Characterization and Interpretation of the Aluminum Zone Refining through Infrared Thermographic Analysis
Abstract
1. Introduction
2. Experimental Procedure
2.1. Experimental Design
2.2. Data Analysis
2.2.1. Thermographic Data Analysis
2.2.2. Impurity Concentration Analysis
3. Results and Discussion
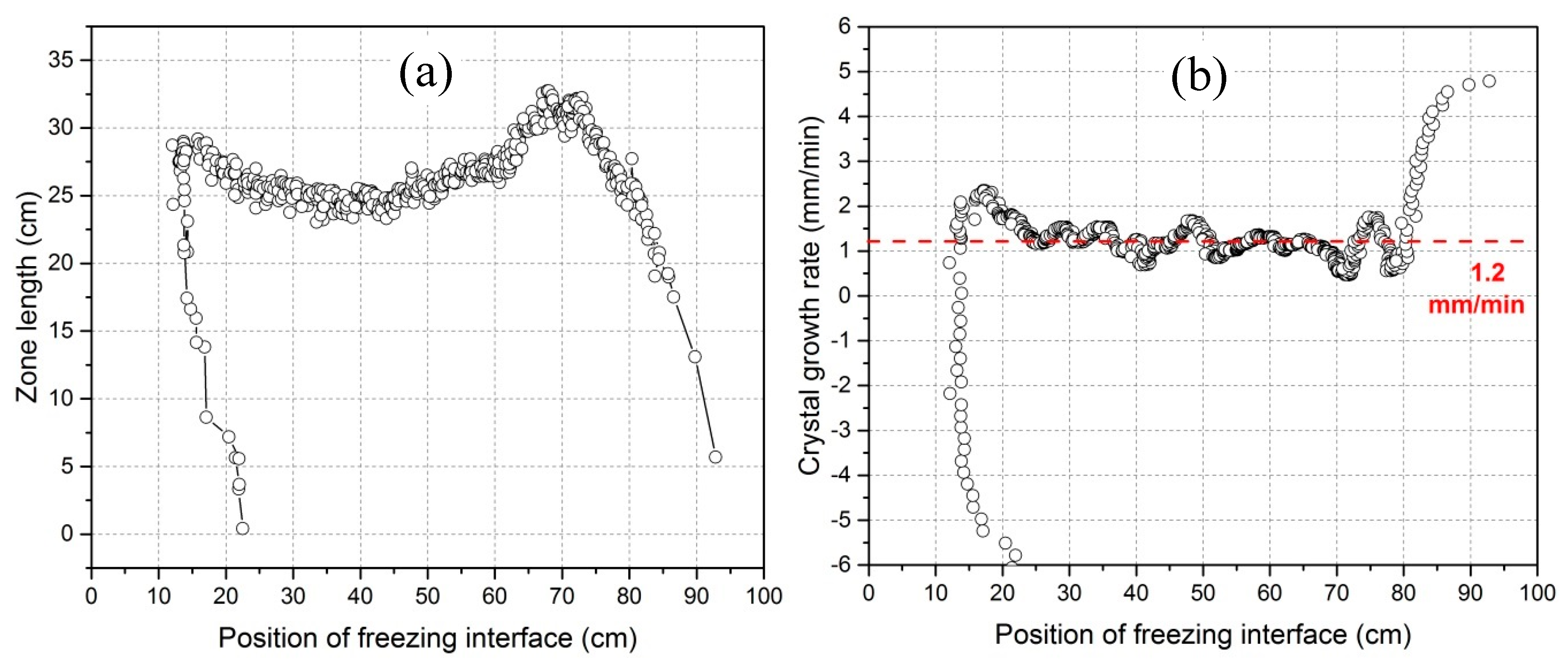
3.1. Characterization of the Zone Refining Process
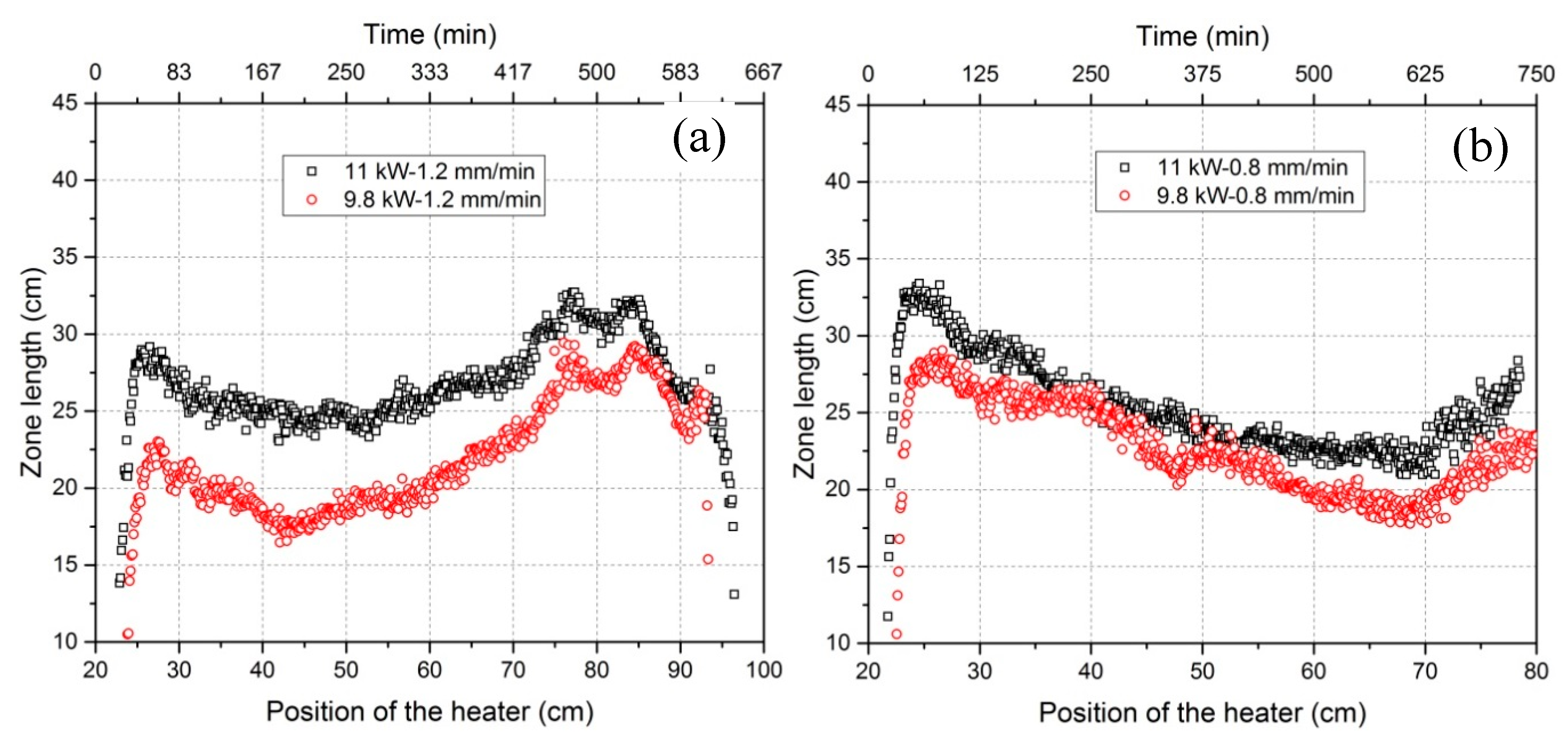
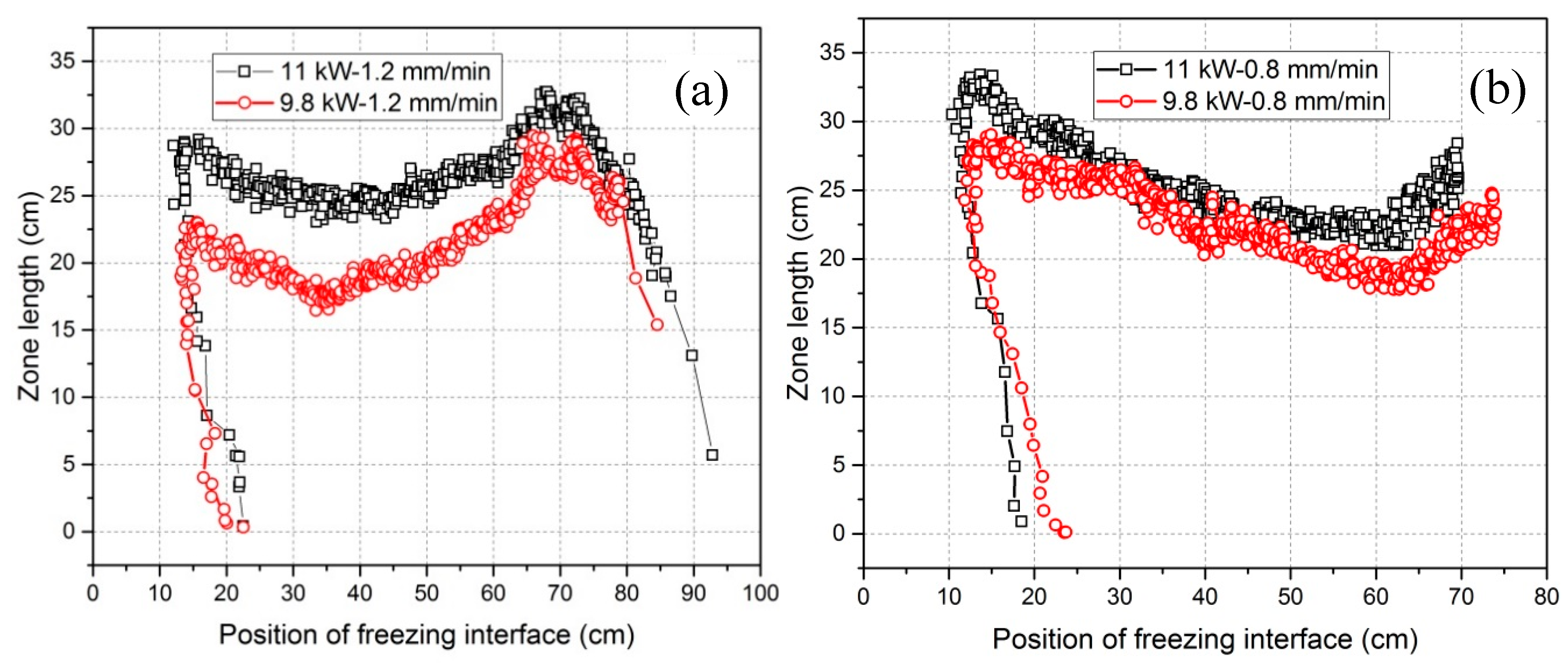
3.2. Dependency of the Zone Length on Power and Heater Movement Velocity
3.3. Crystal Growth Rate Variation
4. Conclusions and Outlook
- The application of an IR camera is a good way to control and characterize the zone refining process, which can derive the current position of the molten zone, the size of zone length and the actual crystal growth rate online.
