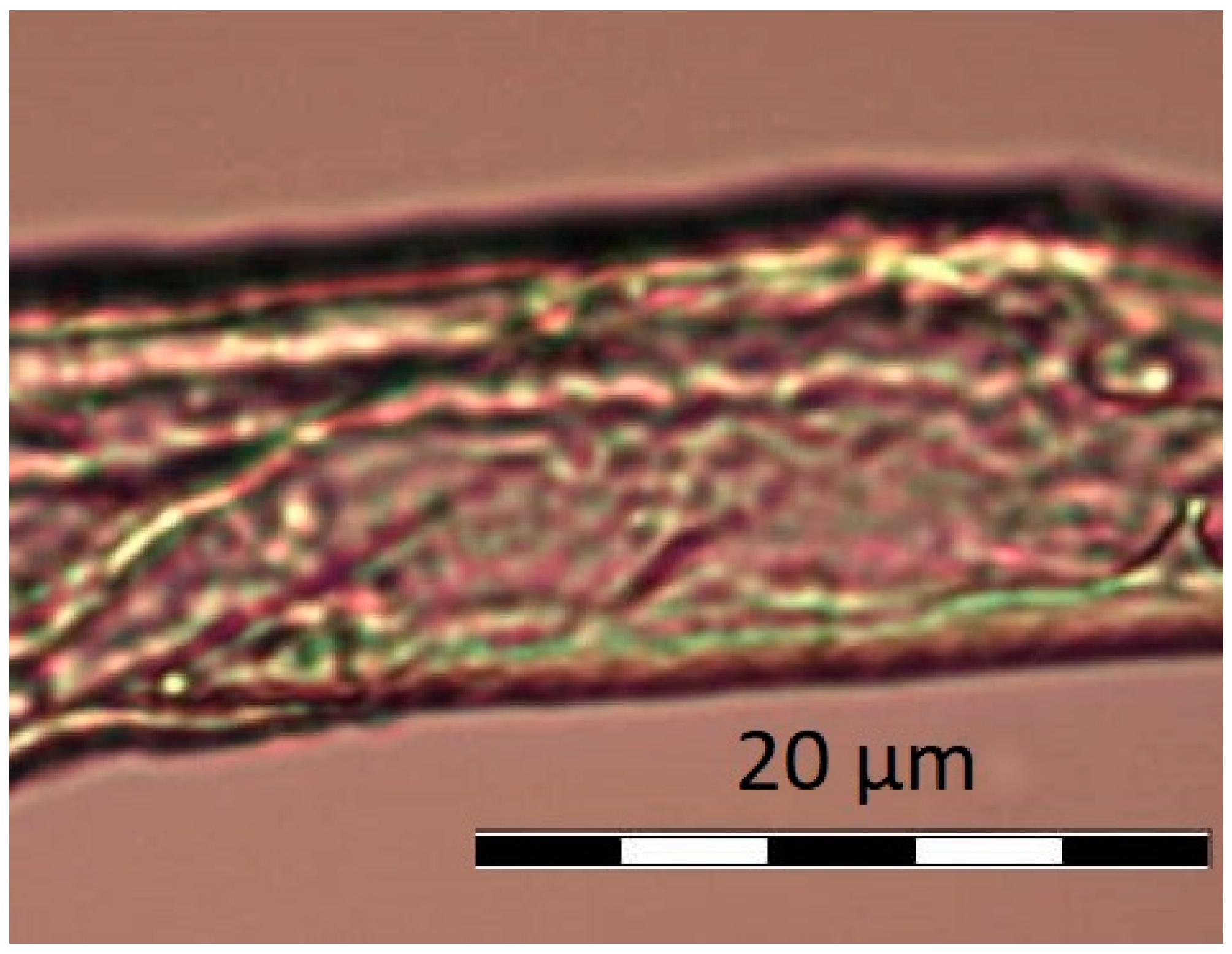
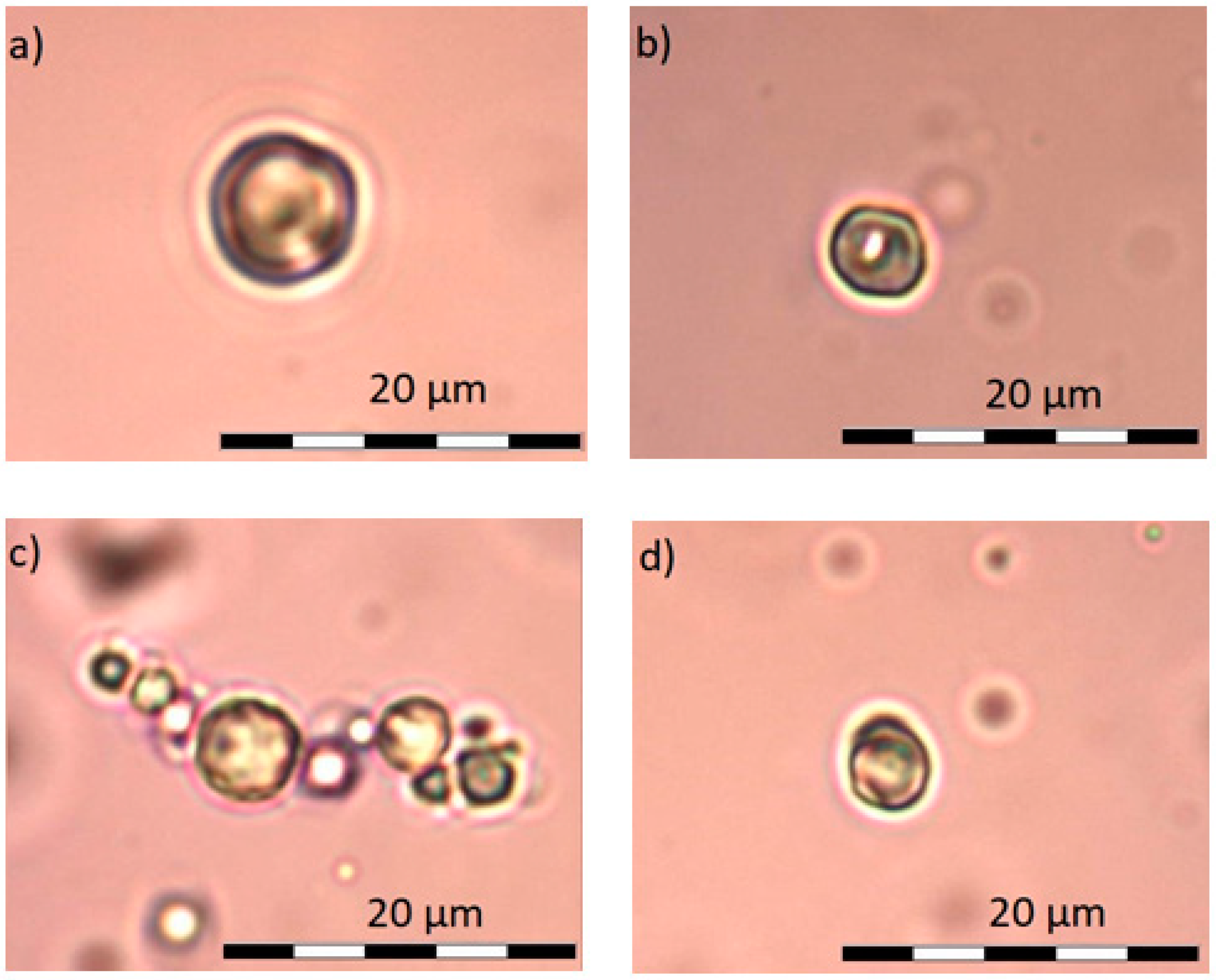
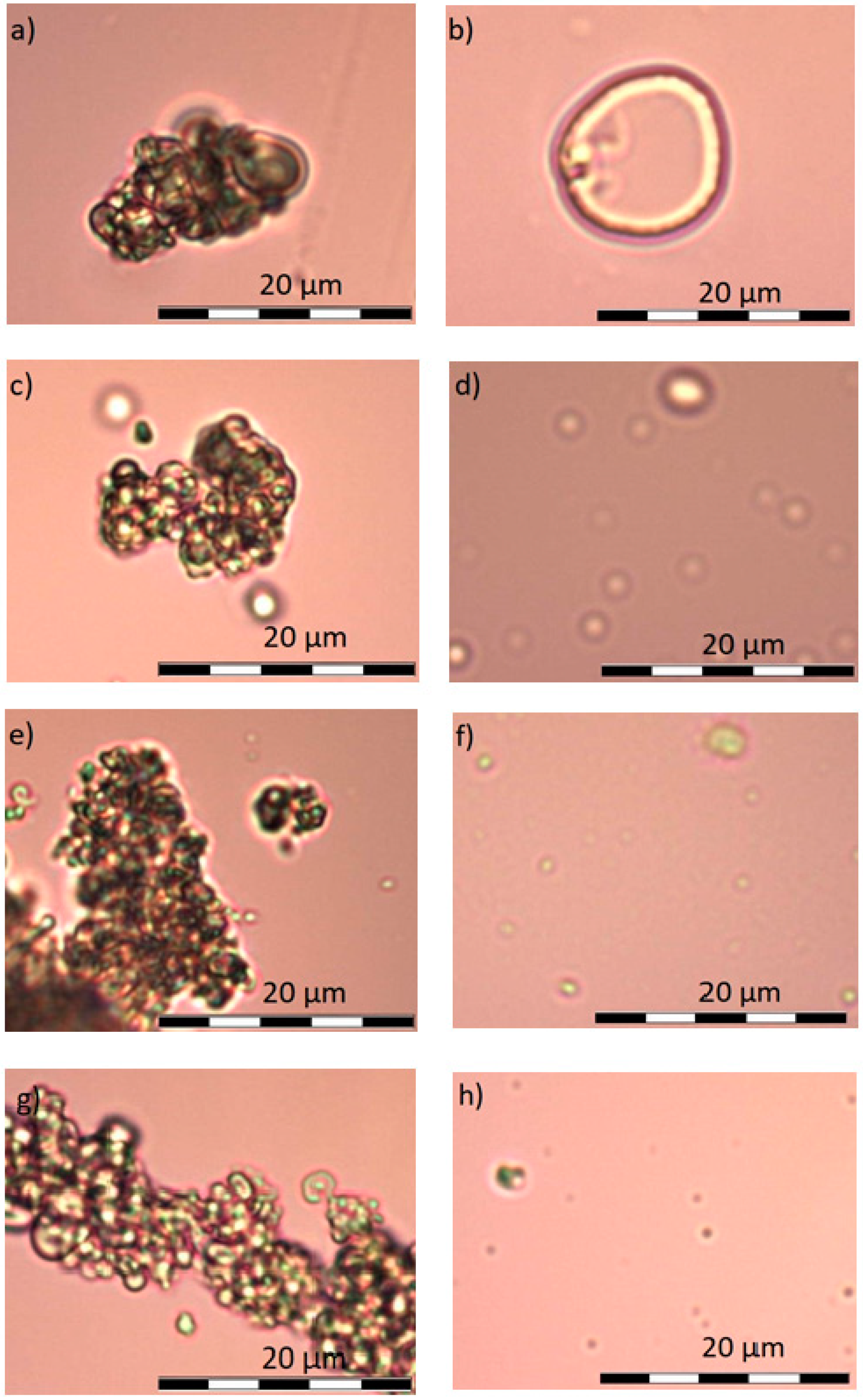
4.2. Optical Microscopy
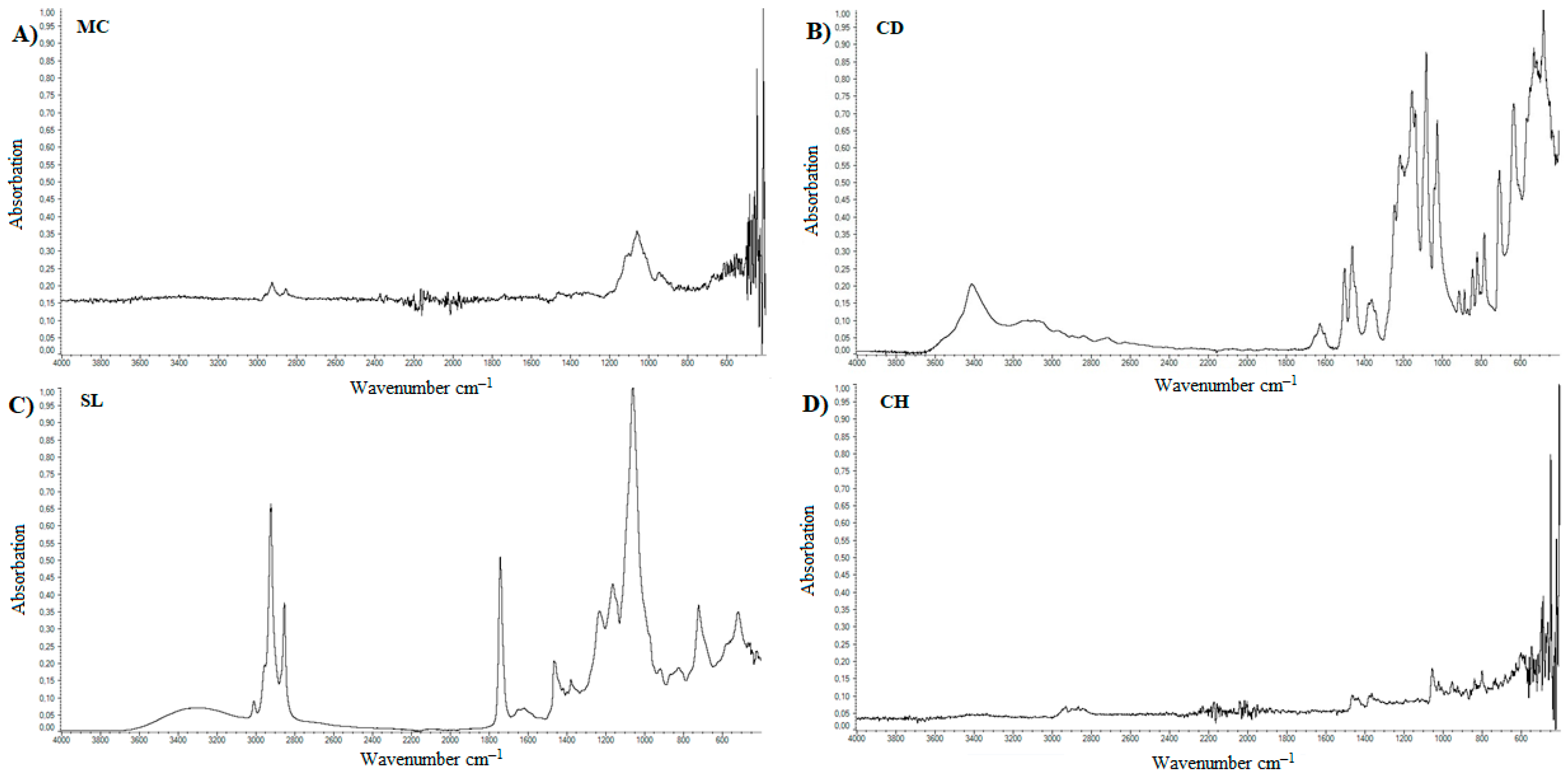
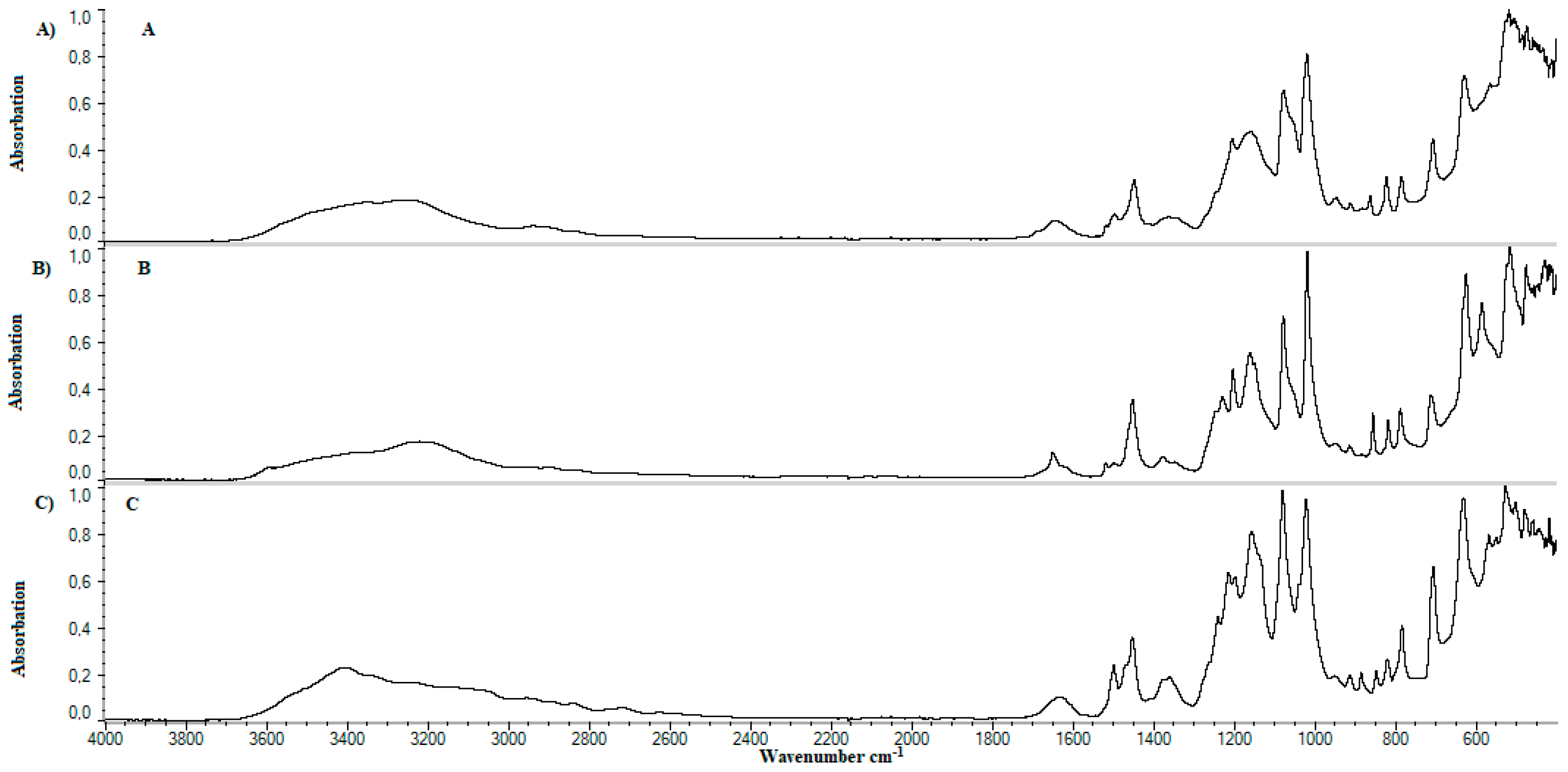
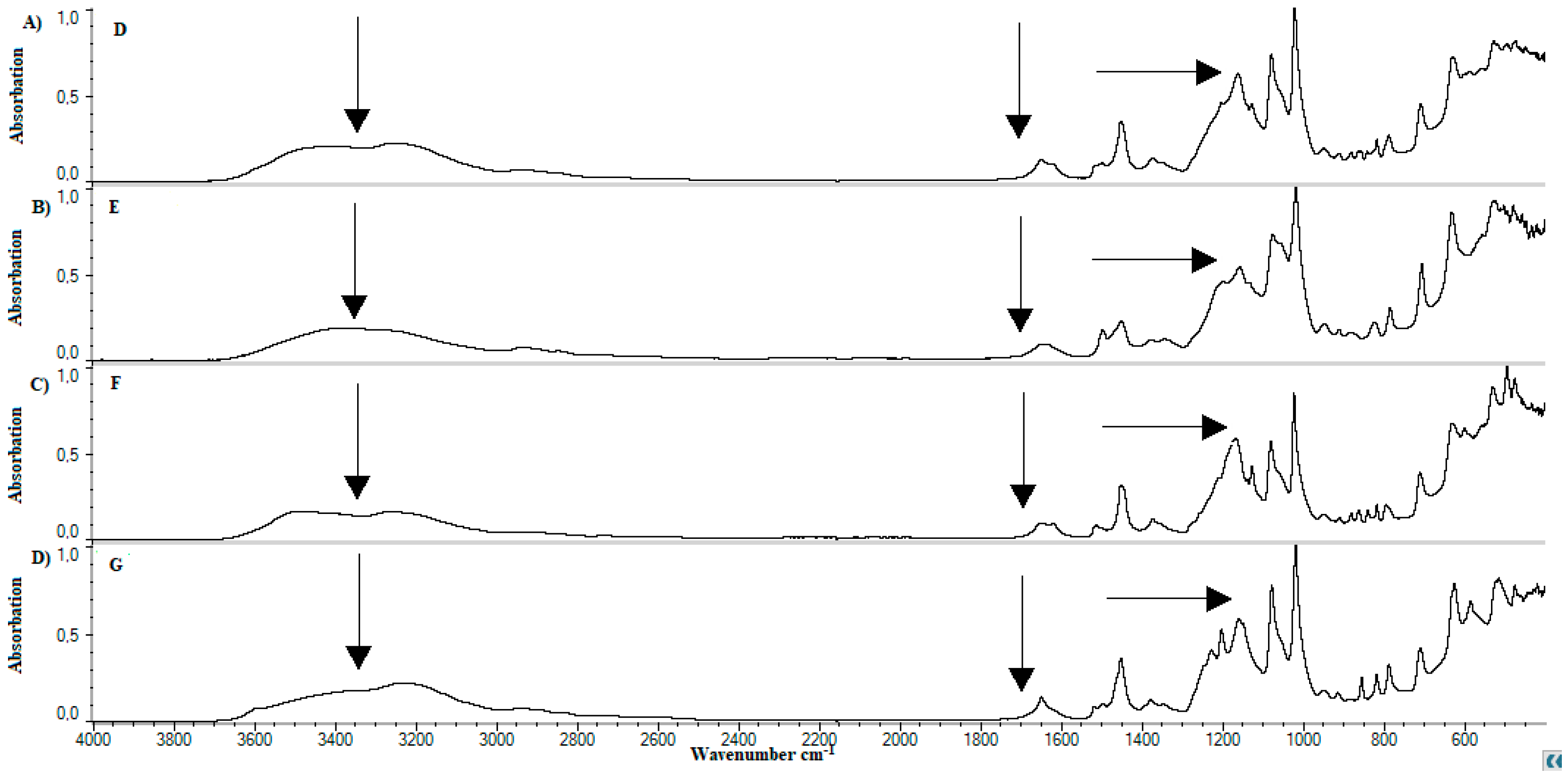
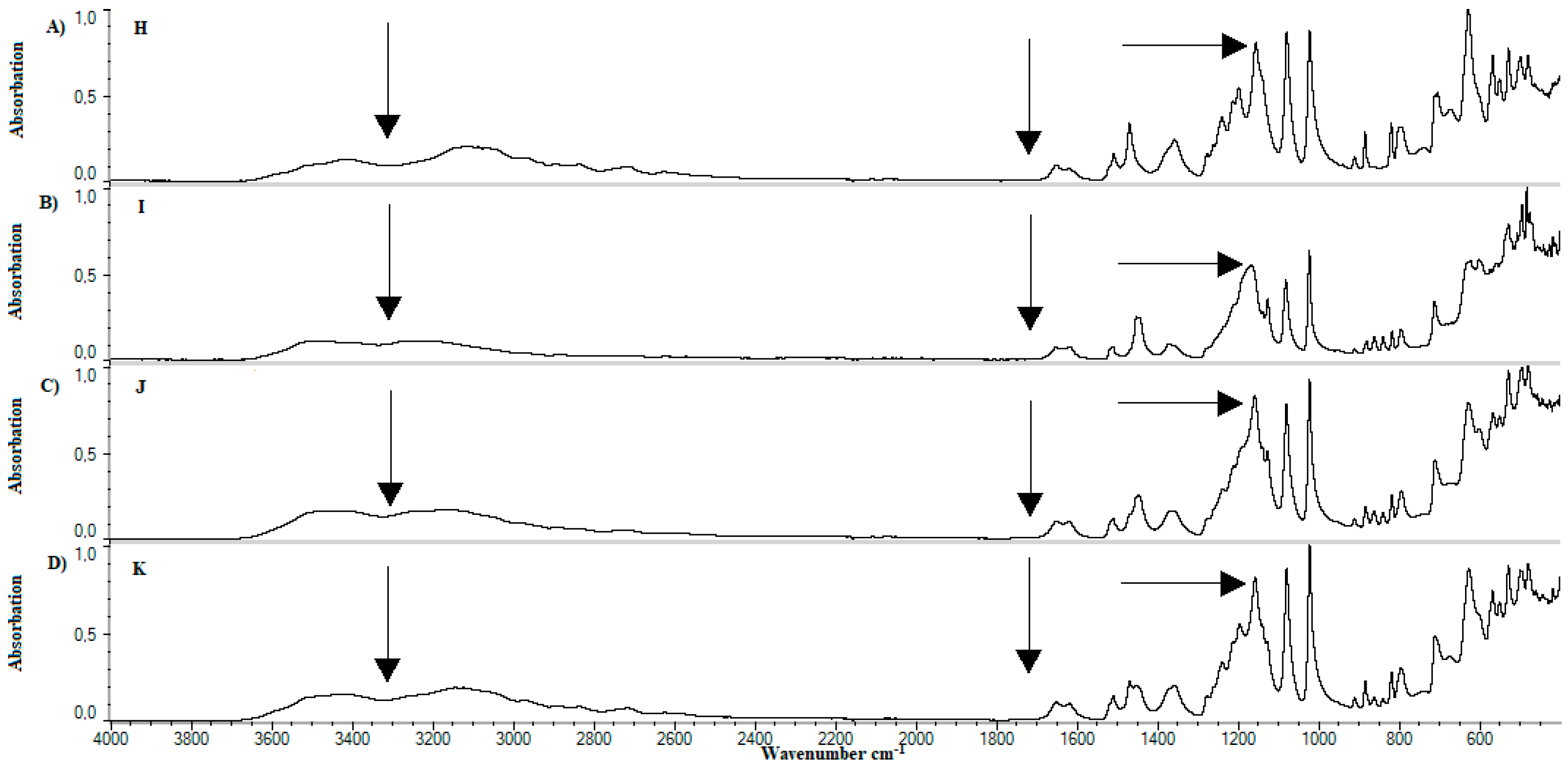
4.4. FTIR
5. Discussion
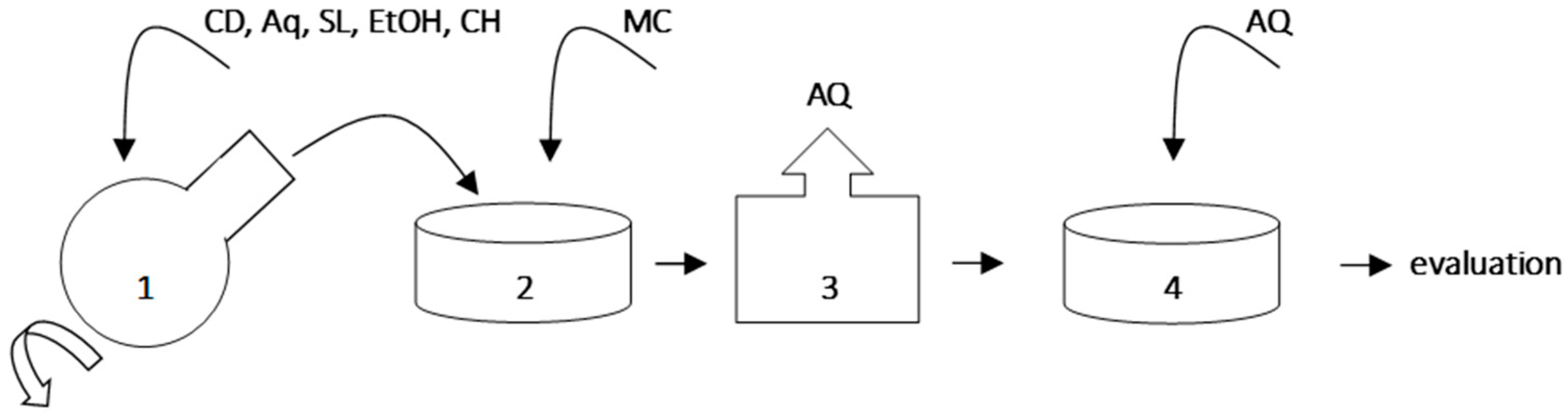
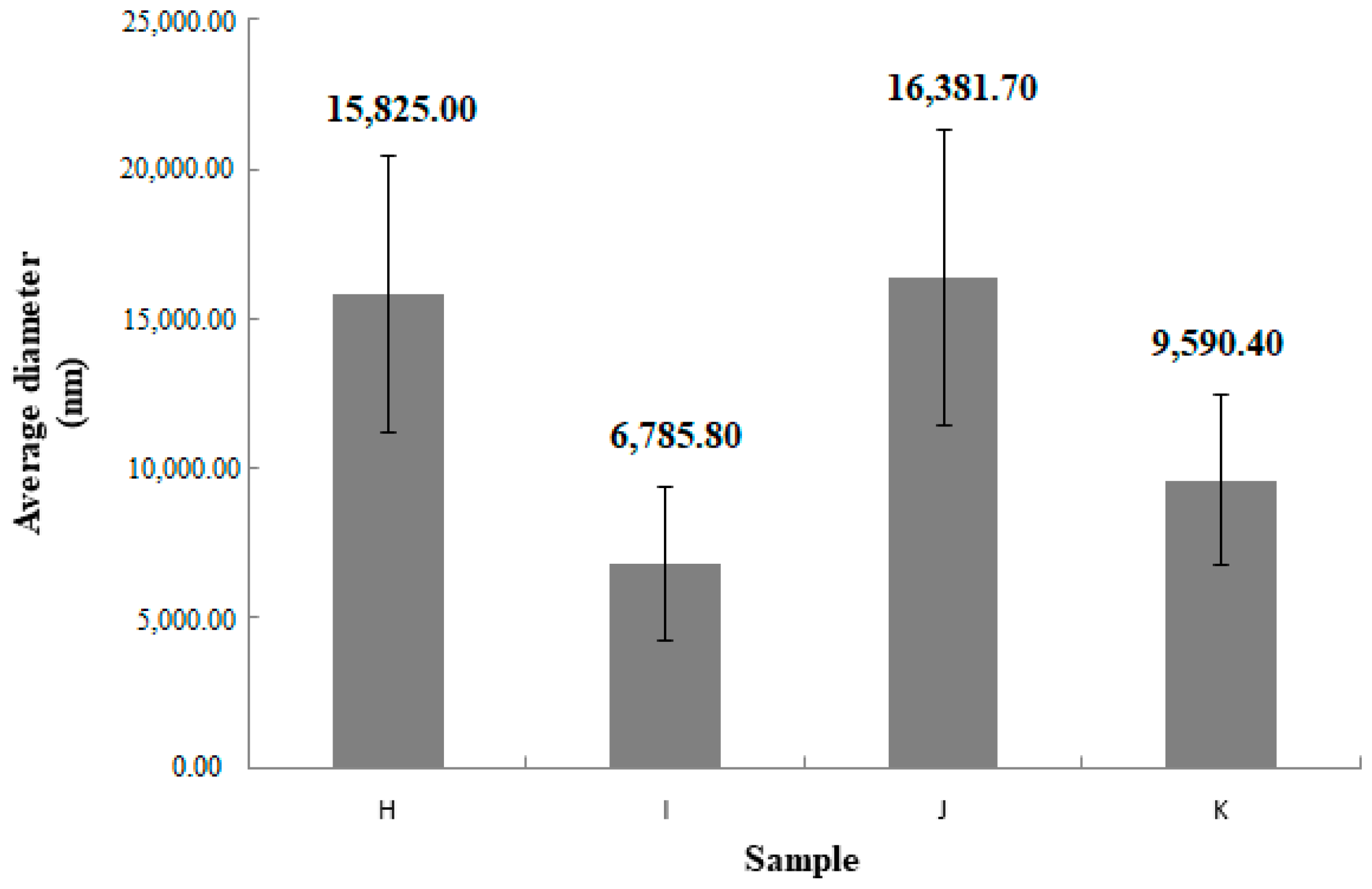
5.1. Hydrodynamic Diameter of Particles
5.2. Optical Microscopy
5.3. FTIR
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gala, R.P.; Ru, Z.; D’Souza, M.J.; Zughaier, S.M. Novel Whole-Cell Inactivated Neisseria Gonorrhoeae microparticles as vaccine formulation in microneedle-based transdermal immunization. Vaccines 2018, 6, 60. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2009, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Mitragotri, S.; Langer, R. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug Discov. 2004, 3, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Benson, H. Transdermal Drug Delivery: Penetration Enhancement Techniques. Curr. Drug Deliv. 2005, 2, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Abdulbaqi, I.M.; Darwis, Y.; Khan, N.A.K.; Assi, R.A.; Khan, A.A. Ethosomal nanocarriers: The impact of constituents and formulation techniques on ethosomal properties, in vivo studies, and clinical trials. Int. J. Nanomed. 2016, 11, 2279–2304. [Google Scholar] [CrossRef] [PubMed]
