The Influence of Mayenite Employed as a Functional Component on Hydration Properties of Ordinary Portland Cement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Preparing Methods
2.2. Instruments
2.3. Hydration Kinetic Model
3. Results
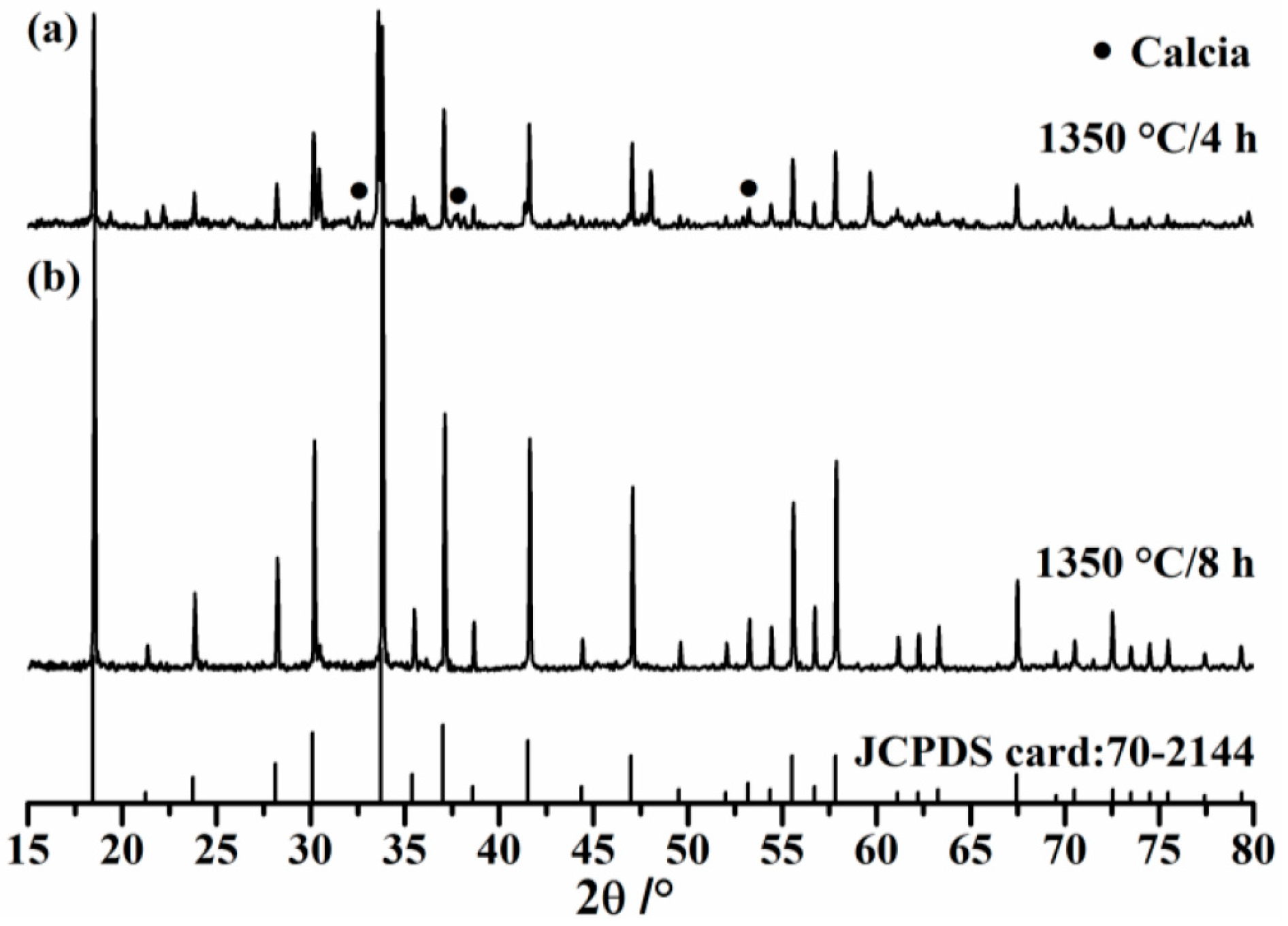
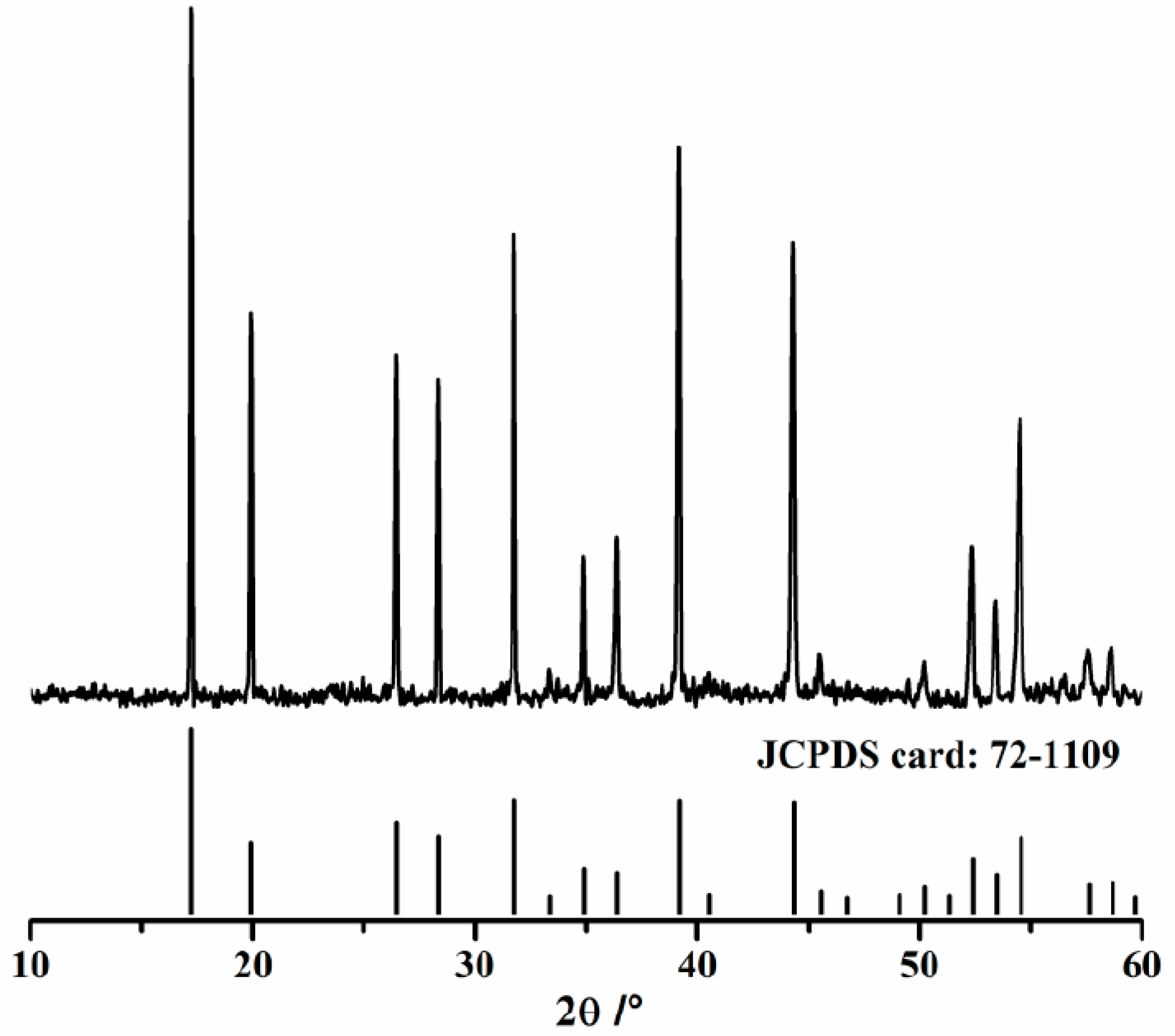
3.1. Hydration Properties of Pure C12A7
3.2. Effect of C12A7 on Heat-Releasing Behavior
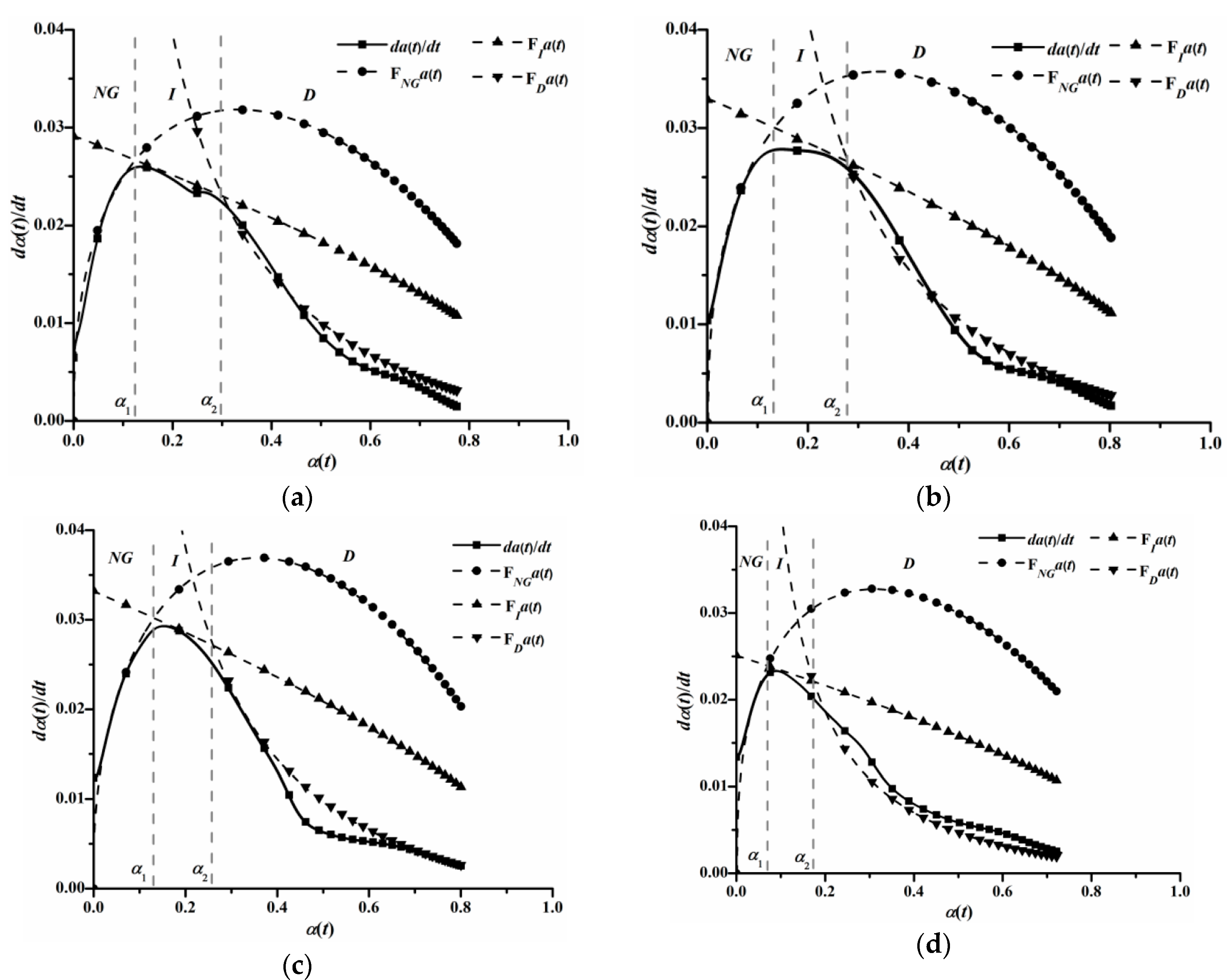
3.3. Effect of C12A7 on Hydration Kinetic Processes
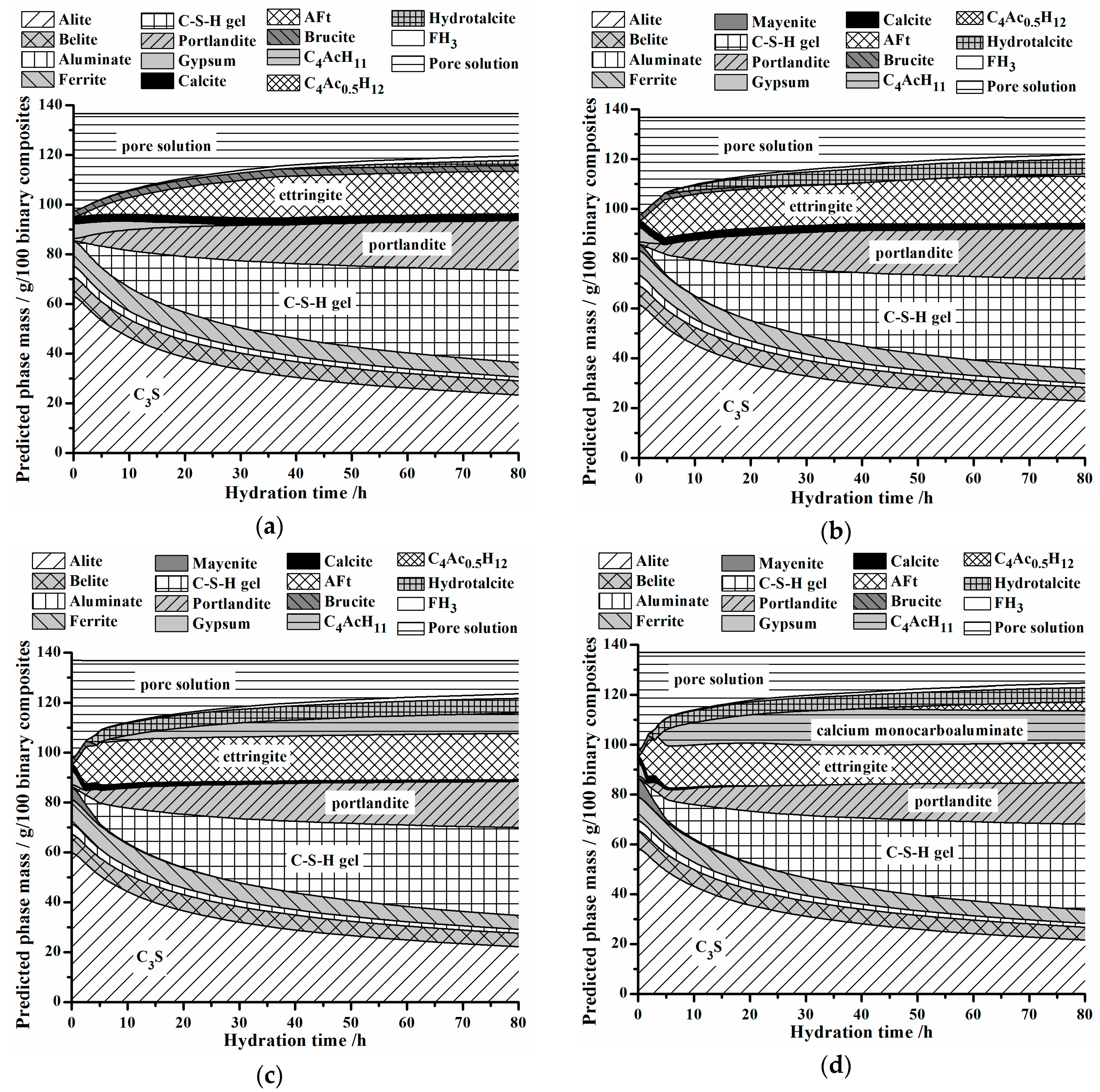
3.4. Effect of C12A7 on Hydrate Assemblages
3.5. Effect of C12A7 on Mechanical Strehgthes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mohamed, B.M.; Sharp, J.H. Kinetics and mechanism of formation of tricalcium aluminate, Ca3Al2O6. Thermochim. Acta 2002, 388, 105–114. [Google Scholar] [CrossRef]
- Nishio, Y.; Nomura, K.; Miyakawa, M.; Hayashi, K.; Yanagi, H.; Kamiya, T.; Hosono, H. Fabrication and transport properties of 12CaO·7Al2O3 (C12A7) electride nanowire. Phys. Status Solidi A Appl. Res. 2008, 205, 2047–2051. [Google Scholar] [CrossRef]