- Zone length varies with the tendency to firstly decrease and then increase for all power and heater movement velocity combinations (see Figure 9). The percentage difference of zone length between maximum and minimum can be depressed by increasing the power. Lowering the heater movement velocity delays the freezing interface position where the zone length becomes minimal.
- Effective refinement (50% to 75% Fe/Si-reduction) of aluminum happens for all experiments with only one zone pass, but higher power and lower velocity result in the highest refining efficiency. However, the impurity concentration distribution profile after zone refining fluctuates along the bar in each case.
- Crystal growth rate is not equal to the heater movement velocity and instead, fluctuates around the heater movement velocity. The fluctuations of the impurity concentration profile after refinement mainly results from the variation of crystal growth rate.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Cheung, N.; Bertazzoli, R.; Garcia, A. Experimental impurity segregation and numerical analysis based on variable solute distribution coefficients during multi-pass zone refining of aluminum. J. Cryst. Growth 2008, 310, 1274–1280. [Google Scholar] [CrossRef]
- Hashimoto, E.; Ueda, Y.; Kino, T.; Hashimoto, E.; Ueda, Y.; Purification, T.K.; Purity, U.; Journal, A. Purification of Ultra-High Purity Aluminum. J. Phys. IV Colloq. 1995, 5, 153–157. [Google Scholar] [CrossRef]
- Nakamura, M.; Watanabe, M.; Tanaka, K.; Kirihata, A.; Sumomogi, T.; Hiroaki, H.; Tanaka, I. Zone Refining of Aluminum and its Simulation. Mater. Trans. 2014, 55, 664–670. [Google Scholar] [CrossRef]
- Yang, G.; Govani, J.; Mei, H.; Guan, Y.; Wang, G.; Huang, M.; Mei, D. Investigation of influential factors on the purification of zone-refined germanium ingot. Cryst. Res. Technol. 2014, 49, 269–275. [Google Scholar] [CrossRef]
- Ghosh, K.; Mani, V.N.; Dhar, S. A modeling approach for the purification of group III metals (Ga and In) by zone refining. J. Appl. Phys. 2008, 104, 024904. [Google Scholar] [CrossRef]