- Pierre, M.B.R.; Costa, I.S.M. Liposomal systems as drug delivery vehicles for dermal and transdermal applications. Arch. Dermatol. Res. 2011, 303, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Schreier, H.; Bouwstra, J. Liposomes and niosomes as topical drug carriers: Dermal and transdermal drug delivery. J. Control. Release 1994, 30, 1–15. [Google Scholar] [CrossRef]
- Manosroi, A.; Podjanasoonthon, K.; Manosroi, J. Development of novel topical tranexamic acid liposome formulations. Int. J. Pharm. 2002, 235, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Golmohammadzadeh, S.; Imani, F.; Hosseinzadeh, H.; Jaafari, M.R. Preparation, characterization and evaluation of sun protective and moisturizing effects of nanoliposomes containing safranal. Iran. J. Basic Med. Sci. 2011, 14, 521–533. [Google Scholar] [PubMed]
- Kanjickal, D.; Lopina, S.; Evancho-Chapman, M.M.; Schmidt, S.; Donovan, D. Improving delivery of hydrophobic drugs from hydrogels through cyclodextrins. J. Biomed. Mater. Res. 2005, 74, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Nishinari, K.; Hofmann, K.E.; Moritaka, H.; Kohyama, K.; Nishinari, N. Gel-sol transition of methylcellulose. Macromol. Chem. Phys. 1997, 198, 1217–1226. [Google Scholar] [CrossRef]
- Shtenberg, Y.; Goldfeder, M.; Prinz, H.; Shainsky, J.; Ghantous, Y.; El-Naaj, I.A.; Schroeder, A.; Bianco-Peled, H. Mucoadhesive alginate pastes with embedded liposomes for local oral drug delivery. Int. J. Biol. Macromol. 