- Tong, Z.F.; Xie, S.L.; Zhang, L.H.; Chen, T.; Liu, W. Preparation and application of the 12CaO·7Al2O3 material. Nonferr. Met. Sci. Eng. 2011, 2, 16–21. [Google Scholar]
- Hirao, H.; Yokoyama, S. Behavior of chloride ions in hardened eco-cement: A new type portland cement made from municipal waste incinerator ash. Concr. Res. Technol. 2003, 13, 129–138. [Google Scholar] [CrossRef]
- Xie, Y.G.; Sheng, X.; Wang, Y.C.; Li, L.H. Study on sulfate treatment in water by mayenite. China Water Waste Water 2008, 24, 92–94. [Google Scholar]
- Wang, Q.; Yang, J.W.; Yan, P.Y. Influence of initial alkalinity on the hydration of steel slag. Sci. China Technol. Sci. 2012, 55, 3378–3387. [Google Scholar] [CrossRef]
- Han, F.H.; Wang, D.M.; Yan, P.Y. Hydration kinetics of composite binder containing different content of slag or fly ash. J. China Ceram. Soc. 2014, 42, 613–620. [Google Scholar] [CrossRef]
- Edmonds, R.N.; Majumdar, A.J. The hydration of 12CaO.7Al2O3 at different temperatures. Cem. Concr. Res. 1988, 18, 473–478. [Google Scholar] [CrossRef]