- Spim, J.A.; Bernadou, M.J.S.; Garcia, A. Numerical modeling and optimization of zone refining. J. Alloys Compd. 2000, 298, 299–305. [Google Scholar] [CrossRef]
- Burris, L., Jr.; Stockman, C.H.; Dillon, I.G. Contribution to mathematics of zone refining. J. Miner. Met. Mater. Soc. 1955, 7, 1017–1023. [Google Scholar] [CrossRef]
- Pfann, W.G. Zone Melting, 2nd ed.; Wiley: New York, NY, USA, 1966. [Google Scholar]
- Ho, C.; Yeh, H.; Yeh, T. Numerical analysis on optimal zone lengths for each pass in multipass zone-refining processes. Can. J. Chem. Eng. 1998, 76, 3–9. [Google Scholar] [CrossRef]
- Rodway, G.H.; Hunt, J.D. Optimizing zone refining. J. Cryst. Growth 1989, 97, 680–688. [Google Scholar] [CrossRef]
- Chang, C.E.; Wilcox, W.R. Heat transfer in vertical zone melting of poor thermal conductors. J. Cryst. Growth 1975, 28, 288–294. [Google Scholar] [CrossRef]
- Kobayashi, N. Power required to form a floating zone and the zone shape. J. Cryst. Growth 1978, 43, 417–424. [Google Scholar] [CrossRef]