2018, 111, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Mura, P.; Mennini, N.; Nativi, C.; Richichi, B. In situ mucoadhesive-thermosensitive liposomal gel as a novel vehicle for nasal extended delivery of opiorphin. Eur. J. Pharm. Biopharm. 2018, 122, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.H.; Jeong, Y.J.; Kim, A.Y.; Hong, I.K.; Lee, N.H.; Park, S.N. Preparation and Physicochemical Properties of a Cysteine Derivative-Loaded Deformable Liposomes in Hydrogel for Enhancing Whitening Effects. Eur. J. Lipid Sci. Technol. 2018, 120, 1800125. [Google Scholar] [CrossRef]
- Sylvester, B.; Porfire, A.; Van Bockstal, P.-J.; Porav, S.; Achim, M.; De Beer, T.; Tomuta, I. Formulation Optimization of Freeze-Dried Long-Circulating Liposomes and In-Line Monitoring of the Freeze-Drying Process Using an NIR Spectroscopy Tool. J. Pharm. Sci. 2018, 107, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, Q.; Wu, H.; Wang, K.; Li, L.; Zhou, C.; Ao, N. Preparation and characterization of in-situ formable liposome/chitosan composite hydrogels. Mater. Lett. 2018, 220, 289–292. [Google Scholar] [CrossRef]
- Liversidge, G.G.; West, C.; Phillips, C.P.; Cundy, K.C. Method to Reduce Particle Size Growth during Lyophilization. U.S. Patent 5,302,401, 12 April 1994. [Google Scholar]
- Dua, J.S.; Rana, A.C.; Bhandari, A.K. Liposome methods of preparation and applications. Int. J. Pharm. Stud. Res. 2012, 3, 14–20. [Google Scholar]
- Abdelwahed, W.; Degobert, G.; Stainmesse, S.; Fessi, H. Freeze-drying of nanoparticles: Formulation, process and storage considerations. Adv. Drug Deliv. Rev. 2006, 58, 1688–1713. [Google Scholar] [CrossRef] [PubMed]