- Damidot, D.; Rettel, A. Effect of gypsum on CA and C12A7 Hydration at room temperature. In Proceedings of the 11th International Congress on the Chemistry of Cement (ICCC), Durban, South Africa, 11–16 May 2003. [Google Scholar]
- Park, C.K. Characteristics and hydration of C12−xA7·x(CaF2) (x = 0~1.5) minerals. Cem. Concr. Res. 1998, 28, 1357–1362. [Google Scholar] [CrossRef]
- Park, H.G.; Sung, S.K.; Park, C.G.; Won, J.P. Influence of a C12A7 mineral-based accelerator on the strength and durability of shotcrete. Cem. Concr. Res. 2008, 38, 379–385. [Google Scholar] [CrossRef]
- Won, J.P.; Hwang, U.J.; Kim, C.K.; Lee, S.J. Mechanical performance of shotcrete made with a high-strength cement-based mineral accelerator. Constr. Build. Mater. 2013, 49, 175–183. [Google Scholar] [CrossRef]
- Won, J.P.; Hwang, U.J.; Lee, S.J. Enhanced long-term strength and durability of shotcrete with high-strength C12A7 mineral-based accelerator. Cem. Concr. Res. 2015, 76, 121–129. [Google Scholar] [CrossRef]
- Rovnanik, P. Influence of C12A7 admixture on setting properties of fly ash geopolymer. Ceram. Silikaty 2011, 54, 362–367. [Google Scholar]
- Taylor, H.F.W. Portland cement and its major constituent phase. In Cement Chemistry, 2nd ed.; Thomas Telford Services Ltd.: London, UK, 1997; p. 77. [Google Scholar]
- Castel, E.; Ik Shin, T.; Fourcade, S.; Decourt, R.; Maglione, M.; García-Víllora, E.; Shimamura, K. Effect of annealing under O2 and H2 on the piezoelectric parameters of the Ca12Al14O33 single crystals. J. Appl. Phys. 2012, 111, 054107. [Google Scholar] [CrossRef]
- GB/T 17671-1999. The Quality and Technology Supervision Bureau. Method of Testing Cements—Determination of Strength; China Standard Press: Beijing, China, 2014. [Google Scholar]
- GB/T 175-2007. The Quality and Technology Supervision Bureau. Common Portland Cement; China Standard Press: Beijing, China, 2015. [Google Scholar]
- Dong, J.H.; Li, Z.Y. Effect of Temperature on Heat Release Behavior of Hydration of Cement. J. Build. Mat. 2010, 13, 676–677. [Google Scholar] [CrossRef]
