Wear Resistance Mechanism of Alumina Ceramics Containing Gd2O3
Abstract
:1. Introduction
2. Materials and Methods
3. Results
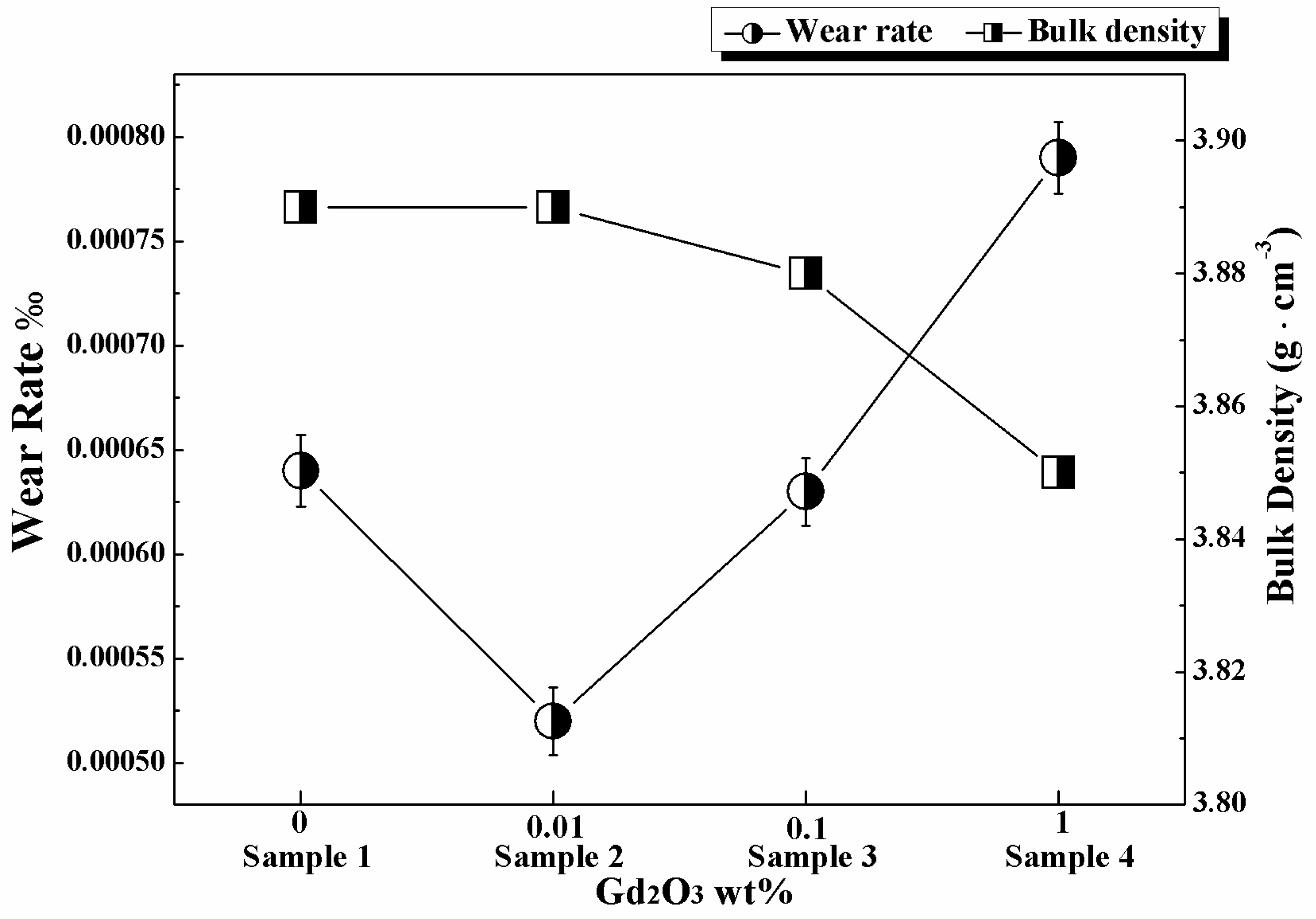
3.1. Wear Rate and Bulk Density
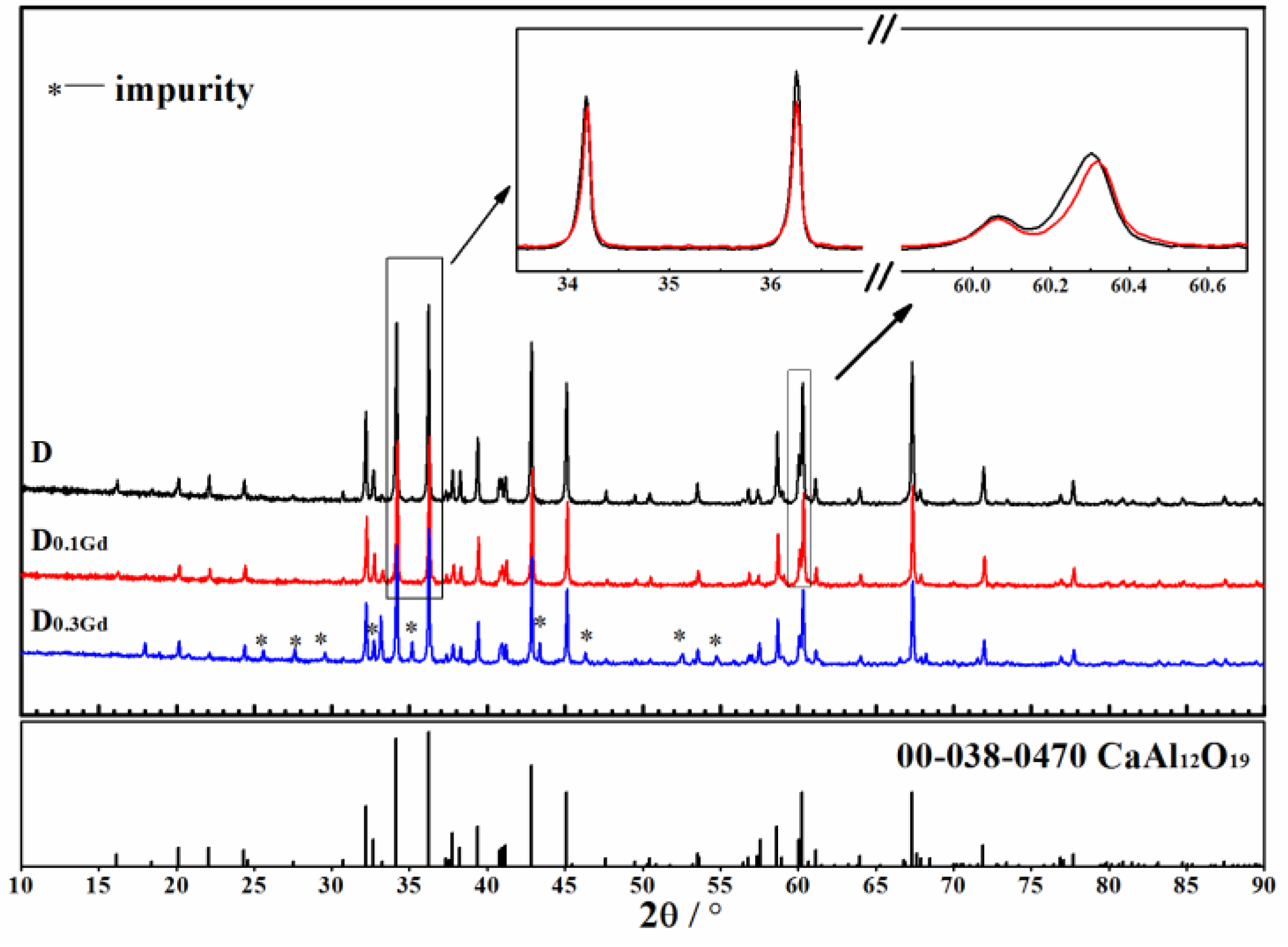
3.2. Phase Composition
3.3. Microstructure
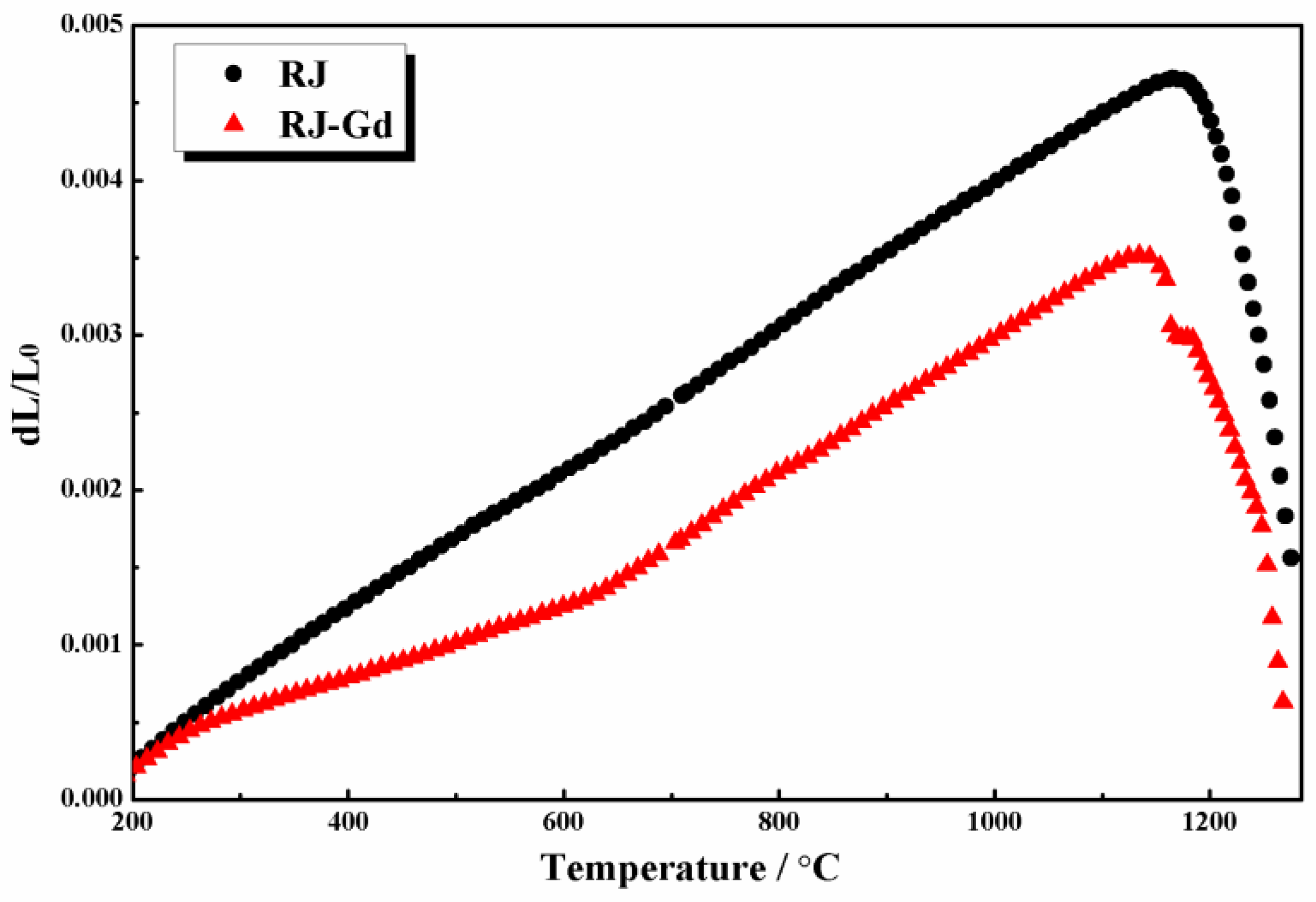
3.4. Effect of Gd2O3 on the Thermal Expansion of Glass Phase
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Moore, M.A. Abrasive wear. Int. J. Mater. Eng. Appl. 1978, 1, 97–111. [Google Scholar] [CrossRef]
- Medvedovski, E. Wear-resistant engineering ceramics. Wear 2001, 249, 821–828. [Google Scholar] [CrossRef]
- Ucar, V.; Ozel, A.; Mimaroglu, A.; Callı, I.; Gur, M. Influence of SiO2 and MnO2 additives on the dry friction and wear performance of Al2O3 ceramic. Mater. Des. 2001, 22, 171–175. [Google Scholar] [CrossRef]
- Esposito, L.; Tucci, A. Microstructural dependence of friction and wear behaviours in low purity alumina ceramics. Wear 1997, 205, 88–96. [Google Scholar] [CrossRef]
- Doğan, C.; Hawk, J. Role of composition and microstructure in the abrasive wear of high-alumina ceramics. Wear 1999, 225, 1050–1058. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, X.; Zhang, Y.; Dong, Y. Friction and Wear Behavior of Fine-grained Alumina Ceramic Under Articular Biomimetic Synovial Fluid Conditions. J. Chin. Ceram. Soc. 2013, 41, 890–894. [Google Scholar]
- Dimond, C. The specification and installation of alumina ceramics in industry. Mater. Des. 1987, 8, 152–155. [Google Scholar] [CrossRef]
- Goswami, A.P.; Roy, S.; Mitra, M.K.; Das, G.C. Influence of powder, chemistry and intergranular phases on the wear resistance of liquid-phase-sintered Al2O3. Wear 2000, 244, 1–14. [Google Scholar] [CrossRef]