- Wang, W. Lyophilization and development of solid protein pharmaceuticals. Int. J. Pharm. 2000, 203, 1–60. [Google Scholar] [CrossRef]
- Arun, A.; Amrish, C. Fast Drug Delivery Systems: A Review. Der Pharm. Lett. 2010, 2, 350–361. [Google Scholar]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Khadka, P.; Ro, J.; Kim, H.; Kim, I.; Kim, J.T.; Kim, H.; Lee, J. Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. Asian J. Pharm. Sci. 2014, 9, 304–316. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Han, D.; Cai, C.; Tang, X. An overview of liposome lyophilization and its future potential. J. Control. Release 2010, 142, 299–311. [Google Scholar] [CrossRef] [PubMed]
- El-Nesr, O.H.; Yahiya, S.A.; El-Gazayerly, O.N. Effect of formulation design and freeze-drying on properties of fluconazole multilamellar liposomes. Saudi Pharm. J. 2010, 18, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.H.; Ghosh, B. Fast-Dissolving-Drug-Delivery-Systems: A Review of the Literature. Indian J. Pharm. Sci. 2002, 64, 331–336. [Google Scholar]
- Chang, H.-I.; Yeh, M.-K. Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy. Int. J. Nanomed. 2012, 7, 49–60. [Google Scholar] [CrossRef]
- Gala, R.P.; Khan, I.; Elhissi, A.M.A.; Alhnan, M.A. A comprehensive production method of self-cryoprotected nano-liposome powders. Int. J. Pharm. 2015, 486, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.A.; Anyarambhatla, G.; Ma, L.; Ugwu, S.; Xuan, T.; Sardone, T.; Ahmad, I. Development and characterization of a novel Cremophorw EL free liposome-based paclitaxel (LEP-ETU) formulation. Eur. J Pharm. Biopharm. 