- Hewlett, P.C. The constitution and specification of portland cement. In Lea's Chemistry of Cement and Concrete, 4th ed.; Butterworth-Heinemann: Oxford, UK, 2004; p. 324. [Google Scholar]
- Krstulović, R.; Dabić, P. A conceptual model of the cement hydration process. Cem. Concr. Res. 2000, 30, 693–698. [Google Scholar] [CrossRef]
- Yan, P.; Zheng, F. Kinetics model for the hydration mechanism of cementitious materials. J. Ceram. Soc. 2006, 34, 555–559. [Google Scholar]
- He, Z.; Yang, H.; Hu, S.; Liu, M. Hydration mechanism of silica fume-sulphoaluminate cement. J. Wuhan Univer. Technol. Mater. Sci. Ed. 2013, 28, 1128–1133. [Google Scholar] [CrossRef]
- He, Z.; Yang, H.M.; Liu, M.Y. Hydration Mechanism of Sulphoaluminate Cement. J. Wuhan Univer. Technol. Mater. Sci. Ed. 2014, 29, 70–74. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, J.; Liu, J.; Li, C. Hydration kinetics process of low alkalinity sulphoaluminate cement and its thermodynamical properties. Procedia Eng. 2012, 27, 323–331. [Google Scholar] [CrossRef]