- Roussopoulos, G.S.; Rubini, P.A. A thermal analysis of the horizontal zone refining of indium antimonide. J. Cryst. Growth 2004, 271, 333–340. [Google Scholar] [CrossRef]
- Wang, J.H.; Kim, D.H. Numerical analysis of melt/solid interface shape in zone melting recrystallization process. J. Cryst. Growth 1997, 173, 201–209. [Google Scholar] [CrossRef]
- Louchev, O.A. The influence of natural convection on the formation of a molten zone under optical heating. J. Cryst. Growth 1993, 133, 261–266. [Google Scholar] [CrossRef]
- Munirathnam, N.R.; Prasad, D.S.; Sudheer, C.H.; Rao, J.V.; Prakash, T.L. Zone refining of cadmium and related characterization. Bull. Mater. Sci. 2005, 28, 209–212. [Google Scholar] [CrossRef]
- Curtolo, D.; Zhang, X.; Rojas, M.J.R.; Friedrich, S.; Friedrich, B. Realization of the measurement and control of zone length via implementation of an infrared camera during a zone melting refining process. Appl. Sci 2018, 8, 875. [Google Scholar] [CrossRef]
- Zhang, X.; Friedrich, S.; Friedrich, B. Investigation of Influencing Parameters on Zone Melting Refining of Aluminium, Part One Impu-rities: Iron and Silicon. Proc. EMC 2017, 1, 327–334. [Google Scholar]
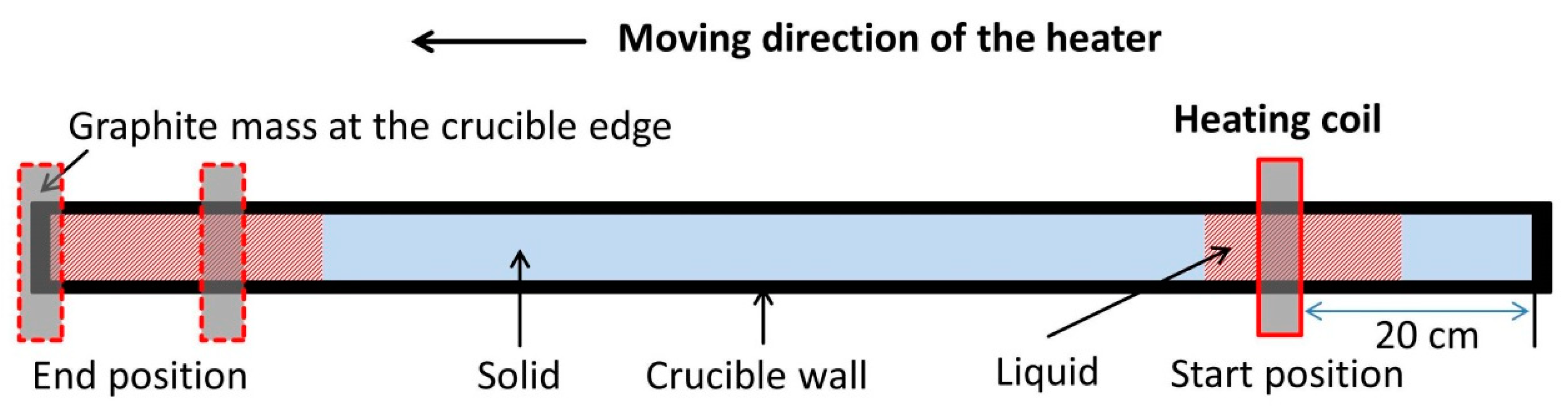

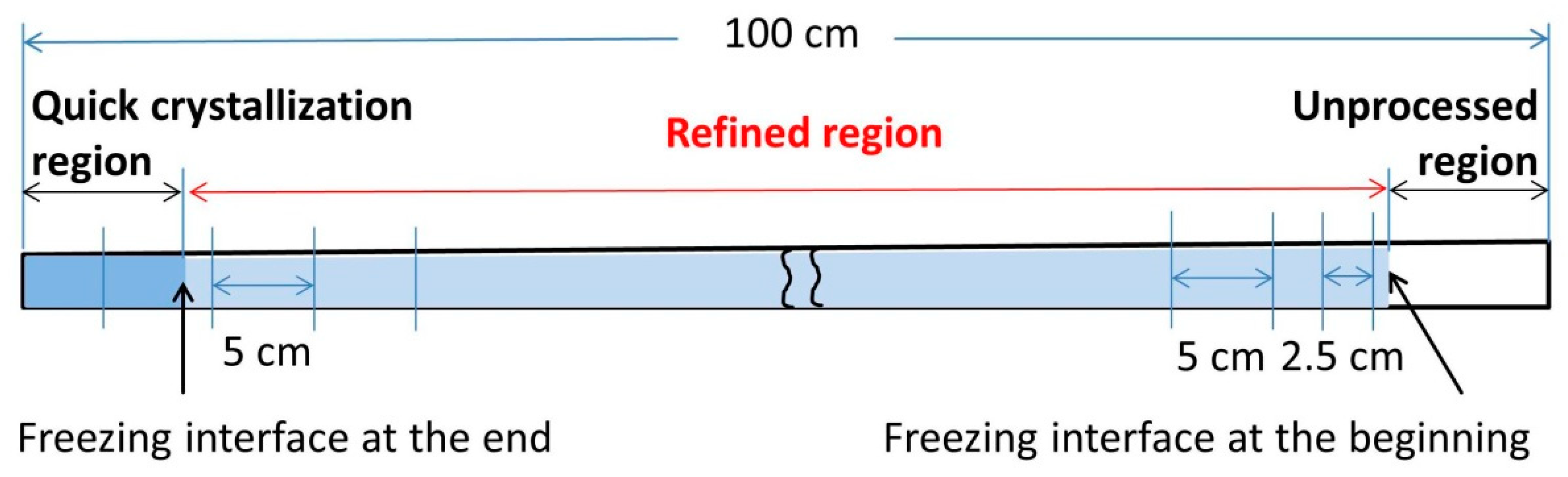
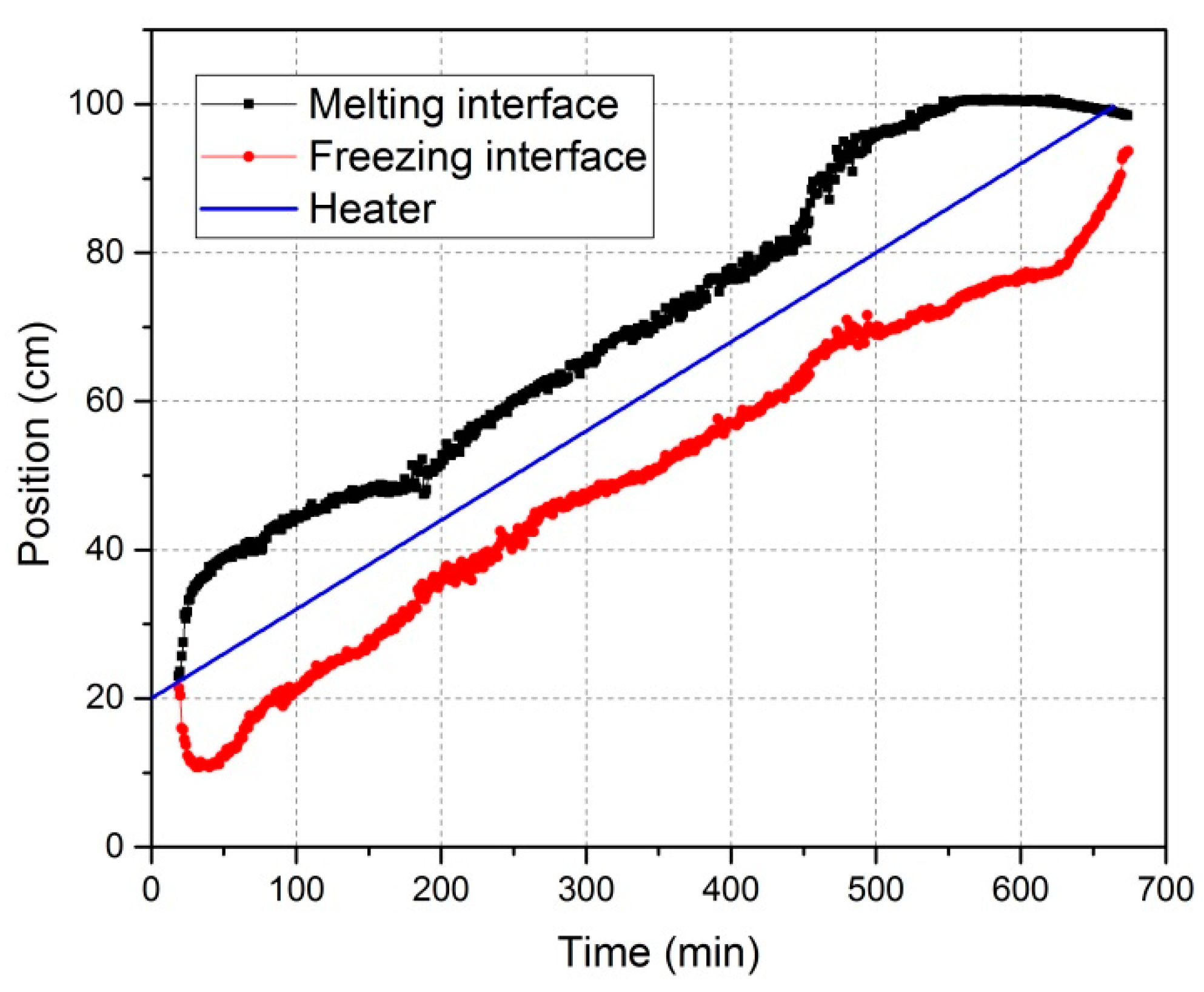

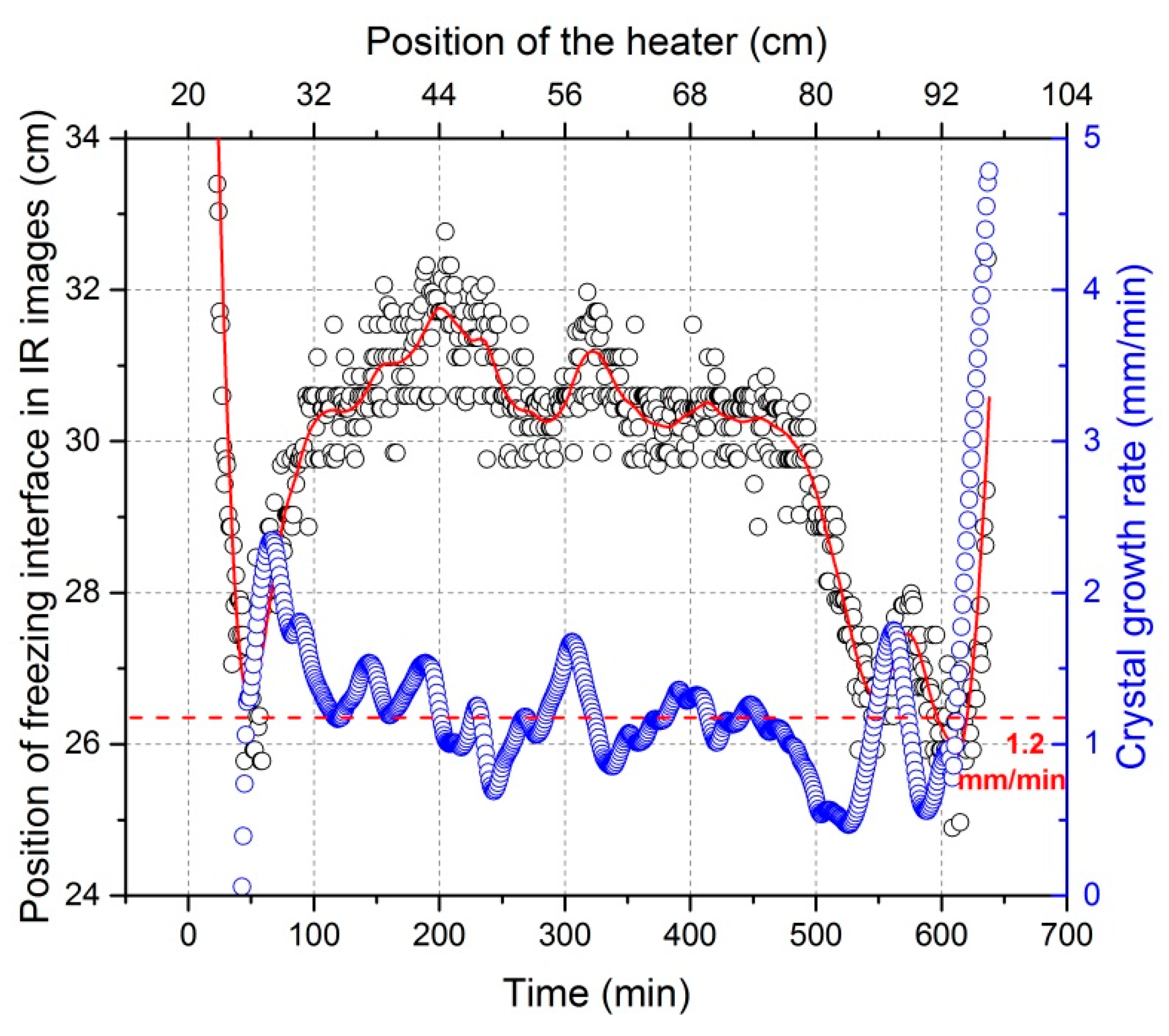


| Power | Heater Movement Velocity | |
|---|---|---|
| 11 kW | 1.2 mm/min | 0.8 mm/min |
| 9.8 kW | 1.2 mm/min | 0.8 mm/min |
| Characteristic Values | 11 kW-1.2 mm/min | 9.8 kW-1.2 mm/min | 11 kW-0.8 mm/min | 9.8 kW-0.8 mm/min |
|---|---|---|---|---|
| Peak of zone length at the beginning (Lp1) | 28 cm | 22 cm | 32 cm | 28 cm |
| Peak of zone length at the end (Lp2) | 32 cm | 28 cm | 26 cm | 23 cm |
| Minimum zone length (Lmin) | 24 cm | 18 cm | 22 cm | 19 cm |
| 1-(Lmin/Lp1) | 14.3% | 18.2% | 31.2% | 32.1% |
| 1-(Lmin/Lp2) | 25.0% | 35.7% | 15.4% | 17.4% |
| Position of freezing interface for Lmin | 24 cm | 21 cm | 49 cm | 50 cm |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Friedrich, S.; Friedrich, B. Characterization and Interpretation of the Aluminum Zone Refining through Infrared Thermographic Analysis. Materials 2018, 11, 2039. https://doi.org/10.3390/ma11102039
Zhang X, Friedrich S, Friedrich B. Characterization and Interpretation of the Aluminum Zone Refining through Infrared Thermographic Analysis. Materials. 2018; 11(10):2039. https://doi.org/10.3390/ma11102039
Chicago/Turabian StyleZhang, Xiaoxin, Semiramis Friedrich, and Bernd Friedrich. 2018. "Characterization and Interpretation of the Aluminum Zone Refining through Infrared Thermographic Analysis" Materials 11, no. 10: 2039. https://doi.org/10.3390/ma11102039
APA StyleZhang, X., Friedrich, S., & Friedrich, B. (2018). Characterization and Interpretation of the Aluminum Zone Refining through Infrared Thermographic Analysis. Materials, 11(10), 2039. https://doi.org/10.3390/ma11102039