2005, 59, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Ghanbarzadeh, S.; Valizadeh, H.; Zakeri-Milani, P. The effects of lyophilization on the physico-chemical stability of sirolimus liposomes. Adv. Pharm. Bull. 2013, 3, 25–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Feng, J.; Wu, P. Preparation of organically dispersible graphene nanosheet powders through a lyophilization method and their poly(lactic acid) composites. Carbon 2010, 48, 3834–3839. [Google Scholar] [CrossRef]
- Pehkonen, K.S.; Roos, Y.H.; Miao, S.; Ross, R.P.; Stanton, C. State transitions and physicochemical aspects of cryoprotection and stabilization in freeze-drying of Lactobacillus rhamnosus GG (LGG). J. Appl. Microbiol. 2008, 104, 1732–1743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.K.; Srinivasan, K.K.; Gowthamarajan, K.; Prakash, D.; Gaikwad, N.B. Investigation of preparation parameters of solid dispersion and lyophilization technique in order to enhance the dissolution of poorly soluble glyburide. J. Pharm. Res. 2011, 4, 2718–2723. [Google Scholar]
- Yadava, P.; Gibbs, M.; Castro, C.; Hughes, J.A. Effect of Lyophilization and Freeze-thawing on the Stability of siRNA-liposome Complexes. AAPS Pharm. Sci. Technol. 2008, 9, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Handa, T.; Takeuchi, H.; Ohokubo, Y.; Kawashima, Y. Lyophilized Liposomes Prepared by a Modified Reversed-Phase Evaporation Method. Chem. Pharm. Bull. 1987, 35, 748–755. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Zhang, Z.R.; Yang, H.; Tan, Q.Y.; Qin, S.R.; Qiu, X.L. Lyophilized paclitaxel magnetoliposomes as a potential drug delivery system for breast carcinoma via parenteral administration: In vitro and in vivo studies. Pharm. Res. 2005, 22, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Abidian, M.; Martin, D.C. Conducting polymers grown in hydrogel scaffolds coated on neural prosthetic devices. J. Biomed. Mater. Res. A 2004, 71, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Crowe, C.S.; Chattopadhyay, A.; McGoldrick, R.; Chiou, G.; Pham, H.; Chang, J. Characteristics of Reconstituted Lyophilized Tendon Hydrogel: An Injectable Scaffold for Tendon Regeneration. Plast. Reconstr. Surg. 2016, 137, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Marefati, A.; Rayner, M.; Timgren, A.; Dejmek, P.; Sjöö, M. Freezing and freeze-drying of Pickering emulsions stabilized by starch granules. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 512–520. [Google Scholar] [CrossRef]
- Fang, J.Y.; Hong, C.T.; Chiu, W.T.; Wang, Y.Y. Effect of liposomes and niosomes on skin permeation of enoxacin. Int. J. Pharm. 2001, 219, 61–72. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.P.; Misra, A. Liposomal Amikacin dry powder inhaler: Effect of fines on in vitro performance. Pharmazie 2004, 59, 812–813. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, E.; Lorente, R.; Tejerina, T. Effects of calcium dobesilate on the synthesis of endothelium-dependent relaxing factors in rabbit isolated aorta. Br. J. Pharmacol. 