- Tian, Y.; Jin, X.Y.; Jin, N.G. Micro expressions of cement hydration kinetics. Rare Met. Mater. Eng. 2010, 39, 216–219. [Google Scholar]
- Damidot, D.; Sorrentino, F. Modification of the hydration process from C12A7 to C3A at 20 °C. In Proceedings of the 10th International Congress on the Chemistry of Cement (ICCC), Goteborg, Sweden, 2–6 June 1997. [Google Scholar]
- Kirby, D.M.; Biernacki, J.J. The Effect of water-to-cement ratio on the hydration kinetics of tricalcium silicate cements: Testing the two-step hydration hypothesis. Cem. Concr. Res. 2012, 42, 1147–1156. [Google Scholar] [CrossRef]
- Das, S.K.; Mitra, A.; Das, P.P.K. Thermal analysis of hydrated calcium aluminates. J. Therm. Anal. Calorim. 1996, 47, 765–774. [Google Scholar] [CrossRef]
- Thoenen, T.; Kulik, D. Nagra/PSI chemical thermodynamic database 01/01 for the GEM-Selektor (V.2-PSI) Geochemical Modeling Code, PSI. Available online: http://les.web.psi.ch/Software/GEMS-PSI/doc/pdf/TM-44-03-04-web.pdf. 2003. (accessed on 10 September 2018).