- Zhou, H.; Zeng, G.; Liu, J.; Zhou, Y. Rare earth oxides La2O3/Y2O3-toughened & Reinforced ZTA ceramic and its abrasion resistance. Mater. Rev. 2016, 30, 14–17. [Google Scholar]
- Lee, D.G.; Lee, H.; Kim, P.; Bang, K. Micro-drilling of alumina green bodies with diamond grit abrasive micro-drills. Int. J. Mach. Tools Manuf. 2003, 43, 551–558. [Google Scholar] [CrossRef]
- Matsuzaki, H.; Wako, Y.; Watanabe, M. Studies on the characteristics of wear in micro-beads, and food-safety of fine particles caused by wear in a micro-bead mill. Powder Technol. 2013, 240, 74–78. [Google Scholar] [CrossRef]
- Davidge, R.; Twigg, P.; Riley, F. Effects of silicon carbide nano-phase on the wet erosive wear of polycrystalline alumina. J. Eur. Ceram. Soc. 1996, 16, 799–802. [Google Scholar] [CrossRef]
- Franco, A.; Roberts, S. Controlled wet erosive wear of polycrystalline alumina. J. Eur. Ceram. Soc. 1996, 16, 1365–1375. [Google Scholar] [CrossRef]
- Rainforth, W.M. The sliding wear of ceramics. Ceram. Int. 1996, 22, 365–372. [Google Scholar] [CrossRef]
- Badmos, A.Y.; Ivey, D.G. Characterization of structural alumina ceramics used in ballistic armour and wear applications. J. Mater. Sci. 2001, 36, 4995–5005. [Google Scholar] [CrossRef]
- Qiu, G.; Li, X.; Qiu, T.; Zhao, H.; Yu, H.; Ma, R. Application of rare earths in advanced ceramic materials. J. Rare Earths 2007, 25, 281–286. [Google Scholar]
- Fang, J.; Thompson, A.M.; Harmer, M.P.; Chan, H.M. Effect of Yttrium and Lanthanum on the Final-Stage Sintering Behavior of Ultrahigh-Purity Alumina. J. Am. Ceram. Soc. 1997, 80, 2005–2012. [Google Scholar] [CrossRef]