1997, 121, 711–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, M.L.; Seres, A.I.; Carneiro, A.M.; Stur, M.; Zourdani, A.; Caillon, P.; Cunha-Vaz, J.G. Effect of calcium dobesilate on progression of early diabetic retinopathy: A randomised double-blind study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2006, 244, 1591–1600. [Google Scholar] [CrossRef] [PubMed]
- Flota-Cervera, F.; Flota-Ruiz, C.; Treviño, C.; Berber, A. Randomized, double blind, placebo-controlled clinical trial to evaluate the lymphagogue effect and clinical efficacy of calcium dobesilate in chronic venous disease. Angiology 2008, 59, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Ciapponi, A.; Laffaire, E.; Roque, M. Calcium dobesilate for chronic venous insufficiency: A systematic review. Angiology 2004, 55, 147–154. [Google Scholar] [CrossRef] [PubMed]
- McMahon, A.M.; Carati, C.J.; Piller, N.B.; Gannon, B.J. The effects of radiation on the contractile activity of guinea pig mesenteric lymphatics. Lymphology 1994, 27, 193–200. [Google Scholar] [PubMed]
- Allain, H.; Ramelet, A.A.; Polard, E.; Bentué-Ferrer, D. Safety of calcium dobesilate in chronic venous disease, diabetic retinopathy and haemorrhoids. Drug Saf. 2004, 27, 649–660. [Google Scholar] [CrossRef] [PubMed]
- FSP Galena Karta Charakterystyki. Available online: http://www.galena.pl/api/calcium-dobesilate-monohydrate/ (accessed on 1 September 2018).