- Matschei, T.; Lothenbach, B.; Glasser, F.P. Thermodynamic properties of portland cement hydrates in the system CaO-Al2O3-SiO2-CaSO4-CaCO3-H2O. Cem. Concr. Res. 1997, 37, 1397–1410. [Google Scholar] [CrossRef]
- Lothenbach, B.; Matschei, T.; Möschner, G.; Glasser, F.P. Thermodynamic modelling of the effect of temperature on the hydration and porosity of portland cement. Cem. Concr. Res. 2008, 38, 1–18. [Google Scholar] [CrossRef]
- Han, F.H.; Zhang, Z.Q.; Yan, P.Y. Early hydration properties of steel slag under high alkalinity. J. Chin. Electron Micros. Soc. 2012, 33, 343–348. [Google Scholar]
- Lothenbach, B.; Winnefeld, F. Thermodynamic modelling of the hydration of portland cement. Cem. Concr. Res. 2006, 36, 209–226. [Google Scholar] [CrossRef]
- Schmidt, T.; Lothenbach, B.; Romer, M.; Scrivener, K. A thermodynamic and experimental study of the conditions of thaumasite formation. Cem. Concr. Res. 2008, 38, 337–349. [Google Scholar] [CrossRef]
- Paul, S.C.; van Rooyen, A.S.; van Zijl, G.P. Properties of cement-based composites using nanoparticles: A comprehensive review. Constr. Build. Mater. 2018, 189, 1019–1034. [Google Scholar] [CrossRef]
- He, Z.; Cao, G.X.; Li, Y.; Liu, L.L. Influence of potassium-sodium ratio on the early age cracking sensitivity of cementitious materials with high alkalinity. J. Hydraul. Eng. 2016, 47, 1–9. [Google Scholar] [CrossRef]
- Paul, S.C.; Šavija, B.; Babafemi, A.J. A comprehensive review on mechanical and durability properties of cement-based materials containing waste recycle glass. J. Clean. Prod. 2018, 198, 891–906. [Google Scholar] [CrossRef]





| Chemical Composition | Normative Phase Composition | ||||
|---|---|---|---|---|---|
| /g | /mmol | /g | /mmol | ||
| CaO | 63.525 | 1132.7 | alite | 62.9 | 275.34 |
| SiO2 | 19.25 | 320.41 | belite | 7.8 | 45.37 |
| Al2O3 | 3.82 | 37.47 | aluminate | 4.4 | 16.31 |
| Fe2O3 | 3.371 | 21.11 | ferrite | 10.2 | 21.09 |
| MgO | 2.76 | 68.49 | free-CaO | 0.875 | 15.603 |
| K2O | 0.746 | 7.92 | CaCO3 | 2.809 | 28.062 |
| Na2O | 0.12 | 1.94 | CaSO4 d | 5.396 | 39.636 |
| CO2 | 1.235 | 28.06 | K2SO4 s | 0.2898 | 1.66 |
| TiO2 | 0.201 | 2.52 | TiO2 | 0.201 | 2.517 |
| P2O5 | 0.11 | 0.77 | P2O5 | 0.11 | 0.775 |
| SrO | 0.207 | 2.00 | SrO | 0.207 | 1.998 |
| MnO | 0.103 | 1.45 | MnO | 0.103 | 1.452 |
| SO3 | 3.45 | 49.09 | Na2SO4 s | 0.3351 | 0.81 |
| free-CaO | 0.875 | 10.16 | K2O b | 0.157 | 1.663 |
| readily soluble alkalis c | Na2O b | 0.0504 | 0.813 | ||
| K2O | 0.589 | 5.785 | MgO b | 1.84 | 45.7 |
| Na2O | 0.07 | 0.984 | SO3 b | 0.078 | 0.98 |
| Mineral Phases | Elements /(mmol/100 g) | |||
|---|---|---|---|---|
| Na | K | Mg | S | |
| Alite | 0.48 | 0.08 | 27.1 | 1.06 |
| Belite | 0.17 | 2.9 | 3.2 | 0.38 |
| Aluminate | 0.52 | 1.3 | 4.9 | — |