- Yao, Y.; Qiu, T.; Jiao, B.; Shen, C. The Effects of Rare Earth Oxide on the Properties of Alumina Ceramics. Vac. Electron. 2004, 4, 28–31. [Google Scholar]
- West, G.D.; Perkins, J.M.; Lewis, M.H. Characterisation of fine-grained oxide ceramics. J. Mater. Sci. 2004, 39, 6687–6704. [Google Scholar] [CrossRef]
- Yang, Q.; Zeng, Z.; Xu, J.; Zhang, H.; Ding, J. Effect of La2O3 on Microstructure and Transmittance of Transparent Alumina Ceramics. J. Rare Earths 2006, 24, 72–75. [Google Scholar] [CrossRef]
- Loudjani, M.; Huntz, A.; Cortes, R. Influence of yttrium on microstructure and point defects in α-Al2O3 in relation to oxidation. J. Mater. Sci. 1993, 28, 6466–6473. [Google Scholar] [CrossRef]
- Thompson, A.M.; Soni, K.K.; Chan, H.M.; Harmer, M.P.; Williams, D.B.; Chabala, J.M.; Levi-Setti, R. Dopant distributions in rare earth doped alumina. J. Am. Ceram. Soc. 1997, 80, 373–376. [Google Scholar] [CrossRef]
- Guo, H.; Dong, N.; Yin, M.; Zhang, W.; Lou, L.; Xia, S. Visible Upconversion in Rare Earth Ion-Doped Gd2O3 Nanocrystals. J. Phys. Chem. B 2004, 108, 19205–19209. [Google Scholar] [CrossRef]
- Luo, N.; Yang, C.; Tian, X.M.; Xiao, J.; Liu, J.; Chen, F.; Zhang, D.; Xu, D.; Zhang, Y.; Yang, G.W. A general top-down approach to synthesize rare earth doped-Gd2O3 nanocrystals as dualmodal contrast agents. J. Mater. Chem. B 2014, 2, 5891–5897. [Google Scholar] [CrossRef]
- Wang, H.; Huang, H.; Liang, J.; Liu, J. Preparation of ZrO2/Gd2O3 composite ceramic materials by coprecipitation method. Ceram. Int. 2014, 40, 3995–3999. [Google Scholar] [CrossRef]