- Lisik, A.; Wojcik-Pastuszka, D.; Twarda, M.; Berkowski, R.; Musial, W. Effect of standard and reversible arrangements of Ph.Eur./USP extraction cells during dissolution tests of calcium dobesilate in hydrogel formulation. Curr. Issues Pharm. Med. Sci. 2015, 28, 136–141. [Google Scholar] [CrossRef]
- Pilch, E.; Lisik, A.; Słowiak, L.; Musiał, W. The Influence of Non-Unified Liposomal Fraction on the Release Kinetics of Calcium Dobesilate from Hydrophilic Gel. Am. J. Pharm. 2017, 36, 2241–2250. [Google Scholar]
- Gasztych, M.; Gola, A.; Kobryń, J.; Musiał, W. Synthesis and Formulation of Thermosensitive Drug Carrier for Temperature Triggered Delivery of Naproxen Sodium. Molecules 2016, 21, 1473. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Kakuda, Y.; Cui, W. Hydrocolloids in emulsions: Particle size distribution and interfacial activity. Food Hydrocoll. 2001, 15, 533–542. [Google Scholar] [CrossRef]
- Bendas, E.R.; Tadros, M.I. Enhanced transdermal delivery of salbutamol sulfate via ethosomes. AAPS Pharm. Sci. Technol. 2007, 8, E1–E8. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Mao, G.; Ng, K.Y.S. Mechanical properties and stability measurement of cholesterol-containing liposome on mica by atomic force microscopy. J. Colloid Interface Sci. 2004, 278, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-C.; Yeh, G.H.-C.; Chiang, B.-H. Antimicrobial and physicochemical properties of methylcellulose and chitosan films containing a preservative. J. Food Process. Preserv. 1996, 20, 379–390. [Google Scholar] [CrossRef]
- Trandum, C.; Westh, P.; Jørgensen, K.; Mouritsen, O.G. Association of ethanol with lipid membranes containing cholesterol, sphingomyelin and ganglioside: A titration calorimetry study. Biochim. Biophys. Acta Biomembr. 1999, 1420, 179–188. [Google Scholar] [CrossRef]
- Rangelova, N.; Radev, L.; Nenkova, S.; Salvado, I.M.M.; Fernandes, M.H.V.; Herzog, M. Methylcellulose/SiO2hybrids: Sol-gel preparation and characterization by XRD, FTIR and AFM. Cent. Eur. J. Chem. 2011, 9, 112–118. [Google Scholar] [CrossRef]
- Rokhade, A.P.; Shelke, N.B.; Patil, S.A.; Aminabhavi, T.M. Novel interpenetrating polymer network microspheres of chitosan and methylcellulose for controlled release of theophylline. Carbohydr. Polym. 2007, 69, 678–687. [Google Scholar] [CrossRef]
- Turhan, K.N.; Sahbaz, F.; Güner, A. A Spectrophotometric Study of Hydrogen Bonding in Methylcellulose-based Edible Films Plasticized by Polyethylene Glycol. J. Food Sci. 2001, 66, 59–62. [Google Scholar] [CrossRef]
- Sullad, A.G.; Manjeshwar, L.S.; Aminabhavi, T.M. Blend microspheres of chitosan and polyurethane for controlled release of water-soluble antihypertensitive drugs. Polym. Bull. 2014, 72, 265–280. [Google Scholar] [CrossRef]
- Nzai, J.M.; Proctor, A. Soy Lecithin Phospholipid Determination by Fourier Transform Infrared Spectroscopy and the Acid Digest/Arseno-Molybdate Method: A Comparative Study. J. Am. Oil Chem. Soc. 1999, 76, 61–66. [Google Scholar] [CrossRef]
- Zhang, T.; Xi, K.; Gu, M.; Jiang, Z.S. Phosphoryl choline-grafted water-soluble carbon nanotube. Chin. Chem. Lett. 2008, 19, 105–109. [Google Scholar] [CrossRef]
- Meade, A.D.; Lyng, F.M.; Knief, P.; Byrne, H.J. Growth substrate induced functional changes elucidated by FTIR and Raman spectroscopy in in-vitro cultured human keratinocytes. Anal. Bioanal. Chem. 2007, 387, 1717–1728. [Google Scholar] [CrossRef] [PubMed]
- Kamelska, A.M.; Pietrzak-Fiećko, R.; Bryl, K. Variation of the cholesterol content in breast milk during 10 days collection at early stages of lactation. Acta Biochim. Pol. 2012, 59, 243–247. [Google Scholar] [PubMed]
| Formulation | Components (g) | |||||
|---|---|---|---|---|---|---|
| CD | MC | EtOH | SL | CH | AQ | |
| A | 2.5 | 1.5 | 0.0 | 0.0 | 0.0 | 96.000 |
| B | 2.5 | 1.5 | 20.0 | 0.0 | 0.0 | 76.000 |
| C | 2.5 | 1.5 | 48.0 | 0.0 | 0.0 | 48.000 |
| D | 2.5 | 1.5 | 20.0 | 0.041 | 0.0 | 75.959 |
| E | 2.5 | 1.5 | 48.0 | 0.041 | 0.0 | 47.959 |
| F | 2.5 | 1.5 | 20.0 | 0.041 | 0.004 | 75.955 |
| G | 2.5 | 1.5 | 48.0 | 0.041 | 0.004 | 47.955 |
| H | 2.5 | 0.0 | 20.0 | 0.041 | 0.0 | 77.459 |
| I | 2.5 | 0.0 | 48.0 | 0.041 | 0.0 | 49.459 |
| J | 2.5 | 0.0 | 20.0 | 0.041 | 0.004 | 77.455 |
| K | 2.5 | 0.0 | 48.0 | 0.041 | 0.004 | 49.455 |
| Sample | Average Diameter (nm) | SD | PDI | SD |
|---|---|---|---|---|
| H | 15,825.0 | 4594.4 | 0.354 | 0.239 |
| I | 6785.8 | 2570.9 | 0.702 | 0.392 |
| J | 16,381.7 | 4958.2 | 0.854 | 0.230 |
| K | 9590.4 | 2854.5 | 0.516 | 0.299 |
| Lp. | Substance | References | Observed | Group | Literature |
|---|---|---|---|---|---|
| cm−1 | |||||
| 1. | Methylcellulose | 1126 | 1125 | C–O (oxygen stretching) | [57] |
| 1647 | - | C−O (carbonyl stretching) | [57] | ||
| 2843, 2932 | 2925 | C–H | [57,58,59] | ||
| 3459 | - | O–H | [57,58,59] | ||
| 2. | Calcium dobesilate | 822 | 800−925 | Aromatic C–H | [60] |
| 1023, 1361 | 1050, 1100, 1350 | S=O, C–O | [61] | ||
| 3155 | 3100 | Aromatic C–H | [62] | ||
| 3431 | 3400 | O–H | [63] | ||
| 3. | Soy lecithin | 970−1200 | 1050 | P–O–C; PO2 | [61] |
| 1145−1200 | 1150 | PO2 | [61,62] | ||
| 1540 | 1450 | P–O–alkyl and amide groups | [62] | ||
| 1630–1640 | 1625 | C–N, N–H | [63] | ||
| 1720–1765 | 1725 | C=O | [61] | ||
| 2850–2960 | 2850, 2925 | C–H | [61] | ||
| 3015 | 3015 | =C–H | [61] | ||
| 4. | Cholesterol | 2800–3200 | 2850–2950 | C–H | [64] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilch, E.; Musiał, W. Selected Physicochemical Properties of Lyophilized Hydrogel with Liposomal Fraction of Calcium Dobesilate. Materials 2018, 11, 2143. https://doi.org/10.3390/ma11112143
Pilch E, Musiał W. Selected Physicochemical Properties of Lyophilized Hydrogel with Liposomal Fraction of Calcium Dobesilate. Materials. 2018; 11(11):2143. https://doi.org/10.3390/ma11112143
Chicago/Turabian StylePilch, Ewa, and Witold Musiał. 2018. "Selected Physicochemical Properties of Lyophilized Hydrogel with Liposomal Fraction of Calcium Dobesilate" Materials 11, no. 11: 2143. https://doi.org/10.3390/ma11112143
APA StylePilch, E., & Musiał, W. (2018). Selected Physicochemical Properties of Lyophilized Hydrogel with Liposomal Fraction of Calcium Dobesilate. Materials, 11(11), 2143. https://doi.org/10.3390/ma11112143