| Ferrite | 0.07 | 0.4 | 10.6 | — |
| Compounds | △Hf° /(kJ·mol−1) | △Gf° /(kJ·mol−1) | S /(J·mol−1·K−1) | Cp /(J·mol−1·K−1) |
|---|---|---|---|---|
| Ca12Al14O33 (s) | −19,414.43 G | −18,451.44 G | 1044.74 G | 1084.83 G |
| H2O (l) | −286 M | −237.2 M | 70 M | 75 M |
| Ca3Al2O6·6H2O (s) | −5537.25 G | −5008.15 G | 421.7 G | 445.60 G |
| Al(OH)3 (am.) | −1289 L | −1151.0 L | 70 L | 36 L |
| Ca(OH)2 (s) | −985 L | −897.01 L | 83.4 L | 187.51 L |
| CaAl2O4·10H2O (s) | −5320 M | −4622.4 M | 501 M | 151 M |
| Ca2Al2O5·8H2O (s) | −5433 L | −4812.8 L | 440 L | 392 L |
| Reaction | Enthalpy /(kJ·mol−1) | Gibbs Free Energy/(kJ·mol−1) | ||||
|---|---|---|---|---|---|---|
| Reactants | Products | Differences | Reactants | Products | Difference | |
| (5) | −34,000.43 | −35,176 | −1175.57 | −30,548.64 | −31,178.8 | −630.16 |
| (7) | −36,574.43 | −37,805 | −1230.57 | −32,683.44 | −3308.8 | −625.36 |
| (8) | −16,299 | −16,226.5 | 72.5 | −14,438.4 | −14,453.1 | −14.7 |
| (9) | −15,960 | −15,841.25 | 118.75 | −13,867.2 | −13,881.75 | −14.55 |
| (11) | −28,852.43 | −29,883 | −1030.57 | −26,279.04 | −26,938.6 | −659.56 |
| Sample | NG | I | D | ||||
|---|---|---|---|---|---|---|---|
| n | KNG/(μm2·h−1) | sd | KI/(μm2·h−1) | sd | KD/(μm2·h−1) | sd | |
| M0 | 1.67523 | 0.040978 | 0.00237 | 0.009704 | 0.00528 | 0.002187 | 0.00326 |
| M2 | 1.715074 | 0.045435 | 0.0027 | 0.010967 | 0.00484 | 0.002263 | 0.00191 |
| M5 | 1.792231 | 0.046102 | 0.00252 | 0.011083 | 0.00457 | 0.002136 | 0.00238 |
| M7 | 1.591035 | 0.043168 | 0.00186 | 0.008372 | 0.00367 | 0.001025 | 0.002 |
| Sample | Hydration Degree | Duration of Each Hydration Stage | |||
|---|---|---|---|---|---|
| α1 | α2 | NG | I | D | |
| M0 | 0.1258 | 0.2997 | 0.1258 | 0.1739 | 0.7003 |
| M2 | 0.1328 | 0.2775 | 0.1328 | 0.1447 | 0.7225 |
| M5 | 0.1296 | 0.2617 | 0.1296 | 0.1321 | 0.7383 |
| M7 | 0.0750 | 0.1680 | 0.0750 | 0.0930 | 0.8320 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Li, Y. The Influence of Mayenite Employed as a Functional Component on Hydration Properties of Ordinary Portland Cement. Materials 2018, 11, 1958. https://doi.org/10.3390/ma11101958
He Z, Li Y. The Influence of Mayenite Employed as a Functional Component on Hydration Properties of Ordinary Portland Cement. Materials. 2018; 11(10):1958. https://doi.org/10.3390/ma11101958
Chicago/Turabian StyleHe, Zhen, and Yang Li. 2018. "The Influence of Mayenite Employed as a Functional Component on Hydration Properties of Ordinary Portland Cement" Materials 11, no. 10: 1958. https://doi.org/10.3390/ma11101958
APA StyleHe, Z., & Li, Y. (2018). The Influence of Mayenite Employed as a Functional Component on Hydration Properties of Ordinary Portland Cement. Materials, 11(10), 1958. https://doi.org/10.3390/ma11101958