- Wu, T.T.; Zhou, J.; Wu, B.L.; Liu, J.C. Effect of Rare-earth Lu2O3 on the Wear Resistance of Alumina Ceramics for Grinding Media. Powder Technol. 2016, 303, 27–32. [Google Scholar] [CrossRef]
- Wu, T.T.; Zhou, J.; Wu, B.L.; Li, W.J. Effect of La2O3 content on wear resistance of alumina ceramics. J. Rare Earths 2016, 34, 288–294. [Google Scholar] [CrossRef]
- Zhou, Y. Science of Ceramic, 2nd ed.; Science Press: Wuhan, China, 2004; pp. 444–457. ISBN 7-5629-1244-0. [Google Scholar]
- Kingery, W.D.; Bowen, H.K.; Uhlmann, D.R. Introduction to Ceramics; Higher Education Press: Beijing, China, 2010; pp. 118–140. ISBN 9787040256000. [Google Scholar]
- Salomão, R.; Ferreira, V.L.; Oliveira, I.R.D.; Souza, A.D.V.; Correr, W.R. Mechanism of pore generation in calcium hexaluminate (CA6) ceramics formed in situ from calcined alumina and calcium carbonate aggregates. J. Eur. Ceram. Soc. 2016, 36, 4225–4235. [Google Scholar] [CrossRef]
- Altay, A.; Carter, C.B.; Rulis, P.; Ching, W.Y.; Arslan, I.; Gülgün, M.A. Characterizing CA2 and CA6 using ELNES. J Solid State Chem. 2010, 183, 1776–1784. [Google Scholar] [CrossRef]
- Coble, R.L. Sintering Crystalline Solids. I. Intermediate and Final State Diffusion Models. J. Appl. Phys. 1961, 32, 787–792. [Google Scholar] [CrossRef]
- Pillai, S.K.C.; Baron, B.; Pomeroy, M.J.; Hampshire, S. Effect of oxide dopants on densification, microstructure and mechanical properties of alumina-silicon carbide nanocomposite ceramics prepared by pressureless sintering. J. Eur. Ceram. Soc. 2004, 24, 3317–3326. [Google Scholar] [CrossRef]
- Bae, S.I.; Baik, S. Determination of Critical Concentrations of Silica and/or Calcia for Abnormal Grain Growth in Alumina. J. Am. Ceram. Soc. 1993, 76, 1065–1067. [Google Scholar] [CrossRef]
- Guo, J.K.; Kou, H.M.; Li, J. Study on High Temperature Structural Ceramics; Science Press: Beijing, China, 2011; pp. 47–50, 72–88. ISBN 9787030307347. [Google Scholar]
- Deng, Y.C. Effects of Adding Eu3+, La3+ on the Structures and Properties of Alumina Ceramics; Suzhou University: Suzhou, China, 2009. [Google Scholar]
- Koyama, T.; Nishiyama, A.; Niihara, K. Effect of grain morphology and grain size on the mechanical properties of Al2O3 ceramics. J. Mater. Sci. 1994, 29, 3949–3954. [Google Scholar] [CrossRef]
- Dogan, C.; Hawk, J. Effect of grain boundary glass composition and devitrification on the abrasive wear of Al2O3. Wear 1995, 181, 129–137. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Wang, J.; Sun, B. Synthesis, Properties and Application of Calcium Hexaluminat. Bull. Chin. Ceram. Soc. 2009, 28, 201–205. [Google Scholar]
- Evans, A.; Marshall, D. Wear mechanisms in ceramics. In Proc. of Int. Conf. on Fundaments of Friction and Wear of Materials; AMSE: Pittsburgh, PA, USA, 1980; pp. 439–452. [Google Scholar]
- Kong, Y.; Gong, J. Friction and Sliding Wear of Structural Ceramics. Bull. Chin. Ceram. Soc. 1988, 17, 32–38. [Google Scholar]
- West, G.; Perkins, J.; Lewis, M. The effect of rare earth dopants on grain boundary cohesion in alumina. J. Eur. Ceram. Soc. 2007, 27, 1913–1918. [Google Scholar] [CrossRef]
- Ikuhara, Y.; Yoshida, H.; Sakuma, T. Impurity effects on grain boundary strength in structural ceramics. Mater. Sci. Eng. A 2001, 319, 24–30. [Google Scholar] [CrossRef]
- Meng, F.; Fu, Z.; Wang, W.; Zhang, Q. Microstructural evolution of nanocrystalline Al2O3 sintered at a high heating rate. Ceram. Int. 2010, 36, 555–559. [Google Scholar] [CrossRef]
- Powell-Doǧan, C.A.; Heuer, A.H. Microstructure of 96% Alumina Ceramics: III, Characterization of High-Calcia Boundary Glasses. J. Am. Ceram. Soc. 1990, 73, 3684–3691. [Google Scholar] [CrossRef]




| No. | Chemical Formula | x | y |
|---|---|---|---|
| D | CaAl12O19 | - | - |
| D0.1Gd | (Gd0.1,Ca0.9)Al11.97O19 | 0.1 | 11.97 |
| D0.3Gd | (Gd0.3,Ca0.7)Al11.9O19 | 0.3 | 11.9 |
| No. | Stoichiometric Formula | Lattice Parameters | Lattice Volume/V | |
|---|---|---|---|---|
| a = b | c | |||
| D | CaAl12O19 | 5.55272 | 21.88913 | 584.48 |
| D0.1Gd | (Gd0.1,Ca0.9)Al11.97O19 | 5.5533 | 21.88022 | 584.37 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.; Su, J.; Li, Y.; Zhao, H.; Zhang, Y.; Zhang, M.; Wu, B. Wear Resistance Mechanism of Alumina Ceramics Containing Gd2O3. Materials 2018, 11, 2054. https://doi.org/10.3390/ma11102054
Wu T, Su J, Li Y, Zhao H, Zhang Y, Zhang M, Wu B. Wear Resistance Mechanism of Alumina Ceramics Containing Gd2O3. Materials. 2018; 11(10):2054. https://doi.org/10.3390/ma11102054
Chicago/Turabian StyleWu, Tingting, Jianxiu Su, Yongfeng Li, Hongyuan Zhao, Yaqi Zhang, Mingming Zhang, and Bolin Wu. 2018. "Wear Resistance Mechanism of Alumina Ceramics Containing Gd2O3" Materials 11, no. 10: 2054. https://doi.org/10.3390/ma11102054
APA StyleWu, T., Su, J., Li, Y., Zhao, H., Zhang, Y., Zhang, M., & Wu, B. (2018). Wear Resistance Mechanism of Alumina Ceramics Containing Gd2O3. Materials, 11(10), 2054. https://doi.org/10.3390/ma11102054






